
Transcriber's note:
This book contains many abbreviations. Abbreviations of words have been expanded using the title attribute; screenreader users may wish to set their computer to read only the title attribute. Abbreviations used to identify parts of illustrations are spelled out.
OF
VOL. I.
Memorial Edition.
Cambridge:
PRINTED BY C. J. CLAY, M.A. AND SON,
AT THE UNIVERSITY PRESS.

Memorial Edition.
OF
M.A., LL.D., F.R.S.,
FELLOW OF TRINITY COLLEGE,
AND PROFESSOR OF
ANIMAL MORPHOLOGY IN THE UNIVERSITY OF
CAMBRIDGE.
EDITED BY
PROFESSOR OF PHYSIOLOGY IN THE UNIVERSITY OF CAMBRIDGE;
AND
FELLOW AND LECTURER OF TRINITY COLLEGE, CAMBRIDGE.
VOL. I.
SEPARATE MEMOIRS.
London:
MACMILLAN AND CO.
1885
[The Right of Translation is reserved.]
Upon the death of Francis Maitland Balfour, a desire very naturally arose among his friends and admirers to provide some memorial of him. And, at a public meeting held at Cambridge in October 1882, the Vice-Chancellor presiding, and many distinguished men of science being present, it was decided to establish a 'Balfour Fund' the proceeds of which should be applied: firstly to maintain a studentship, the holder of which should devote himself to original research in Biology, especially in Animal Morphology, and secondly, 'by occasional grants of money, to further in other ways original research in the same subject'. The sum of £8446 was subsequently raised; this was, under certain conditions, entrusted to and accepted by the University of Cambridge; and the first 'Balfour student' was appointed in October 1883.
The publication of Balfour's works in a collected form was not proposed as an object on which part of the fund should be expended, since his family had expressed their wish to take upon themselves the charge of arranging for a memorial edition of their brother's scientific writings. [Pg ii] That edition, with no more delay than circumstances have rendered necessary, is now laid before the public. It comprises four volumes.
The first volume contains, in chronological order, all Balfour's scattered original papers, including those published by him in conjunction with his pupils, as well as the Monograph on the Elasmobranch Fishes. The last memoir in the volume, that on the Anatomy and Development of Peripatus Capensis, was published after his death, from his notes and drawings, with additions by Prof. Moseley and Mr Adam Sedgwick, who prepared the manuscript for publication. To the volume is prefixed an introductory biographical notice.
The second and third volumes are the two volumes of the Comparative Embryology reprinted from the original edition without alteration, save the correction of obvious misprints and omissions.
The fourth volume contains the plates illustrating the memoirs contained in Vol. 1. We believe that we are consulting the convenience of readers in adopting this plan, rather than in distributing the plates among the memoirs to which they belong. To assist the reader the explanations of these plates have been given twice: at the end of the memoir to which they belong (in the case of the Monograph on Elasmobranch Fishes at the end of each separate chapter), and in the volume of plates.
All the figures of these plates had to be redrawn on the stone, and our best thanks are due to the Cambridge Scientific Instrument Company for the pains which they have taken in executing this work. We are also indebted to the Committee of Publication of the Zoological Society for the gift of electrotypes of the woodcuts illustrating memoir no. XX. of Vol. 1. [Pg iii]
Several photographs of Balfour, taken at different times of his life, the last shortly before his death, are in the possession of his relatives and friends; but these, in the opinion of many, leave much to be desired.
There is also a portrait of him in oils painted since his death by Mr John Collier, A.R.A., and Herr Hildebrand of Florence has executed a posthumous bust in bronze[1]. The portrait which forms the frontispiece of Vol. 1. has been drawn on stone by Mr E. Wilson of the Cambridge Scientific Instrument Company, after the latest photograph. Should it fail, in the eyes of those who knew Balfour well, to have reproduced with complete success his features and expression, we would venture to ask them to bear in mind the acknowledged difficulties of posthumous portraiture.
[1] In possession of the family. Copies also exist in the Library of Trinity College, and in the Morphological Laboratory, at Cambridge.
| TABLE OF CONTENTS. | ||
|---|---|---|
| PAGE | ||
| Preface | i | |
| Introduction | 1 | |
| 1872 | ||
| I. | On some points in the Geology of the East Lothian Coast. By G. W. and F. M. Balfour | 25 |
| 1873 | ||
| II. | The development and growth of the layers of the blastoderm. With Plate 1 | 29 |
| III. | On the disappearance of the Primitive Groove in the Embryo Chick. With Plate 1 | 41 |
| IV. | The development of the blood-vessels of the Chick. With Plate 2 | 47 |
| 1874 | ||
| V. | A preliminary account of the development of the Elasmobranch Fishes. With Plates 3 and 4 | 60 |
| 1875 | ||
| VI. | A comparison of the early stages in the development of Vertebrates. With Plate 5 | 112 |
| VII. | On the origin and history of the urinogenital organs of Vertebrates | 135 |
| VIII. | On the development of the spinal nerves in Elasmobranch Fishes. With Plates 22 and 23 | 168 |
| 1876 [Pg vi] | ||
| IX. | On the spinal nerves of Amphioxus | 197 |
| 1876-78 | ||
| X. | A Monograph on the development of Elasmobranch Fishes. With Plates 6-21 | 203 |
| 1878 | ||
| XI. | On the phenomena accompanying the maturation and impregnation of the ovum | 521 |
| XII. | On the structure and development of the vertebrate ovary. With Plates 24, 25, 26 | 549 |
| 1879 | ||
| XIII. | On the existence of a Head-kidney in the Embryo Chick, and on certain points in the development of the Müllerian duct. By F. M. Balfour and A. Sedgwick. With Plates 27 and 28 | 618 |
| XIV. | On the early development of the Lacertilia, together with some observations on the nature and relations of the primitive Streak. With Plate 29 | 644 |
| XV. | On certain points in the Anatomy of Peripatus Capensis | 657 |
| XVI. | On the morphology and systematic position of the Spongida | 661 |
| 1880 | ||
| XVII. | Notes on the development of the Araneina. With Plates 30, 31, 32 | 668 |
| XVIII. | On the spinal nerves of Amphioxus | 696 |
| XIX. | Address to the Department of Anatomy and Physiology of the British Association for the Advancement of Science | 698 |
| 1881 | ||
| XX. | On the development of the skeleton of the paired fins of Elasmobranchii, considered in relation to its bearings on the nature of the limbs of the Vertebrata. With Plate 33 | 714 |
| XXI. | On the evolution of the Placenta, and on the possibility of employing the characters of the Placenta in the classification of the Mammalia | 734 |
| 1882 [Pg vii] | ||
| XXII. | On the structure and development of Lepidosteus. By F. M. Balfour and W. N. Parker. With Plates 34-42 | 738 |
| XXIII. | On the nature of the organ in Adult Teleosteans and Ganoids which is usually regarded as the Head-kidney or Pronephros | 848 |
| XXIV. | A renewed study of the germinal layers of the Chick. By F. M. Balfour and F. Deighton. With Plates 43, 44, 45 | 854 |
| Posthumous, 1883 | ||
| XXV. | The Anatomy and Development of Peripatus Capensis. Edited by H. N. Moseley and A. Sedgwick. With Plates 46-53 | 871 |
Francis Maitland Balfour, the sixth child and third son of James Maitland Balfour of Whittinghame, East Lothian, and Lady Blanche, daughter of the second Marquis of Salisbury, was born at Edinburgh, during a temporary stay of his parents there, on the 10th November, 1851. He can hardly be said to have known his father, who died of consumption in 1856, at the early age of thirty-six, and who spent the greater part of the last two years of his life at Madeira, separated from the younger children who remained at home. He fancied at one time that he had inherited his father's constitution; and this idea seems to have spurred him on to achieve early what he had to do. But, though there was a period soon after he went to College, during which he seemed delicate, and the state of his health caused considerable anxiety to his friends, he eventually became fairly robust, and that in spite of labours which greatly taxed his strength.
The early years of his life were spent chiefly at Whittinghame under the
loving care of his mother. She made it a point to attempt to cultivate in
all her children some taste for natural science, especially for natural
history, and in this she was greatly helped by the boys' tutor, Mr J. W.
Kitto. They were encouraged to make collections and to form a museum, and
the fossils found in the gravel spread in front of the house served as the
nucleus of a geological series. Frank soon became greatly interested in
these things, and indeed they may be said to have formed the beginnings of
his scientific career. At all events there was thus awakened in him a love
for geology, which science continued to be his favorite study all through
his [Pg 2]
boyhood, and interested him to the last. He was most assiduous in searching
for fossils in the gravel and elsewhere, and so great was his love for his
collections that while as yet quite a little boy the most delightful
birthday present he could think of was a box with trays and divisions to
hold his fossils and specimens. His mother, thinking that his fondness for
fossils was a passing fancy and that he might soon regret the purchase of
the box, purposely delayed the present. But he remained constant to his
wish and in time received his box. He must at this time have been about
seven or eight years old. In the children's museum, which has been
preserved, there are specimens labelled with his childish round-hand, such
as a piece of stone with the label marks of some shels;
and his
sister Alice, who was at that time his chief companion, remembers
discussing with him one day after the nursery dinner, when he was about
nine years old, whether it were better to be a geologist or a naturalist,
he deciding for the former on the ground that it was better to do one thing
thoroughly than to attempt many branches of science and do them
imperfectly.
Besides fossils, he collected not only butterflies, as do most boys at some time or other, but also birds; and he with his sister Alice, being instructed in the art of preparing and preserving skins, succeeded in making a very considerable collection. He thus acquired before long not only a very large but a very exact knowledge of British birds.
In the more ordinary work of the school-room he was somewhat backward. This may have been partly due to the great difficulty he had in learning to write, for he was not only left-handed but, in his early years, singularly inapt in acquiring particular muscular movements, learning to dance being a great trouble to him. Probably however the chief reason was that he failed to find any interest in the ordinary school studies. He fancied that the family thought him stupid, but this does not appear to have been the case.
In character he was at this time quick tempered, sometimes even violent, and the energy which he shewed in after life even thus early manifested itself as perseverance, which, when he was crossed, often took on the form of obstinacy, causing at times no little trouble to his nurses and tutors. But he was at the [Pg 3] same time warm-hearted and affectionate; full of strong impulses, he disliked heartily and loved much, and in his affections was wonderfully unselfish, wholly forgetting himself in his thought for others, and ready to do things which he disliked to please those whom he loved. Though, as we have said, somewhat clumsy, he was nevertheless active and courageous; in learning to ride he shewed no signs of fear, and boldly put his pony to every jump which was practicable.
In 1861 he was sent to the Rev. C. G. Chittenden's preparatory school at Hoddesden in Hertfordshire, and here the qualities which had been already visible at home became still more obvious. He found difficulty not only in writing but also in spelling, and in the ordinary school-work he took but little interest and made but little progress.
In 1865 he was moved to Harrow and placed in the house of the Rev. F. Rendall. Here, as at Hoddesden, he did not
show any great ability in the ordinary school studies, though as he grew
older his progress became more marked. But happily he found at Harrow an
opportunity for cultivating that love of scientific studies which was
yearly growing stronger in him. Under the care of one of the Masters, Mr G.
Griffith, the boys at Harrow were even then taught the elements of natural
science. The lessons were at that time, so to speak, extra-academical,
carried on out of school hours; nevertheless, many of the boys worked at
them with diligence and even enthusiasm, and among these Balfour became
conspicuous, not only by his zeal but by his ability. Griffith was soon
able to recognize the power of his new pupil, and thus early began to see
that the pale, earnest, somewhat clumsy-handed lad, though he gave no
promise of being a scholar in the narrower sense of the word, had in him
the makings of a man of science. Griffith chiefly confined his teaching to
elementary physics and chemistry with some little geology, but he also
encouraged natural history studies and began the formation of a museum of
comparative anatomy. Balfour soon began to be very zealous in dissecting
animals, and was especially delighted when the Rev. A. C. Eaton, the well-known entomologist, on a visit
to Harrow, initiated Griffith's pupils in the art of dissecting under
water. The dissection of a caterpillar in this way was probably an [Pg 4] epoch in
Balfour's life. Up to that time his rough examination of such bodies had
revealed to him nothing more than what in school-boy language he spoke of
as squash;
but when under Eaton's deft hands the intricate organs of
the larval Arthropod floated out under water and displayed themselves as a
labyrinth of threads and sheets of silvery whiteness a new world of
observation opened itself up to Balfour, and we may probably date from this
the beginning of his exact morphological knowledge.
While thus learning the art of observing, he was at the same time
developing his power of thinking. He was by nature fond of argument, and
defended with earnestness any opinions which he had been led to adopt. He
was very active in the Harrow Scientific Society, reading papers, taking
part in the discussions, and exhibiting specimens. He gained in 1867 a
prize for an essay on coal, and when, in 1868, Mr Leaf offered a prize (a
microscope) for the best account of some locality visited by the writer
during the Easter Holidays,
two essays sent in, one by Balfour, the
other by his close friend, Mr Arthur Evans, since well known for his
researches in Illyria, were found to be of such unusual merit that Prof.
Huxley was specially requested to adjudicate between them. He judged them
to be of equal merit, and a prize was given to each. The subject of
Balfour's essay was The Geology and Natural History of East Lothian.
When biological subjects were discussed at the Scientific Society, Balfour
appears to have spoken as a most uncompromising opponent of the views of Mr
Charles Darwin, little thinking that in after life his chief work would be
to develop and illustrate the doctrine of evolution.
The years at Harrow passed quickly away, Balfour making fair, but
perhaps not more than fair, progress in the ordinary school learning. In
due course however he reached the upper sixth form, and in his last year,
became a monitor. At the same time his exact scientific knowledge was
rapidly increasing. Geology still continued to be his favorite study, and
in this he made no mean progress. During his last years at Harrow he and
his brother Gerald worked out together some views concerning the geology of
their native county. These views they ultimately embodied in a paper, which
was published in their joint names in the Geological Magazine
for 1872, under the title [Pg 5] of Some Points in the Geology of the East
Lothian Coast,
and which was in itself a work of considerable promise.
Geology however was beginning to find a rival in natural history. Much of
his holiday time was now spent in dredging for marine animals along the
coast off Dunbar. Each specimen thus obtained was carefully determined and
exact records were kept of the various 'finds,' so that the dredgings
(which were zealously continued after he had left Harrow and gone to
Cambridge) really constituted a serious study of the fauna of this part of
the coast. They also enabled him to make a not inconsiderable collection of
shells, in the arrangement of which he was assisted by his sister Evelyn,
of crustacea and of other animals.
Both to the masters and to his schoolfellows he became known as a boy of great force of character. Among the latter his scrupulous and unwavering conscientiousness made him less popular perhaps than might have been expected from his bright kindly manner and his unselfish warmheartedness. In the incidents of school life a too strict conscience is often an inconvenience, and the sternness and energy with which Balfour denounced acts of meanness and falsehood were thought by some to be unnecessarily great. He thus came to be feared rather than liked by many, and comparatively few grew to be sufficiently intimate with him to appreciate the warmth of his affections and the charm of his playful moments.
At the Easter of 1870 he passed the entrance examination at Trinity College, Cambridge, and entered into residence in the following October. His college tutor was Mr J. Prior, but he was from the first assisted and guided in his studies by his friend, Mr Marlborough Pryor, an old Harrow boy, who in the same October had been, on account of his distinction in Natural Science, elected a Fellow of the College, in accordance with certain new regulations which then came into action for the first time, and which provided that every three years one of the College Fellowships should be awarded for excellence in some branch or branches of Natural Science, as distinguished from mathematics, pure or mixed. During the whole of that year and part of the next Mr Marlborough Pryor remained in residence, and his influence in wisely directing Balfour's studies had a most beneficial effect on the latter's progress.
[Pg 6] During his first term Balfour was occupied in preparation for the Previous Examination; and this he successfully passed at Christmas. After that he devoted himself entirely to Natural Science, attending lectures on several branches. During the Lent term he was a very diligent hearer of the lectures on Physiology which I was then giving as Trinity Prælector, having been appointed to that post in the same October that Balfour came into residence. At this time he was not very strong, and I remember very well noticing among my scanty audience, a pale retiring student, whose mind seemed at times divided between a desire to hear the lecture and a feeling that his frequent coughing was growing an annoyance to myself and the class. This delicate-looking student, I soon learnt, was named Balfour, and when the Rev. Coutts Trotter, Mr Pryor and myself came to examine the candidates for the Natural Science Scholarships which were awarded at Easter, we had no difficulty in giving the first place to him. In point of knowledge, and especially in the thoughtfulness and exactitude displayed in his papers and work, he was very clearly ahead of his competitors.
During the succeeding Easter term and the following winter he appears to have studied physics, chemistry, geology and comparative anatomy, both under Mr Marlborough Pryor and by means of lectures. He also continued to attend my lectures, but though I gradually got to know him more and more we did not become intimate until the Lent term of 1872. He had been very much interested in some lectures on embryology which I had given, and, since Marlborough Pryor had left or was about to leave Cambridge, he soon began to consult me a good deal about his studies. He commenced practical histological and embryological work under me, and I remember very vividly that one day when we were making a little excursion in search of nests and eggs of the stickleback in order that he might study the embryology of fishes, he definitely asked my opinion as to whether he might take up a scientific career with a fair chance of success. I had by this time formed a very high opinion of his abilities, and learning then for the first time that he had an income independent of his own exertions, my answer was very decidedly a positive one. Soon after, feeling more and [Pg 7] more impressed with his power and increasingly satisfied both with his progress in biological studies and his sound general knowledge of other sciences, anxious also, it may be, at the same time that as much original inquiry as possible should be carried on at Cambridge in my department, I either suggested to him or acquiesced in his own suggestion that he should at once set to work on some distinct research; and as far as I remember the task which I first proposed to him was an investigation of the layers of the blastoderm in the chick. It must have been about the same time that I proposed to him to join me in preparing for publication a small work on Embryology, the materials for this I had ready to hand in a rough form as lectures which I had previously given. To this proposal he enthusiastically assented, and while the lighter task of writing what was to be written fell to me, he undertook to work over as far as was possible the many undetermined points and unsatisfactory statements across which we were continually coming.
During his two years at College his health had improved; though still hardly robust and always in danger of overworking himself, he obviously grew stronger. He rejoiced exceedingly in his work, never tiring of it, and was also making his worth felt among his fellow students, and especially perhaps among those of his own college whose studies did not lie in the same direction as his own. At this time he must have been altogether happy, but a sorrow now came upon him. His mother, to whom he was passionately attached, and to whose judicious care in his early days not only the right development of his strong character but even his scientific leanings were due, had for some time past been failing in health, though her condition caused no immediate alarm. In May 1872, however, she died quite suddenly from unsuspected heart disease. Her loss was a great blow to him, and for some time afterward I feared his health would give way; but he bore his grief quietly and manfully and threw himself with even increased vigour into his work.
During the academic session of 1872-3, he continued steadily at work at his investigations, and soon began to make rapid progress. At the beginning he had complained to me about what he considered his natural clumsiness, and expressed a fear [Pg 8] that he should never be able to make satisfactory microscopic sections; as to his being able to make drawings of his dissections and microscopical preparations, he looked upon that at first as wholly impossible. I need hardly say that in time he acquired great skill in the details of microscopical technique, and that his drawings, if wanting in so-called artistic finish, were always singularly true and instructive. While thus struggling with the details which I could teach him, he soon began to manifest qualities which no teacher could give him. I remember calling his attention to Dursy's paper on the Primitive Streak, and suggesting that he should work the matter over, since if such a structure really existed, it must, most probably, have great morphological significance. I am free to confess that I myself rather doubted the matter, and a weaker student might have been influenced by my preconceptions. Balfour, however, thus early had the power of seeing what existed and of refusing to see what did not exist. He was soon able to convince me that Dursy's streak was a reality, and the complete working out of its significance occupied his thoughts to the end of his days.
The results of these early studies were made known in three papers which appeared in the Quarterly Journal of Microscopical Science for July 1873, and will be found in the beginning of this volume. The summer and autumn of that year were spent partly in a visit to Finland, in company with his friend and old school-fellow Mr Arthur Evans, and partly in formal preparation for the approaching Tripos examination. Into this preparation Balfour threw himself with characteristic energy, and fully justified my having encouraged his spending so much of the preceding time in original research, not only by the rapidity with which he accumulated the stock of knowledge of various kinds necessary for the examination but also by the manner in which he acquitted himself at the trial itself. At that time the position of the candidates in the Natural Sciences Tripos was determined by the total number of marks, and Balfour was placed second, the first place being gained by H. Newell Martin of Christ's College, now Professor at Baltimore, U.S.A. In the examination, in which I took part, Balfour did not write much, and he had not yet learnt the art of putting his statements in the best [Pg 9] possible form; he won his position chiefly by the firm thought and clear insight which was present in almost all his answers.
The examination was over in the early days of Dec. 1873 and Balfour was now free to devote himself wholly to his original work. Happily, the University had not long before secured the use of two of the tables at the then recently founded Stazione Zoologica at Naples. And upon the nomination of the University, Balfour, about Christmas, started for Naples in company with his friend Mr A. G. Dew-Smith, also of Trinity College. The latter was about to carry on some physiological observations; Balfour had set himself to work out as completely as he could the embryology of Elasmobranch fishes, about which little was at that time known, but which, from the striking characters of the adult animals could not help proving of interest and importance.
From his arrival there at Christmas 1873 until he left in June 1874, he worked assiduously, and with such success, that as the result of the half-year's work he had made a whole series of observations of the greatest importance. Of these perhaps the most striking were those on the development of the urogenital organs, on the neurenteric canal, on the development of the spinal nerves, on the formation of the layers and on the phenomena of segmentation, including a history of the behaviour of nuclei in cell division. He returned home laden with facts and views both novel and destined to influence largely the progress of embryology.
In August of the same year he attended the meeting of the British Association for the Advancement of Science at Belfast; and the account he then gave of his researches formed one of the most important incidents at the Biological Section on that occasion.
In the September of that year the triennial fellowship for Natural Science was to be awarded at Trinity College, and Balfour naturally was a candidate. The election was, according to the regulations, to be determined partly by the result of an examination in various branches of science, and partly by such evidence of ability and promise as might be afforded by original work, published or in manuscript. He spent the remainder of the autumn in preparation for this examination. But when the [Pg 10] examination was concluded it was found that in his written answers he had not been very successful; he had not even acquitted himself so well as in the Tripos of the year before, and had the election been determined by the results of the examination alone, the examiners would have been led to choose the gentleman who was Balfour's only competitor. The original work however which Balfour sent in, including a preliminary account of the discoveries made at Naples, was obviously of so high a merit and was spoken of in such enthusiastic terms by the External Referee Prof. Huxley, that the examiners did not hesitate for a moment to neglect altogether the formal written answers (and indeed the papers of questions were only introduced as a safeguard, or as a resource in case evidence of original power should be wanted) and unanimously recommended him for election. Accordingly he was elected Fellow in the early days of October.
Almost immediately after, the little book on Embryology appeared, on which he and I had been at work, he doing his share even while his hands and mind were full of the Elasmobranch inquiry. The title-page was kept back some little time in order that his name might appear on it with the addition of Fellow of Trinity, a title of which he was then, and indeed always continued to be, proud. He also published in the October number of the Quarterly Journal of Microscopical Science a preliminary account of his Elasmobranch researches.
He and his friends thought that after these almost incessant labours, and the excitement necessarily contingent upon the fellowship election, he needed rest and change. Accordingly on the 17th of October he started with his friend Marlborough Pryor on a voyage to the west coast of South America. They travelled thither by the Isthmus of Panama, visited Peru and Chili, and returned home along the usual route by the Horn; reaching England some time in Feb. 1875.
Refreshed by this holiday, he now felt anxious to complete as far as
possible his Elasmobranch work, and very soon after his return home, in
fact in March, made his way again to Naples, where he remained till the hot
weather set in in May. [Pg 11] On his return to Cambridge, he still
continued working on the Elasmobranchii, receiving material partly from
Naples, partly from the Brighton Aquarium, the then director of which, Mr
Henry Lee, spared no pains to provide him both with embryo and adult
fishes. While at Naples, he communicated to the Philosophical Society at
Cambridge a remarkable paper on The Early Stages of Vertebrates,
which was published in full in the Quarterly Journal of Microscopical
Science, July, 1875; he also sent me a paper on The Development
of the Spinal Nerves
, which I communicated to the Royal Society, and
which was subsequently published in the Philosophical
Transactions of 1876. He further wrote in the course of the summer
and published in the Journal of Anatomy and Physiology in
October, 1875, a detailed account of his Observations and Views on the
Development of the Urogenital Organs.
Some time in August of the same year he started in company with Mr Arthur Evans and Mr J. F. Bullar for a second trip to Finland, the travellers on this occasion making their way into regions very seldom visited, and having to subsist largely on the preserved provisions which they carried with them, and on the produce of their rods and guns. From a rough diary which Balfour kept during this trip it would appear that while enjoying heartily the fun of the rough travelling, he occupied himself continually with observations on the geology and physical phenomena of the country, as well as on the manners, antiquities, and even language of the people. It was one of his characteristic traits, a mark of the truly scientific bent of his mind, of his having, as Dohrn soon after Balfour's first arrival at Naples said, 'a real scientific head,' that every thing around him wherever he was, incited him to careful exact observation, and stimulated him to thought.
In the early part of the Long Vacation of the same year he had made his first essay in lecturing, having given a short course on Embryology in a room at the New Museums, which I then occupied as a laboratory. Though he afterwards learnt to lecture with great clearness he was not by nature a fluent speaker, and on this occasion he was exceedingly [Pg 12] nervous. But those who listened to him soon forgot these small defects as they began to perceive the knowledge and power which lay in their new teacher.
Encouraged by the result of this experiment, he threw himself, in spite of the heavy work which the Elasmobranch investigation was entailing, with great zeal into an arrangement which Prof. Newton, Mr J. W. Clark and myself had in course of the summer brought about, that he and Mr A. Milnes Marshall, since Professor at Owens College, Manchester, should between them give a course on Animal Morphology, with practical instruction, Prof. Newton giving up a room in the New Museums for the purpose.
In the following October (1875) upon Balfour's return from Finland, these lectures were accordingly begun and carried on by the two lecturers during the Michaelmas and Lent Terms. The number of students attending this first course, conducted on a novel plan, was, as might be expected, small, but the Lent Term did not come to an end before an enthusiasm for morphological studies had been kindled in the members of the class.
The ensuing Easter term (1876) was spent by Balfour at Naples, in order that he might carry on towards completion his Elasmobranch work. He had by this time determined to write as complete a monograph as he could of the development of these fishes, proposing to publish it in instalments in the Journal of Anatomy and Physiology, and subsequently to gather together the several papers into one volume. The first of these papers, dealing with the ovum, appeared in Jan. 1876; most of the numbers of the Journal during that and the succeeding year contained further portions; but the complete monograph did not leave the publisher's hands until 1878.
He returned to England with his pupil and friend Mr J. F. Bullar some time in the summer; on their way home they passed through Switzerland, and it was during the few days which he then spent in sight of the snow-clad hills that the beginnings of a desire for that Alpine climbing, which was destined to be so disastrous, seem to have been kindled in him.
In October, 1876, he resumed the lectures on Morphology, taking the whole course himself, his colleague, Mr Marshall, [Pg 13] having meanwhile left Cambridge. Indeed, from this time onward, he may be said to have made these lectures, in a certain sense, the chief business of his life. He lectured all three terms, devoting the Michaelmas and Lent terms to a systematic course of Animal Morphology, and the Easter term to a more elementary course of Embryology. These lectures were given under the auspices of Prof. Newton; but Balfour's position was before long confirmed by his being made a Lecturer of Trinity College, the lectures which he gave at the New Museums, and which were open to all students of the University, being accepted in a liberal spirit by the College as equivalent to College Lectures. He very soon found it desirable to divide the morphological course into an elementary and an advanced course, and to increase the number of his lectures from three to four a week. Each lecture was followed by practical work, the students dissecting and examining microscopically, an animal or some animals chosen as types to illustrate the subject-matter of the lecture; and although Balfour had the assistance at first of one[2], and ultimately of several demonstrators, he himself put his hand to the plough, and after the lecture always spent some time in the laboratory among his pupils. Had Balfour been only an ordinary man, the zeal and energy which he threw into his lectures, and into the supervision of the practical work, added to the almost brotherly interest which he took in the individual development of every one of the pupils who shewed any love whatever for the subject, would have made him a most successful teacher. But his talents and powers were such as could not be hid even from beginners. His extensive and exact knowledge, the clearness with which in spite of, or shall I not rather say, by help of a certain want of fluency, he explained difficult and abstruse matters, the trenchant way in which he lay bare specious fallacies, and the presence in almost his every word of that power which belongs only to the man who has thought out for himself everything which he says, these things aroused and indeed could hardly fail to arouse in his hearers feelings which, except in the case of the very dullest, grew to be those of [Pg 14] enthusiasm. His class, at first slowly, but afterwards more rapidly, increased in numbers, and, what is of more importance, grew in quality. The room allotted to him soon became far too small and steps were taken to provide for him, for myself, whose wants were also urgent, and for the biological studies generally, adequate accommodation; but it was not until Oct. 1877 that we were able to take possession of the new quarters.
Even this new accommodation soon became insufficient, and in the spring of 1882 a new morphological laboratory was commenced in accordance with plans suggested by himself. He was to have occupied them in the October term, 1883, but did not live to see them finished.
As might have been expected from his own career, he regarded the mere teaching of what is known as a very small part of his duties as Lecturer; and as soon as any of his pupils became sufficiently advanced, he urged or rather led them to undertake original investigations; and he had the satisfaction before his death of seeing the researches of his pupils (such as those by Messrs. Bullar, Sedgwick, Mitzikuri, Haddon, Scott, Osborne, Caldwell, Heape, Weldon, Parker, Deighton and others) carried to a successful end. In each of these inquiries he himself took part, sometimes a large part, generally suggesting the problem to be solved, indicating the methods, and keeping a close watch over the whole progress of the study. Hence in many cases the published account bears his name as well as that of the pupil.
In the year 1878 his Monograph on Elasmobranch Fishes was published as a complete volume, and in the same year he received the honour of being elected a Fellow of the Royal Society, a distinction which now-a-days does not often fall to one so young. No sooner was the Monograph completed than in spite of the labours which his lectures entailed, he set himself to the great task of writing a complete treatise on Comparative Embryology. This not only laid upon him the heavy burden of gathering together the observations of others, enormous in number and continually increasing, scattered through many journals and books, and recorded in many different languages, as well as of putting them in orderly array, and of winnowing [Pg 15] out the grain from the chaff (though his critical spirit found some relief in the latter task), but also caused him much labour, inasmuch as at almost every turn new problems suggested themselves, and demanded inquiry before he could bring his mind to writing about them. This desire to see his way straight before him, pursued him from page to page, and while it has resulted in giving the book an almost priceless value, made the writing of it a work of vast labour. Many of the ideas thus originated served as the bases of inquiries worked out by himself or his pupils, and published in the form of separate papers, but still more perhaps never appeared either in the book or elsewhere and were carried with him undeveloped and unrecorded to the grave.
The preparation of this work occupied the best part of his time for the next three years, the first volume appearing in 1880, the second in 1881.
In the autumn of 1880, he attended the Meeting at Swansea of the British Association for the Advancement of Science, having been appointed Vice-President of the Biological Section with charge of the Department of Anatomy and Physiology. At the Meetings of the Association, especially of late years, much, perhaps too much, is expected in the direction of explaining the new results of science in a manner interesting to the unlearned. Popular expositions were never very congenial to Balfour, his mind was too much occupied with the anxiety of problems yet to be solved; he was therefore not wholly at his ease, in his position on this occasion. Yet his introductory address, though not of a nature to interest a large mixed audience, was a luminous, brief exposition of the modern development and aims of embryological investigation.
During these years of travail with the Comparative Embryology the amount of work which he got through was a marvel to his friends, for besides his lectures, and the researches, and the writing of the book, new labours were demanded of him by the University for which he was already doing so much. Men at Cambridge, and indeed elsewhere as well, soon began to find out that the same clear insight which was solving biological problems could be used to settle knotty [Pg 16] questions of policy and business. Moreover he united in a remarkable manner, the power of boldly and firmly asserting and maintaining his own views, with a frank courteousness which went far to disarm opponents. Accordingly he found himself before long a member of various Syndicates, and indeed a very great deal of his time was thus occupied, especially with the Museums and Library Syndicates, in both of which he took the liveliest interest. Besides these University duties his time and energy were also at the service of his College. In the preparation of the New Statutes, with which about this time the College was much occupied, the Junior Fellows of the College took a conspicuous share, and among these Junior Fellows Balfour was perhaps the most active; indeed he was their leader, and he threw himself into the investigation of the bearings and probable results of this and that proposed new statute with as much zeal as if he were attacking some morphological problem.
While he was in the midst of these various labours, his friends often feared for his strength, for though gradually improving in health after his first year at Cambridge, he was not robust, and from time to time he seemed on the point of breaking down. Still, hard as he was working, he was in reality wisely careful of himself, and as he grew older, paid more and more attention to his health, daily taking exercise in the form either of bicycle rides or of lawn-tennis. Moreover he continued to spend some part of his vacations in travel. Combining business with pleasure, he made frequent visits to Germany and France, and especially to Naples. The Christmas of 1876-7 he spent in Greece, that of 1878-9 at Ragusa, where his old school-fellow and friend Mr Arthur Evans was at that time residing, and the appointment of his friend Kleinenberg to a Professorship at Messina led to a journey there. Early in the long vacation of 1880, he went with his sister, Mrs H. Sidgwick, and her husband to Switzerland, and was joined there for a short time by his friend and pupil Adam Sedgwick. During this visit he took his first lessons in Alpine climbing, making several excursions, some of them difficult and dangerous; and the love of mountaineering laid so firm a hold upon him, that he returned to Switzerland later on in the autumn of the same year, in company with his [Pg 17] brother Gerald, and spent some weeks near Zermatt in systematic climbing, ascending, among other mountains, the Matterhorn and the Weisshorn. In the following summer, 1881, he and his brother Gerald again visited the Alps, dividing their time between the Chamonix district and the Bernese Oberland. On this occasion some of the excursions which they made were of extreme difficulty, and such as needed not only great presence of mind and bodily endurance, but also skilful and ready use of the limbs. As a climber indeed Balfour soon shewed himself fearless, indefatigable, and expert in all necessary movements as well as full of resources and expedients in the face of difficulties, so much so that he almost at once took rank among the foremost of distinguished mountaineers. In spite of his apparent clumsiness in some matters, he had even as a lad proved himself to be a bold and surefooted climber. Moreover he had been perhaps in a measure prepared for the difficulties of Alpine climbing by his experience in deer-stalking. This sport he had keenly and successfully pursued for many years at his brother's place in Rosshire. When however about the year 1877, the question of physiological experiments on animals became largely discussed in public, he felt that to continue the pursuit of this or any other sport involving, for the sake of mere pleasure, the pain and death of animals, was inconsistent with the position which he had warmly taken up, as an advocate of the right to experiment on animals; and he accordingly from that time onward wholly gave it up.
His fame as an investigator and teacher, and as a man of brilliant and powerful parts, was now being widely spread. Pupils came to him, not only from various parts of England, but from America, Australia and Japan. At the York Meeting of the British Association for the Advancement of Science, in August, 1881, he was chosen as one of the General Secretaries. In April, 1881, the honorary degree of LL.D. was conferred upon him by the University of Glasgow, and in November of the same year the Royal Society gave him one of the Royal Medals in recognition of his embryological discoveries, and at the same time placed him on its Council.
At Cambridge he was chosen, in the autumn of 1880, President of the Philosophical Society, and in the December of that [Pg 18] year a brilliant company were gathered together at the Annual Dinner to do honour to their new young President. Otherwise nothing as yet had been done for him in his own University in the way of recognition of his abilities and services; and he still remained a Lecturer of Trinity College, giving lectures in a University building. An effort had been made by some of his friends to urge the University to take some step in this direction; but it was thought at that time impossible to do anything. In 1881 a great loss fell upon the sister University of Oxford in the death of Prof. George Rolleston; and soon after very vigorous efforts were made to induce Balfour to become a candidate for the vacant chair. The prospect was in many ways a tempting one, and Balfour seeing no very clear way in the future for him at his own University, was at times inclined to offer himself, but eventually he decided to remain at Cambridge. Hardly had this temptation if we may so call it been overcome when a still greater one presented itself. Through the lamented death of Sir Wyville Thomson in the winter of 1881-2, the chair of Natural History at Edinburgh, perhaps the richest and most conspicuous biological chair in the United Kingdom, became vacant. The post was in many ways one which Balfour would have liked to hold. The teaching duties were it is true laborious, but they had in the past been compressed into a short time, occupying only the summer session and leaving the rest of the year free, and it seemed probable that this arrangement might be continued with him. The large emolument would also have been grateful to him inasmuch as he would have felt able to devote the whole of it to scientific ends; and the nearness to Whittinghame, his native place and brother's home, added to the attractions; but what tempted him most was the position which it would have given him, and the opportunities it would have afforded, with the rich marine Fauna of the north-eastern coast close at hand, to develop a large school of Animal Morphology. The existing Professors at Edinburgh were most desirous that he should join them, and made every effort to induce him to come. On the part of the Crown, in whose hands the appointment lay, not only were distinct offers made to him, but he was repeatedly pressed to accept the post. Nor was it until after a considerable [Pg 19] struggle that he finally refused, his love for his own University in the end overcoming the many inducements to leave; he elected to stay where he was, trusting to the future opening up for him some suitable position. In this decision he was undoubtedly influenced by the consideration that Cambridge, besides being the centre of his old friendships, had become as it were a second home for his own family. By the appointment of Lord Rayleigh to the chair of Experimental Physics his sister Lady Rayleigh had become a resident, his sister Mrs Sidgwick had lived there now for some years, and his brother Gerald generally spent the summer there; their presence made Cambridge doubly dear to him.
At the close of the Michaelmas term, with feelings of relief at having completed his Comparative Embryology, the preparation of the second volume of which had led to almost incessant labour during the preceding year, he started to spend the Christmas vacation with his friend Kleinenberg at Messina. Stopping at Naples on his way thither he found his pupil Caldwell, who had been sent to occupy the University table at the Stazione Zoologica, lying ill at Capri, with what proved to be typhoid fever. The patient was alone, without any friend to tend him, and his mother who had been sent for had not yet arrived. Accordingly Balfour (with the kindness all forgetful of himself which was his mark all his life through) stayed on his journey to nurse the sick man until the mother came. He then went on to Messina, and there seemed to be in good health, amusing himself with the ascent of Etna. Yet in January, soon after his return home, he complained of being unwell, and in due time distinct symptoms of typhoid fever made their appearance. The attack at first promised to be severe, but happily the crisis was soon safely passed and the convalescence was satisfactory.
While yet on his sick bed, a last attempt was made to induce him to accept the Edinburgh offer, and for the last time he refused. These repeated offers, and the fact that the dangers of his grave illness had led the University vividly to realize how much they would lose if Balfour were taken away from them, encouraged his friends to make a renewed effort to gain for him some adequate position in the University. This time [Pg 20] the attempt was successful, and the authorities took a step, unusual but approved of by the whole body of resident members of the University; they instituted a new Professorship of Animal Morphology, to be held by Balfour during his life or as long as he should desire, but to terminate at his death or resignation unless it should be otherwise desirable. Accordingly in May, 1882, he was admitted into the Professoriate as Professor of Animal Morphology.
During his illness his lectures had been carried on by his Demonstrator, Mr Adam Sedgwick, who continued to take his place during the remainder of that Lent Term and during the ensuing Easter Term. The spring Balfour spent partly in the Channel Islands with his sister Alice, partly in London with his eldest brother, but in the course of the Easter Term returned to Cambridge and resumed his work though not his lectures. His recovery to health was steady and satisfactory, the only drawback being a swelling over the shin-bone of one leg, due to a blow on the rocks at Sark; otherwise he was rapidly becoming strong. He himself felt convinced that a visit to the Alps, with some mountaineering of not too difficult a kind, would complete his restoration to health. In this view many of his friends coincided; for the experience of former years had shewn them what a wonderfully beneficial effect the Alpine air and exercise had upon his health. He used to go away pale, thin and haggard, to return bronzed, clear, firm and almost stout; nor was there anything in his condition which seemed to forbid his climbing, provided that he was cautious at the outset. Accordingly, early in June he left Cambridge for Switzerland, having long ago, during his illness in fact, engaged his old guide, Johann Petrus, whom he had first met in 1880, and who had always accompanied him in his expeditions since.
His first walking was in the Chamonix district; and here he very soon found his strength and elasticity come back to him. Crossing over from Montanvert to Courmayeur, by the Col du Géant, he was attracted by the peak called the Aiguille Blanche de Peuteret, a virgin peak, the ascent of which had been before attempted but not accomplished. Consulting with Petrus he determined to try it, feeling that the fortnight, which by this [Pg 21] time he had spent in climbing, had brought back to him his old vigour, and that his illness was already a thing of the past.
There is no reason to believe that he regarded the expedition as one of unusual peril; and an incident which at the time of his death was thought by some to indicate this was in reality nothing more than a proof of his kindly foresight. The guide Petrus was burdened by a debt on his land amounting to about £150. In the previous year Balfour and his brother had come to know of this debt; and, seeing that no Alpine ascent is free from danger, that on any expedition some accident might carry them off, had conceived the idea of making some provision for Petrus' family in case he might meet with sudden death in their service. This suggestion of the previous year Balfour carried out on this occasion, and sent home to his brother Gerald a cheque of £150 for this purpose. But the cheque was sent from Montanvert before he had even conceived the idea of ascending the Aiguille Blanche. It was not a provision for any specially dangerous ascent, and must be regarded as a measure prompted not by a sense of coming peril but rather by the donor's generous care for his servant.
On Tuesday afternoon, July 18, he and Petrus, with a porter to carry provisions and firing to their sleeping-place on the rocks, set out from Courmayeur, the porter returning the same night. They expected to get back to Courmayeur some time on the Thursday, but the day passed without their appearing. This did not cause any great anxiety because it was supposed that they might have found it more convenient to pass over to the Chamonix side than to return to Courmayeur. When on Friday however telegrams dispatched to Chamonix and Montanvert brought answers that nothing had been seen of them, it became evident that some accident had happened, and an exploring party set out for the hills. It was not until early on the Sunday morning that this search party found the bodies, both partly covered with snow, lying on the Glacier de Fresney, below the impassable icefall which separates the upper basin of the glacier from the lower portion, and at the foot of a couloir which descends by the side of the icefall. Their tracks were visible on the snow at the top of the couloir. Balfour's neck was broken, and his skull fractured [Pg 22] in three places; Petrus' body was also fractured in many places. The exact manner of their death will never be known, but there can be no doubt that, in Balfour's case at all events, it was instantaneous, and those competent to form a judgment are of opinion that they were killed by a sudden fall through a comparatively small height, slipping on the rocks as they were descending by the side of the ice-fall, and not precipitated from the top of the couloir. There is moreover indirect evidence which renders it probable that in the fatal fall Petrus slipped first and carried Balfour with him. Whether they had reached the summit of the Aiguille and were returning home after a successful ascent or whether they were making their way back disheartened and wearied with failure, is not and perhaps never will be known. Since the provisions at the sleeping-place were untouched, the deaths probably took place on Wednesday the 19th. The bringing down the bodies proved to be a task of extreme difficulty, and it was not till Wednesday the 26th that the remains reached Courmayeur, where M. Bertolini, the master of the hotel, and indeed everyone, not least the officers of a small body of Italian troops stationed there, shewed the greatest kindness and sympathy to Balfour's brothers, Gerald and Eustace, who hastened to the spot as soon as the news of the terrible disaster was telegraphed home. Mr Walter Leaf also and Mr Conway, friends of Balfour, the former a very old one, who had made their way to Courmayeur from other parts of Switzerland as soon as they heard of the accident, rendered great assistance. The body was embalmed, brought to England, and buried at Whittinghame on Saturday, Aug. 5, the Fellows of Trinity College holding a service in the College Chapel at the same time.
In person he was tall, being fully six feet in height, well built though somewhat spare. A broad forehead overhanging deeply set dark brown eyes whose light shining from beneath strongly marked eye-brows told all the changes of his moods, slightly prominent cheek-bones, a pale skin, at times inclined to be even sallow, dark brown hair, allowed to grow on the face only as a small moustache, and slight whiskers, made up a countenance which bespoke at once strength of character and delicacy of constitution. It was an open countenance, hiding [Pg 23] nothing, giving sign at once, both when his body was weary or weak, and when his mind was gladdened, angered or annoyed.
The record of some of his thoughts and work, all that he had given to the world will be found in the following pages. But who can tell the ideas which had passed into his quick brain, but which as yet were known only to himself, of which he had given no sign up to that sad day on which he made the fatal climb? And who can say whither he might not have reached had he lived, and his bright young life ripened as years went on? This is not the place to attempt any judgment of his work: that may be left to other times, and to other hands; but it may be fitting to place here on record a letter which shews how much the greatest naturalist of this age appreciated his younger brother. Among Balfour's papers was found a letter from Charles Darwin, acknowledging the receipt of Vol. II. of the Comparative Embryology in the following words:
"July 6, 1881.
Down, Beckenham, Kent.
My Dear Balfour,
I thank you heartily for the present of your grand book, and I congratulate you on its completion. Although I read almost all of Vol. I, I do not feel that I am worthy of your present, unless indeed the fullest conviction that it is a memorable work makes me worthy to receive it.
* * * * *
Once again accept my thanks, for I am proud to receive a book from you, who, I know, will some day be the chief of the English Biologists.
Believe me,
Yours sincerely,
Charles Darwin."
The loss of him was a manifold loss. He is mourned, and will long be mourned, for many reasons. Some miss only the brilliant investigator; others feel that their powerful and sympathetic teacher is gone; some look back on his memory [Pg 24] and grieve for the charming companion whose kindly courtesy and bright wit made the hours fly swiftly and pleasantly along; and to yet others is left an aching void when they remember that they can never again lean on the friend whose judgment seemed never to fail and whose warm-hearted affection was a constant help. And to some he was all of these. At the news of his death the same lines came to the lips of all of us, so fittingly did Milton's words seem to speak our loss and grief—
"For Lycidas is dead, dead ere his prime,
Young Lycidas, and hath not left his
peer."
M. FOSTER.
[2] His first Demonstrator up to Christmas 1877, was Mr J. F. Bullar. In Jan. 1878, Mr Adam Sedgwick took the post of Senior Demonstrator, and held it until Balfour's death.
By G. W. and F. M. Balfour, Trinity College, Cambridge.
The interesting relation between the Porphyrite of Whitberry Point, at the mouth of the Tyne, near Dunbar, and the adjacent sedimentary rocks, was first noticed, we believe, by Professor Geikie, who speaks of it in the Memoirs of the Geological Survey of East Lothian, pages 40 and 31, and again in the new edition of Jukes's Geology, p. 269. The volcanic mass which forms the point consists of a dark felspathic base with numerous crystals of augite: it is circular in form, and is exposed for two-thirds of its circumference in a vertical precipice facing the sea, about twenty feet in height.
The rock is traversed by numerous joints running both in a horizontal and in a vertical direction. The latter are by far the most conspicuous, and give the face of the cliff, when seen from a distance, a well-marked columnar appearance, though the columns themselves are not very distinct or regular. They are quadrangular in form, and are evidently produced by the intersection at right-angles of the two series of vertical joints.
It is clear that the face of the precipice has been gradually receding in proportion as it yielded to the action of the waves; and that at a former period the volcanic rock extended considerably further than at present over the beds which are seen to dip beneath it. These latter consist of hard fine-grained calcareous sandstones belonging to the Lower Carboniferous formation. Their colour varies from red to white, and their prevailing dip is in a N.W. direction, with an average inclination of 12-20°. If the volcanic mass is a true intrusive rock, we should naturally expect the strata which surround it to dip away in all directions, the amount of their inclination diminishing in [Pg 26] proportion to their distance from it. We find, however, that the case is precisely the reverse: as the beds approach the base of the cliff, they dip towards it from every side at perpetually increasing angles, until at the point of junction the inclination amounts in places to as much as 55 degrees. The exact amount of dip in the various positions will be seen on referring to the accompanying map.
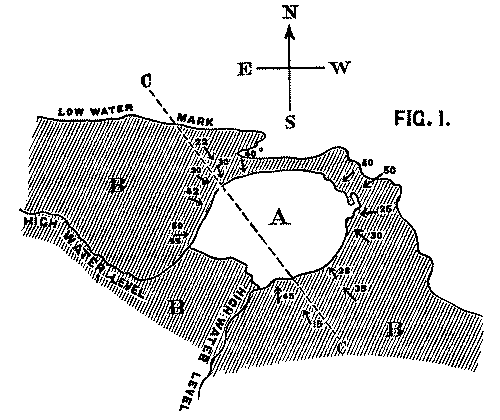
Fig. 1. Map of Strata at Whitberry Point. Scale, 6 in. to the mile.
A. Lava sheet. B. Sandstone Beds, dipping from every side towards the lava. CC. Line of Section along which Fig. 2 is supposed to be drawn.
We conceive that the phenomenon is to be explained by supposing the orifice through which the lava rose and overflowed the surface of the sedimentary strata to have been very much smaller in area than the extent of igneous rock at present visible; and that the pressure of the erupted mass on the soft beds beneath, aided perhaps by the abstraction of matter from below, caused them to incline towards the central point at a gradually increasing angle. The diagram, fig. 2, will serve further to illustrate this hypothesis. A is the neck or orifice by which the melted matter is supposed to ascend. C shews the sheet of lava after it has overspread the surface of the sandstone beds B, so as to cause them to assume their present inclination. The dotted [Pg 27] lines represent the hypothetical extension of the igneous mass and sandstones previous to the denudation which they have suffered from the action of the waves.
Professor Geikie, in his admirable treatise on the Geology of the county[4], adopts a view on this subject which is somewhat different from that which is suggested in this paper. He considers that the whole mass is an intrusive neck of rock with perpendicular sides; and that it once filled up an orifice through the surrounding sedimentary strata, of which it is now the only remnant.
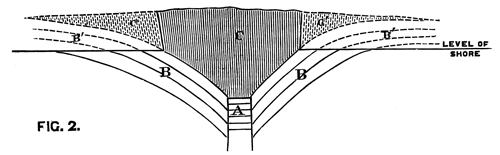
Fig. 2. Vertical Section through CC. Diagram (Fig. 1).
A. Orifice by which the lava ascended. B. Sandstone Beds. B´. Hypothetical extension of ditto. C. Sheet of lava spread over the sandstones B. C´. Hypothetical extension of ditto.
He admits that the inclination of the sandstone beds towards the igneous
mass in the centre is a phenomenon that is somewhat difficult to explain,
and suggests that a subsequent contraction of the column may have tended to
produce such a result. To use his own words: In the case of a solid
column of felstone or basalt, the contraction of the melted mass on cooling
may have had some effect in dragging down the sides of the orifice[5].
But, apart from other objections, it is scarcely conceivable that this result should have been produced by the contraction of the column.
In his recent edition of Jukes's Manual of Geology
(p. 269), in which he also refers to this instance, he states that in
other cases of necks
it is found to be an almost invariable rule,
that [Pg
28] strata are bent down so as to dip into the neck all round its
margin.
We are not aware to what other instances Prof. Geikie may
allude; but on referring to his Memoir on the Geology of East
Lothian, we find that he states in the cases of 'North Berwick Law'
and 'Traprain' (which he compares with the igneous mass at Whitberry
Point), that the beds at the base of these two necks, where exposed, dip
away from them, and that at a high angle.
In support of the hypothesis which we have put forward, the following arguments may be urged:
(1) That in one place at least the sedimentary strata are seen to be actually dipping beneath the superincumbent basalt; and that the impression produced by the general relation of the two rocks is, that they do so everywhere.
(2) Since the columns into which the lava is split are vertical, the cooling surface must have been horizontal: the mass must, therefore, have formed a sheet, and not a dyke; for, in the latter case, the cooling surfaces would have been vertical.
(3) It is difficult to conceive, on the supposition that the volcanic rock is a neck with perpendicular sides, that the marine denudation should have uniformly proceeded only so far as to lay bare the junction between the two formations. We should have expected that in many places the igneous rock itself would have been cut down to the general level, whereas the only signs of such an effect are shown in a few narrow inlets where the rock was manifestly softer than in the surrounding parts.
The last objection is greatly confirmed by the overhanging cliffs and numerous blocks of porphyrite which lie scattered on the beach, as if to attest the former extension of that ancient sheet of which these blocks now form but a small remnant. Indeed, the existence of such remains appears sufficient of itself to condemn any hypothesis which presumes the present face of the cliff to have formed the original boundary of the mass.
It may be fairly objected to our theory, as Prof. Geikie himself has suggested, that the high angle at which the strata dip is difficult to account for. But, in fact, this steep inclination constitutes the very difficulty which any hypothesis on the subject must be framed to explain; and it is a difficulty which is not more easily solved by Prof. Geikie's theory than by our own.
[3] From the Geological Magazine, Vol. IX. No. 4. April, 1872.
[4] Memoirs of Geological Survey of Scotland, sheet 33, pp. 40, 41.
[5] Note on p. 41 of Mem. Geol. Survey of East Lothian.
With Plate 1, figs. 1-5 and 9-12.
The following paper deals with the changes which take place in the cells of the blastoderm of the hen's egg during the first thirty or forty hours of incubation. The subject is one which has, as a general rule, not been much followed up by embryologists, but is nevertheless of the greatest interest, both in reference to embryology itself, and to the growth and changes of protoplasm exhibited in simple embryonic cells. I am far from having exhausted the subject in this paper, and in some cases I shall be able merely to state facts, without being able to give any explanation of their meaning.
My method of investigation has been the examination of sections and surface views. For hardening the blastoderm I have employed, as usual, chromic acid, and also gold chloride. It is, however, difficult to make sections of blastoderms hardened by this latter reagent, and the sections when made are not in all cases satisfactory. For surface views I have chiefly used silver nitrate, which brings out the outlines of the cells in a manner which leaves nothing to be desired as to clearness. If the outlines only of the cells are to be examined, a very short immersion (half a minute) of the blastoderm in a half per cent. solution of silver nitrate is sufficient, but if the immersion lasts for a longer period the nuclei will be brought out also. For studying the latter, however, I have found it better to employ gold chloride or carmine in conjunction with the silver nitrate.
My observations begin with the blastoderm of a freshly laid egg. The
appearances presented by sections of this have been accurately described by
Peremeschko, Ueber die Bildung der [Pg 30] Keimblätter im
Hühnerei,
Sitzungsberichte der K. Akademie der
Wissenschaften in Wien, 1868. Oellacher, Untersuchung
über die Furchung und Blatterbildung im Hühnerei,
Studien aus dem Institut für Experim. Pathologie in Wien, 1870
(pp. 54-74), and Dr Klein, lxiii. Bande der Sitz. der K. Acadamie der Wiss. in
Wien, 1871.
The unincubated blastoderm (Pl. 1, fig. 1) consists of two layers. The upper layer is composed of a single row of columnar cells. Occasionally, however, the layer may be two cells thick. The cells are filled with highly refracting spherules of a very small size, and similar in appearance to the finest white yolk spherules, and each cell also contains a distinct oval nucleus. This membrane rests with its extreme edge on the white yolk, its central portion covering in the segmentation cavity. From the very first it is a distinct coherent membrane, and exhibits with silver nitrate a beautiful hexagonal mosaic of the outlines (Pl. 1, fig. 6) of the cells. The diameter of the cells when viewed from above is from 1/2000 - 1/3000 of an inch. The under layer is very different from this: it is composed of cells which are slightly, if at all, united, and which vary in size and appearance, and in which a nucleus can rarely be seen. The cells of which it is composed fill up irregularly the segmentation cavity, though a distinct space is even at this time occasionally to be found at the bottom of it. Later, when the blastoderm has spread and the white yolk floor has been used as food, a considerable space filled with fluid may generally be found.
The shape of the floor of the cavity varies considerably, but it is usually raised in the middle and depressed near the circumference. In this case the under layer is perhaps only two cells deep at the centre and three or four cells deep near the circumference.
The cells of which this layer is composed vary a good deal in size; the larger cells being, however, more numerous in the lower layers. In addition, there are usually a few very large cells quite at the bottom of the cavity, occasionally separated from the other cells by fluid. They were called formative cells (Bildungselemente) by Peremeschko (loc. cit.); and, according to Oellacher's observations (loc. cit.), some of them, at any rate, fall to the bottom of the segmentation cavity during the later [Pg 31] stages of segmentation. They do not differ from the general lower layer cells except in size, and even pass into them by insensible gradations. All the cells of the lower layer are granular, and are filled with highly refracting spherules precisely similar to the smaller white yolk spherules which line the bottom of the segmentation cavity.
The size of the ordinary cells of the lower layer varies from 1/2000 - 1/1000 of an inch. The largest of the formative cells come up to 1/300 of an inch. It will be seen from this description that, morphologically speaking, we cannot attach much importance to the formative cells. The fact that they broke off from the blastoderm, towards the end of the segmentation—even if we accept it as a normal occurrence, rather than the result of manipulation—is not of much importance, and, except in size, it is impossible to distinguish these cells from other cells of the lower layer of the blastoderm.
Physiologically, however, as will be afterwards shewn, they are of considerable importance.
The changes which the blastoderm undergoes during the first three or four hours of incubation are not very noticeable. At about the sixth or eighth hour, or in some cases considerably earlier, changes begin to take place very rapidly. These changes result in the formation of a hypoblast and mesoblast, the upper layer of cells remaining comparatively unaltered as the epiblast.
To form the hypoblast a certain number of the cells of the lower layer begin to undergo remarkable changes. From being spherical and, as far as can be seen, non-nucleated, they become (vide fig. 2, h) flattened and nucleated, still remaining granular, but with fewer spherules.
Here, then, is a direct change, of which all the stages can be followed, of a cell of one kind into a cell of a totally different character. The new cell is not formed by a destruction of the old one, but directly from it by a process of metamorphosis. These hypoblast cells are formed first at the centre and later at the circumference, so that from the first the cells at the circumference are less flattened and more granular than the cells at the centre. A number of cells of the original lower layer are enclosed between this layer and the epiblast; and, [Pg 32] in addition to these, the formative cells (as has been shewn by Peremeschko, Oellacher, and Klein, whose observations I can confirm) begin to travel towards the circumference, and to pass in between the epiblast and hypoblast.
Both the formative cells, and the lower layer cells enclosed between the hypoblast and epiblast, contribute towards the mesoblast, but the mode in which the mesoblast is formed is very different from that in which the hypoblast originates.
It is in this difference of formation that the true distinction between the mesoblast and hypoblast is to be looked for, rather than in the original difference of the cells from which they are derived.
The cells of the mesoblast are formed by a process which seems to be a kind of free cell formation. The whole of the interior of each of the formative cells, and of the other cells which are enclosed between the epiblast and the hypoblast, become converted into new cells. These are the cells of the mesoblast. I have not been able perfectly to satisfy myself as to the exact manner in which this takes place, but I am inclined to think that some or all of the spherules which are contained in the original cells develop into nuclei for the new cells, the protoplasm of the new cells being formed from that of the original cells.
The stages of formation of the mesoblast cells are shewn in the section (Pl. 1, fig. 2), taken from the periphery of a blastoderm of eight hours.
The first formation of the mesoblast cells takes place in the centre of the blastoderm, and the mass of cells so formed produces the opaque line known as the primitive streak. This is shown in Pl. 1, fig. 9.
One statement I have made in the above description in reference to the origin of the mesoblast cells, viz. that they are only partly derived from the formative cells at the bottom of the segmentation cavity, is to a certain extent opposed to the statements of the three investigators above mentioned. They state that the mesoblast is entirely derived from the formative cells. It is not a point to which I attach much importance, considering that I can detect no difference between these cells and any other cells of the original lower layer except that of size; and even this difference is probably to be explained [Pg 33] by their proximity to the white yolk, whose spherules they absorb. But my reason for thinking it probable that these cells alone do not form the mesoblast are: 1st. That the mesoblast and hypoblast are formed nearly synchronously, and except at the centre a fairly even sprinkling of lower layer cells is from the first to be distinguished between the epiblast and hypoblast. 2nd. That if some of the lower layer cells are not converted into mesoblast, it is difficult to see what becomes of them, since they appear to be too numerous to be converted into the hypoblast alone. 3rd. That the chief formation of mesoblast at first takes place in the centre, while if the formative cells alone took part in its formation, it would be natural to expect that it would begin to be formed at the periphery.
Oellacher himself has shewn (Zeitschrift für
wissenschaftliche Zoologie, 1873, Beiträge zur Entwick. Gesch.
der Knochenfische
) that in osseous fishes the cells which break
away from the blastoderm take no share in the formation of the mesoblast,
so that we can derive no argument from the formation of the mesoblast in
these animals, for believing that in the chick it is derived only from the
formative cells.
In the later stages, however, from the twelfth to the twenty-fifth hour, the growth of the mesoblast depends almost entirely on these cells, and Peremeschko's discovery of the fact is of great value.
Waldeyer (Henle und v. Pfeufer's Zeitschrift, xxxiv. Band, für 1869) has given a different account of the origin of the layers. There is no doubt, however, in opposition to his statements and drawings, that from the very first the hypoblast is distinct from the mesoblast, which is, indeed, most conspicuously shewn in good sections; and his drawings of the derivation of the mesoblast from the epiblast are not very correct.
The changes which have been described are also clearly shewn by means of silver nitrate. Whereas, at first this reagent brought out no outline markings of cells in the lower layer, by the eighth to the twelfth hour the markings (Pl. 1, fig. 3) are very plain, and shew that the hypoblast is a distinct coherent membrane.
In section, the cells of the hypoblast appear generally very thin and spindle shaped, but the outlines brought out by the [Pg 34] silver nitrate shew that they are much expanded horizontally, but very irregular as to size, varying even within a small area from 1/4000 - 1/400 of an inch in the longest diameter.
At about the twelfth hour they are uniformly smaller a short way from each extremity of its longer axis than over the rest of the blastoderm.
It is, perhaps, fair to conclude from this that growth is most rapid at these parts.
At this time the hypoblast, both in sections and from a surface view after treatment with silver nitrate, appears to end abruptly against the white yolk. The surface view also shews that its cells are still filled with highly refractive globules, making it difficult to see the nucleus. In some cases I thought that I could (fig. 3, a) make out that it was hour-glass shaped, and some cells certainly contain two nuclei. Some of the cells (fig. 3, b) shew re-entrant curves, which prove that they have undergone division.
The cells of the epiblast, up to the thirteenth hour, have chiefly undergone change in becoming smaller.
In surface views they are about 1/4000 of an inch in diameter over the centre of the pellucid area, and increase to 1/2000 of an inch over the opaque area.
In the centre of the pellucid area the form of the epiblast cells is more elongated vertically and over the opaque area more flattened than was the case with the original upper layer cells. In the centre the epiblast is two or three cells deep.
Before going on to the further changes of the blastodermic cells it will be well to say a few words in reference to the origin of the mesoblast.
From the description given above it will be clear that in the chick the mesoblast has an independent origin; it can be said neither to originate from the epiblast nor from the hypoblast. It is formed coincidently with the latter out of apparently similar segmentation cells. The hypoblast, as has been long known, shews in the chick no trace of its primitive method of formation by involution, neither does the mesoblast shew any signs of its primitive mode of formation. In so excessively highly differentiated a type as birds we could hardly expect to find, and certainly do not find, any traces of the [Pg 35] primitive origin of the mesoblast, either from the epiblast or hypoblast, or from both. In the chick the mesoblast cells are formed directly from the ultimate products of segmentation. From having a secondary origin in most invertebrates the mesoblast comes to have, in the chick, a primary origin from the segmentation spheres, precisely as we find to be the case with the nervous layer in osseous fishes. It is true we cannot tell which segmentation-cells will form the mesoblast, and which the hypoblast; but the mesoblast and hypoblast are formed at the same time, and both of them directly from segmentation spheres.
The process of formation of the mesoblast in Loligo, as observed by Mr Ray Lankester (Annals and Magazine of Natural History, February, 1873), is still more modified. Here the mesoblast arises independently of the blastoderm, and by a process of free cell-formation in the yolk round the edge of the blastoderm. If Oellacher's observations in reference to the origin of formative cells are correct, then the modes of origin of the mesoblast in Loligo and the chick would have nothing in common; but if the formative cells are in reality derived from the white yolk, and also are alone concerned in the formation of the mesoblast, then the modes of formation of the mesoblast in the chick would be substantially the same as that observed by Mr Ray Lankester in Loligo.
No very important changes take place in the actual forms of the cells during the next few hours. A kind of fusion takes place between the epiblast and the mesoblast along the line of the primitive streak forming the axis-string of His; but the line of junction between the layers is almost always more or less visible in sections. In any case it does not appear that there is any derivation of mesoblast cells from the epiblast; and since the fusion only takes place in the region of the primitive groove, and not in front, where the medullary groove arises (see succeeding paper), it cannot be considered of any importance in reference to the possible origin of the Wolffian duct, &c., from the epiblast (as mooted by Waldeyer, Eierstock und Ei, Leipzig, 1870). The primitive groove, as can be seen in sections, begins to appear very early, generally before the twelfth hour. The epiblast spreads rapidly over the white yolk, and the area pellucida also increases in size.
[Pg 36] From the mesoblast forming at first only a small mass of cells, which lies below the primitive streak, it soon comes to be the most important layer of the blastoderm. Its growth is effected by means of the formative cells. These cells are generally not very numerous in an unincubated blastoderm, but rapidly increase in numbers, probably by division; at the same time they travel round the edge of, and in some cases through, the hypoblast, and then become converted in the manner described into mesoblast cells. They act as carriers of food from the white yolk to the mesoblast till, after the formation of the vascular area, they are no longer necessary. The numerous cases in which two nucleoli and even two nuclei can be seen in one cell prove that the mesoblast cells also increase by division.
The growth of the hypoblast takes place in a very different way. It occurs by a direct conversion, cell for cell, of the white yolk spheres into hypoblast cells. This interpretation of the appearances, which I will describe presently, was first suggested to me by Dr Foster, from an examination of some of my specimens of about thirty-six hours, prepared with silver nitrate. Where there is no folding at the junction between the pellucid and opaque areas, there seems to be a perfect continuity in the silver markings and a gradual transition in the cells, from what would be undoubtedly called white yolk spheres, to as undoubted hypoblast cells (vide Pl. 1, fig. 5). In passing from the opaque to the pellucid areas the number of white yolk spherules in each cell becomes less, but it is not till some way into the pellucid area that they quite cease to be present. I at first thought that this was merely due to the hypoblast cells feeding on the white yolk sphericles, but the perfect continuity of the cells, and the perfect gradation in passing from the white yolk cells to the hypoblast, proves that the other interpretation is the correct one, viz. that the white yolk spheres become directly converted into the hypoblast cells. This is well shewn in sections (vide Pl. 1, fig. 4) taken from embryos of all ages from the fifteenth to the thirty-sixth hour and onwards. But it is, perhaps, most easily seen in embryos of about twenty hours. In such an embryo there is a most perfect gradation: the cells of the hypoblast become, as they approach the edge [Pg 37] of the pellucid area, broader, and are more and more filled with white yolk sphericles, till at the line of junction it is quite impossible to say whether a particular cell is a white-yolk cell (sphere) or a hypoblast cell. The white-yolk cells near the line of junction can frequently be seen to possess nuclei. At first the hypoblast appears to end abruptly against the white yolk; this state of things, however, soon ends, and there supervenes a complete and unbroken continuity between the hypoblast and the white yolk.
Of the mode of increase of the epiblast I have but little to say. The cells undoubtedly increase entirely by division, and the new material is most probably derived directly from the white yolk.
Up to the sixth hour the cells of the upper layer retain their early regular hexagonal pattern, but by the twelfth hour they have generally entirely lost this, and are irregularly shaped and very angular. The cells over the centre of the pellucid area remain the smallest up to the twenty-fifth hour or later, while those over the rest of the pellucid area are uniformly larger.
In the hypoblast the cells under the primitive groove, and on each side as far as the fold which marks off the exterior limit of the protovertebræ are at the eighteenth hour considerably smaller than any other cells of this layer.
In all the embryos between the eighteenth and twenty-third hour which I have examined for the purpose, I have found that at about two-thirds of the distance from the anterior end of the pellucid area, and just external to the side fold, there is a small space on each side in which the cells are considerably larger than anywhere else in the hypoblast. These larger cells, moreover, contain a greater number of highly refractive spherules than any other cells. It is not easy to understand why growth should have been less rapid here than elsewhere, as the position does not seem to correspond to any feature in the embryo. In some specimens the hypoblast cells at the extreme edge of the pellucid area are smaller than the cells immediately internal to them. At about the twenty-third hour these cells begin rapidly to lose the refractive spherules they contained in the earlier stages of incubation, and come [Pg 38] to consist of a nucleus surrounded simply by granular protoplasm.
At about this period of incubation the formative cells are especially numerous at the periphery of the blastoderm, and, no doubt, become converted into the mass of mesoblast which is found at about the twenty-fifth hour in the region of the vascular area. Some of them are lobate, and appear as if they were undergoing division. At this time also the greatest number of formative cells are to be found at the bottom of the now large segmentation cavity.
In embryos of from thirty to forty hours the cells of the hypoblast have, over the central portion of the pellucid area, entirely lost their highly refractive spherules, and in the fresh state are composed of the most transparent protoplasm. When treated with reagents they are found to contain an oval nucleus with one or sometimes two nucleoli, imbedded in a considerable mass of protoplasm. The protoplasm appears slightly granular and generally contains one or two small vacuoles. I have already spoken of the gradation of the hypoblast at the edge of the blastoderm into white yolk. I have, therefore, only to mention the variations in the size of its cells in different parts of the pellucid area. The points where the cells are smallest seem generally to coincide with the points of maximum growth. Over the embryo the cells are more regular than elsewhere. They are elongated and arranged transversely to the long axis of the embryo. They are somewhat hexagonal in shape, and not unlike the longer pieces in the dental plate of a Myliobatis (Pl. 1, fig. 10). This regularity, however, is much more marked in some specimens than in others. These cells are about 1/4000th of an inch in breadth, and 1/1000th in length. On each side of the embryo immediately external to the protovertebræ the cells are frequently about the same size as those over the embryo itself. In the neck, however, and near the end of the sinus rhomboidalis, they are considerably smaller, about 1/4000th inch each way. The reason of this small size is not very clear, but probably shews that the greatest growth is taking place at these two points. The cells, again, are very small at the head fold, but are very much larger in front of this—larger, in fact, than any other cells of the hypoblast. Outside the embryo they gradually increase [Pg 39] in size towards the edge of the pellucid area. Here they are about 1/1000th of an inch in diameter, irregular in shape and rather angular.
The outlines of the cells of the epiblast at this time are easily distinguished from the cells of the hypoblast by being more elongated and angular; they are further distinguished by the presence of numerous small oval cells, frequently at the meeting point of several cells, at other times at points along the lines of junction of two cells (Pl. 1, fig. 12). These small cells look very like the smaller stomata of endothelial membranes, but are shewn to be cells by possessing a nucleus. There is considerable variation in size in the cells in different parts of the epiblast. Between the front lobes of the brain the cells are very small, 1/4000th inch, rising to 1/2000th on each side. They are about the latter size over the greater part of the embryo. But over the sinus rhomboidalis they fall again to from 1/3000th to 1/4000th inch. This is probably to be explained by the growth of the medullary fold at this point, which pushes back the primitive groove. At the sides of the head the cells are larger than anywhere else in the epiblast, being here about 1/1000th inch in diameter. I at present see no explanation of this fact. At the periphery of the pellucid area and over the vascular area the cells are 1/1500th to 1/2000th inch in diameter, but at the periphery of the opaque area they are smaller again, being about the 1/3000th of an inch. This smaller size at the periphery of the area opaca is remarkable, since in the earlier stages the most peripheral epiblast cells were the largest. It, perhaps, implies that more rapid growth is at this time taking place in that part of the epiblast which is spreading over the yolk sac.
[Pg 40] EXPLANATION OF PLATE 1, Figs. 1-5 and 9-12.
Fig. 1. Section through an unincubated blastoderm,
shewing the upper layer, composed of a single row of columnar cells, and
the lower layer, composed of several rows of rounded cells in which no
nucleus is visible. Some of the formative cells,
at the bottom of
the segmentation cavity, are seen at (b).
Fig. 2. Section through the periphery of an eight hours' blastoderm, shewing the epiblast (p), the hypoblast (h), and the mesoblast commencing to be formed (c), partly by lower-layer cells enclosed between the epiblast and hypoblast, and partly by formative cells. Formative cells at the bottom of the segmentation cavity are seen at b. At s is one of the side folds parallel to the primitive groove.
Fig. 3. Portion of the hypoblast of a thirteen hours' blastoderm, treated with silver nitrate, shewing the great variation in the size of the cells at this period. An hour-glass shaped nucleus is seen at a.
Fig. 4. Periphery of a twenty-three hours' blastoderm, shewing cell for cell the junction between the hypoblast (h) and white-yolk spheres (w).
Fig. 5. Junction between the white-yolk spheres and the hypoblast cells at the passage from the area pellucida to the area opaca. The specimen was treated with silver nitrate to bring out the shape of the cells. The line of junction between the opaque and pellucid areas passes diagonally.
Fig. 9. Section through the primitive streak of an eight hours' blastoderm. The specimen shews the mesoblast very much thickened in the immediate neighbourhood of the primitive streak, but hardly formed at all on each side of the streak. It also shews the primitive groove just beginning to be formed (pr), and the fusion between the epiblast and the mesoblast under the primitive groove. The hypoblast is completely formed in the central part of the blastoderm. At f is seen one of the side folds parallel to the primitive groove. Its depth has been increased by the action of the chromic acid.
Fig. 10. Hypoblast cells from the hinder end of a thirty-six hours' embryo, treated with silver nitrate, shewing the regularity and elongated shape of the cells over the embryo and the smaller cells on each side.
Fig. 11. Epiblast cells from an unincubated blastoderm, treated with silver nitrate, shewing the regular hexagonal shape of the cells and the small spherules they contain.
Fig. 12. Portion of the epiblast of a thirty-six hours' embryo, treated with silver nitrate, shewing the small rounded cells frequently found at the meeting-points of several larger cells which are characteristic of the upper layer.
[6] From the Quarterly Journal of Microscopical Science, Vol. XIII., 1873.
With Plate 1, Figs. 6-8 and 13-19.
The investigations of Dursy (Der Primitivstreif des Hühnchens, von Dr E. Dursy. Lahr, 1866) on the primitive groove, shewing that it is a temporary structure, and not connected with the development of the neural canal, have in this country either been ignored or rejected. They are, nevertheless, perfectly accurate; and had Dursy made use of sections to support his statements I do not think they would so long have been denied. In Germany, it is true, Waldeyer has accepted them with a few modifications, but I have never seen them even alluded to in any English work. The observations which I have made corroborating Dr Dursy may, perhaps, under these circumstances be worth recording.
After about twelve hours of incubation the pellucid area of a hen's egg has become somewhat oval, with its longer axis at right angles to the long axis of the egg. Rather towards the hinder (narrower) end of this an opaque streak has appeared, with a somewhat lighter line in the centre. A section made at the time shews that the opaque streak is due partly to a thickening of the epiblast, but more especially to a large collection of the rounded mesoblast cells, which along this opaque line form a thick mass between the epiblast and the hypoblast. The mesoblast cells are in contact with both hypoblast and epiblast, and appear to be fused with the latter. The line of junction between them can, however, almost always be made out.
Soon after the formation of this primitive streak a groove is formed along its central line by a pushing inwards of the epiblast. [Pg 42] The epiblast is not thinner where it lines the groove, but the mass of mesoblast below the groove is considerably thinner than at its two sides. This it is which produces the peculiar appearance of the primitive groove when the blastoderm is viewed by transmitted light as a transparent line in the middle of an opaque one.
This groove, as I said above, is placed at right angles to the long axis
of the egg, and nearer the hind end, that is, the narrower end of the
pellucid area. It was called the primitive groove
by the early
embryologists, and they supposed that the neural canal arose from the
closure of its edges above. It is always easy to distinguish this groove,
in transverse sections, by several well-marked characters. In the first
place, the epiblast and mesoblast always appear more or less fused together
underneath it; in the second place, the epiblast does not become thinner
where it lines the groove; and in the third place, the mesoblast beneath it
never shews any signs of being differentiated into any organ.
As Dursy has pointed out, there is frequently to be seen in fresh specimens, examined as transparent objects, a narrow opaque line running down the centre of this groove. I do not know what this line is caused by, as there does not appear to be any structural feature visible in sections to which it can correspond.
From the twelfth to the sixteenth hour the primitive groove grows rapidly, and by the sixteenth hour is both absolutely and considerably longer than it was at the twelfth hour, and also proportionately longer as compared with the length of the pellucid area.
There is a greater interval between its end and that of the pellucid area in front than behind.
At about the sixteenth hour, or a little later, a thickening of the
mesoblast takes place in front of the primitive groove, forming an opaque
streak, which in fresh specimens looks like a continuation from the
anterior extremity of the primitive groove (vide
Pl. 1, fig. 8). From hardened specimens,
however, it is easy to see that the connection of this streak with the
primitive groove is only an apparent one. Again, it is generally possible
to see that in the central line of this streak there is a narrow [Pg 43] groove. I
do not feel certain that there is no period when this groove may not be
present, but its very early appearance has not been recognized either by
Dursy or by Waldeyer. Moreover, both these authors, as also His, seem to
have mistaken the opaque streak spoken of above for the notochord. This,
however, is not the case, and the notochord does not make its appearance
till somewhat later. The mistake is of very minor importance, and probably
arose in Dursy's case from his not sufficiently making use of sections. At
about the time the streak in front of the primitive groove makes its
appearance a semicircular fold begins to be formed near the anterior
extremity of the pellucid area, against which the opaque streak, or as it
had, perhaps, better be called, the medullary streak,
ends
abruptly.
This fold is the head fold, and the groove along the medullary streak is the medullary groove, which subsequently forms the cavity of the medullary or neural canal.
Everything which I have described above can without difficulty be made out from the examination of fresh and hardened specimens under the simple microscope; but sections bring out still more clearly these points, and also shew other features which could not have been brought to light without their aid. In Pl. 1, figs. 6 and 7, two sections of an embryo of about eighteen hours are shewn. The first of these passes through the medullary groove, and the second of them through the extreme anterior end of the primitive groove. The points of difference in the two sections are very obvious.
From fig. 6 it is clear that a groove has already been formed in the medullary streak, a fact which was not obvious in the fresh specimen. In the second place the mesoblast is thickened both under the groove and also more especially in the medullary folds at the sides of the groove; but shews hardly a sign of the differentiation of the notochord. So that it is clear that the medullary streak is not the notochord, as was thought to be the case by the authors above mentioned. In the third place there is no adhesion between the epiblast and the mesoblast. In all the sections I have cut through the medullary groove I have found this feature to be constant; while (for instance, as in Pl. 1, figs. 7, 9, 17) all sections through the primitive groove [Pg 44] shew most clearly an adhesion between the epiblast and mesoblast. This fact is both strongly confirmatory of the separate origins of the medullary and primitive grooves, and is also important in itself, as leaving no loophole for supposing that in the region of embryo there is any separation of the cells from the epiblast to form the mesoblast.
By this time the primitive groove has attained its maximum growth, and from this time begins both absolutely to become smaller, and also gradually to be pushed more and more backwards by the growth of the medullary groove.
The specimen figured in Pl. 1, fig. 18, magnified about ten diameters, shews the appearance presented by an embryo of twenty-three hours. The medullary groove (mc) has become much wider and deeper than it was in the earlier stage; the medullary folds (A) are also broader and more conspicuous. The medullary groove widens very much posteriorly, and also the medullary folds separate far apart to enclose the anterior end of the primitive groove (pr).
All this can easily be seen with a simple microscope, but the sections taken from the specimen figured also fully bear out the interpretations given above, and at the same time shew that the notochord has at this age begun to appear. The sections marked 13-17 pass respectively through the lines with corresponding numbers in fig. 18. Section 1 (fig. 13) passes through the middle of the medullary canal.
In it the following points are to be noted. (1) That the epiblast becomes very much thinner where it lines the medullary canal (mc), a feature never found in the epiblast lining the primitive groove. (2) That the mesoblast is very much thickened to form the medullary folds at A, A, while there is no adherence between it and the epiblast, below the primitive groove. (3) The notochord (ch) has begun to be formed, though its separation from the rest of the mesoblast is not as yet very distinct[8].
In fig. 14 the medullary groove has become wider and the medullary folds broader, the notochord has also become more expanded: the other features are the same as in section 1. In the third section (fig. 15) the notochord is still more expanded; [Pg 45] the bottom of the now much expanded medullary groove has become raised to form the ridge which separates the medullary from the primitive groove. The medullary folds are also flatter and broader than in the previous section. Section 4 (fig. 16) passes through the anterior end of the primitive groove. Here the notochord is no longer visible, and the adherence between the mesoblast and epiblast below the primitive groove comes out in marked contrast with the entire separation of the two layers in the previous sections.
The medullary folds (A) are still visible outside the raised edges of the primitive groove, and are as distinctly as possible separate and independent formations, having no connection with the folds of the primitive groove. In the last section (fig. 17), which is taken some way behind section 4, no trace of the medullary folds is any longer to be seen, and the primitive groove has become deeper. This series of sections, taken in conjunction with the specimen figured in fig. 18, must remove all possible doubt as to the total and entire independence of the primitive and medullary grooves. They arise in different parts of the blastoderm; the one reaches its maximum growth before the other has commenced to be formed; and finally, they are distinguished by almost every possible feature by which two such grooves could be distinguished.
Soon after the formation of the notochord, the protovertebræ begin to be formed along the sides of the medullary groove (Pl. 1, fig. 19, pv). Each new protovertebra (of those which are formed from before backwards) arises just in front of the anterior end of the primitive groove. As growth continues, the primitive groove becomes pushed further and further back, and becomes less and less conspicuous, till at about thirty-six hours only a very small and curved remnant is to be seen behind the sinus rhomboidalis; but even up to the forty-ninth Dursy has been able to distinguish it at the hinder end of the embryo.
The primitive groove in the chick is, then, a structure which appears very early, and soon disappears without entering directly into the formation of any part of the future animal, and without, so far as I can see, any function whatever. It is clear, therefore, that the primitive groove must be the rudiment of some ancestral feature; but whether it is a rudiment of some [Pg 46] structure which is to be found in reptiles, or whether of some earlier form, I am unable to decide. It is just possible that it is the last trace of that involution of the epiblast by which the hypoblast is formed in most of the lower animals. The fact that it is formed in the hinder part of the pellucid area perhaps tells slightly in favour of this hypothesis, since the point of involution of the epiblast not unfrequently corresponds with the position of the anus.
EXPLANATION OF PLATE 1, Figs. 6-8 and 13-19.
Figs. 6 and 7 are sections through an embryo rather earlier than the one drawn in fig. 8. Fig. 6 passes through the just commencing medullary groove (md), which appears in fresh specimens, as in fig. 8, merely as an opaque streak coming from the end of the primitive groove. The notochord is hardly differentiated, but the complete separation of mesoblast and hypoblast under the primitive groove is clearly shewn. Fig. 7 passes through the anterior end of the primitive groove (pr), and shews the fusion between the mesoblast and epiblast, which is always to be found under the primitive groove.
Fig. 8 is a view from above of a twenty hours' blastoderm, seen as a transparent object. Primitive groove (pr). Medullary groove (md), which passes off from the anterior end of the primitive groove, and is produced by the thickening of the mesoblast. Head fold (pf).
Figs. 13-17 are sections through the blastoderm, drawn in fig. 18 through the lines 1, 2, 3, 4, 5 respectively.
The first section (fig. 13) passes through the true medullary groove (mc); the two medullary folds (A, A) are seen on each side with the thickened mesoblast, and the mesoblast cells are beginning to form the notochord (nc) under the medullary groove. There is no adherence between the mesoblast cells and the epiblast under the medullary groove.
The second (fig. 14) section passes through the medullary groove where it has become wider. Medullary folds, A, A; notochord, ch.
In the third section (fig. 15) the notochord (ch) is broader, and the epiblast is raised in the centre, while the medullary folds are seen far apart at A.
In section fig. 16 the medullary folds (A) are still to be seen enclosing the anterior end of the primitive groove (pr). Where the primitive groove appears there is a fusion of the epiblast and mesoblast, and no appearance of the notochord.
In the last section, fig. 17, no trace is to be seen of the medullary folds.
Figs. 18 and 19 are magnified views of two hardened blastoderms. Fig. 18 is twenty-three hours old; fig. 19 twenty-five hours. They both shew how the medullary canal arises entirely independently of the primitive groove and in front of it, and also how the primitive groove gets pushed backwards by the growth of the medullary groove. pv, Protovertebræ; other references as above. Fig. 18 is the blastoderm from which sections figs. 13-17 were cut.
[7] From the Quarterly Journal of Microscopical Science, Vol. XIII, 1873.
[8] In the figure the notochord has been made too distinct.
With Plate 2.
The development of the first blood-vessels of the yolk-sac of the chick has been investigated by a large number of observers, but with very discordant results. A good historical résumé of the subject will be found in a paper of Dr Klein (liii. Band der K. Akad. der Wissensch. Wien), its last investigator.
The subject is an important one in reference to the homologies of the blood-vascular system of the vertebrata. As I shall shew in the sequel (and on this point my observations agree with those of Dr Klein), the blood-vessels of the chick do not arise as spaces or channels between the cells of the mesoblast; on the contrary, they arise as a network formed by the united processes of mesoblast-cells, and it is through these processes, and not in the spaces between them, that the blood flows. It is, perhaps, doubtful whether a system of vessels arising in this way can be considered homologous with any vascular system which takes its origin from channels hollowed out in between the cells of the mesoblast.
My own researches chiefly refer to the development of the blood-vessels in the pellucid area. I have worked but very slightly at their development in the vascular area; but, as far as my observations go, they tend to prove that the mode of their origin is the same, both for the pellucid and the vascular area.
The method which I have principally pursued has been to examine the blastoderm from the under surface. It is very difficult to obtain exact notions of the mode of development of [Pg 48] the blood-vessels by means of sections, though these come in as a valuable confirmation of the other method.
For the purpose of examination I have employed (1) fresh specimens; (2) specimens treated with spirit, and then mounted in glycerine; (3) specimens treated with chloride of gold for about half a minute, and then mounted in glycerine; and (4) specimens treated with osmic acid.
All these methods bring out the same appearances with varying clearness; but the successful preparations made by means of the gold chloride are the best, and bring out the appearances with the greatest distinctness.
The first traces of the blood-vessels which I have been able to distinguish in the pellucid area are to be seen at about the thirtieth hour or slightly earlier, at about the time when there are four to five protovertebræ on each side.
Fig. 1 shews the appearance at this time. Immediately above the hypoblast there are certain cells whose protoplasm sends out numerous processes. These processes vary considerably in thickness and size, and quickly come in contact with similar processes from other cells, and unite with them.
I have convinced myself, by the use of the hot stage, that these processes continually undergo alteration, sometimes uniting with other processes, sometimes becoming either more elongated and narrower or broader and shorter. In this way a network of somewhat granular protoplasm is formed with nuclei at the points from which the processes start.
From the first a difference may be observed in the character of this network in different parts of the pellucid area. In the anterior part the processes are less numerous and thicker, the nuclei fewer, and the meshes larger; while in the posterior part the processes are generally very numerous, and at first thin, the meshes small, and the nuclei more frequent. As soon as this network commences to be formed the nuclei begin to divide. I have watched this take place with the hot stage. It begins by the elongation of the nucleus and division of the nucleolus, the parts of which soon come to occupy the two ends of the nucleus. The nucleus becomes still longer and then narrows in the centre and divides. By this means the nuclei become much more numerous, and are found in almost all the larger [Pg 49] processes. Whether they are carried out into the processes by the movement of the surrounding protoplasm, or whether they move through the protoplasm, I have been unable to determine; the former view, however, seems to be the most probable.
It is possible that some nuclei arise spontaneously in the protoplasm, but I am much more inclined to think that they are all formed by the division of pre-existing nuclei—a view favoured by the number of nuclei which are seen to possess two nucleoli. Coincidently with the formation of the new nuclei the protoplasm of the processes, as well as that surrounding the nuclei at the starting-points of the processes, begins to increase in quantity.
At these points the nuclei also increase more rapidly than elsewhere, but at first the resulting nuclei seem to be all of the same kind.
In the anterior part of the pellucid area (fig. 4) the increase in the number of nuclei and in the amount of protoplasm at the starting-points of the protoplasm is not very great, but in the posterior part the increase in the amount of the protoplasm at these points is very marked, and coincidently the increase in number of the nuclei is also great. This is shewn in figs. 2 and 3. These are both taken from the tail end of an embryo of about thirty-three hours, with seven or eight protovertebræ. Fig. 3 shews the processes beginning to increase in thickness, and also the protoplasm at the starting-points increasing in quantity; at the same time the nuclei at these points are beginning to become more numerous. Fig. 3 is taken from a slightly higher level, i.e. slightly nearer the epiblast. In it the protoplasm is seen to have increased still more in quantity, and to be filled with nuclei. These nuclei have begun to be slightly coloured, and one of them is seen to possess two nucleoli.
Very soon after this a change in the nuclei begins to be observed, more especially in the hinder part of the embryo. While before this time they were generally elongated, some of them now become more nearly circular. In addition to this, they begin to have a yellowish tinge, and the nuclei, when treated with gold (for in the fresh condition it is not easy to [Pg 50] see them distinctly), have a more jagged and irregular appearance than the nucleoli of the other nuclei.
This change takes place especially at the starting-points of the processes, so that the appearance presented (fig. 5) is that of spherical masses of yellowish nuclei connected with other similar spherical masses by protoplasmic processes, in which nuclei of the original type are seen imbedded. These masses are surrounded by a thin layer of protoplasm, at the edge of which a normal nucleus may here and there be detected, as at fig. 5, a and a´, the latter possessing two nucleoli. Some of these processes are still very delicate, and it is exceedingly probable that they undergo further changes of position before the final capillary system is formed.
These differentiated nuclei are the first stage in the formation of the blood-corpuscles. From their mode of formation it is clear that the blood-corpuscles of the Sauropsida are to be looked upon as nuclei containing nucleoli, rather than as cells containing nuclei; indeed, they seem to be merely ordinary nuclei with red colouring matter.
This would make them truly instead of only functionally homologous with the red corpuscles of the Mammalia, and would well agree with the fact that the red corpuscles of Mammalia, in their embryonic condition, possess what have previously been called nuclei, but which might perhaps more properly be called nucleoli.
In the anterior part of the blastoderm the processes, as I have stated, are longer and thinner, and the spaces enclosed between them are larger. This is clearly brought out in Pl. 2, fig. 4. But, besides these large spaces, there are other smaller spaces, such as that at v. It is, on account of the transparency of the protoplasm, very difficult to decide whether these are vacuoles or simply spaces enclosed by the processes, but I am inclined to think that they are merely spaces. The difficulty of exactly determining this point is increased by the presence of numerous white-yolk spherules in the hypoblast above, which considerably obscure the view. At about the same time that the blood-corpuscles appear in the posterior end of the pellucid area, or frequently a little later, they begin to be formed in the anterior part also. The [Pg 51] masses of them are, however, far smaller and far fewer than in the posterior part of the embryo. It is at the tail end of the pellucid area that the chief formation of blood-corpuscles takes place.
The part of the pellucid area intermediate in position between the anterior and posterior ends of the embryo is likewise intermediate as regards the number of corpuscles formed and the size of the spaces between the processes; the spaces being here larger than at the posterior extremity, but smaller than the spaces in front. Close to the sides of the embryo the spaces are, however, smaller than in any other part of the pellucid area. It is, however, in this part that the first formation of blood-corpuscles takes place, and that the first complete capillaries are formed.
We have then somewhat round protoplasmic masses filled with blood-corpuscles and connected by means of processes, a few of which may begin to contain blood-corpuscles, but the majority of which only contain ordinary nuclei. The next changes to be noticed take place in the nuclei which were not converted into blood-corpuscles, but which were to be seen in the protoplasm surrounding the corpuscles. They become more numerous and smaller, and, uniting with the protoplasm in which they were imbedded, become converted into flat cells (spindle-shaped in section), and in a short time form an entire investment for the masses of blood-corpuscles. The same change also occurs in the protoplasmic processes which connect the masses of corpuscles. In the case of those processes which contain no corpuscles the greater part of their protoplasm seems to be converted into the protoplasm of the spindle-shaped cells. The nuclei arrange themselves so as completely to surround the exterior of the protoplasmic processes. In this way each process becomes converted into a hollow tube, completely closed in by cells formed from the investment of the original nuclei by the protoplasm which previously formed the solid processes. The remainder of the protoplasm probably becomes fluid, and afterwards forms the plasma in which the corpuscles float. While these changes are taking place the formation of the blood-corpuscles does not stand still, and by the time a system of vessels, enclosed by cellular walls, is formed out of [Pg 52] the protoplasmic network, a large number of the connecting processes in this network have become filled with blood-corpuscles. The appearances presented by the network at a slightly later stage than this is shewn in Pl. 2, fig. 6, but in this figure all the processes are seen to be filled with blood-corpuscles.
This investment of the masses of corpuscles by a cellular wall occurs much earlier in some specimens than in others, both in relation to the time of incubation and to the completion of the network. It is generally completed in some parts by the time there are eight or nine protovertebræ, and is almost always formed over a great part of the pellucid area by the thirty-sixth hour. The formation of the corpuscles, as was pointed out above, occurs earliest in the central part of the hour-glass shaped pellucid area, and latest in its anterior part. In the hinder part of the pellucid area the processes, as well as their enlarged starting-points, become entirely filled with corpuscles; this, however, is by no means the case in its anterior part. Here, although the corpuscles are undoubtedly developed in parts as shewn in fig. 7, yet a large number of the processes are entirely without them. Their development, moreover, is in many cases very much later. When the development has reached the stage described, very little is required to complete the capillary system. There are always, of course, a certain number of the processes which end blindly, and others are late in their development, and are not by this time opened; but, as a general rule, when the cellular investment is formed for the masses of corpuscles, there is completed an open network of tubes with cellular walls, which are more or less filled with corpuscles. These become quickly driven into the opaque area in which at that time more corpuscles may almost always be seen than in the pellucid area.
By the formation of a network of this kind it is clear that there must result spaces enclosed between the walls of the capillaries; these spaces have under the microscope somewhat the appearance of being vesicles enclosed by walls formed of spindle-shaped cells. In reality they are only spaces enclosed at the sides, and, as a general rule, not above and below. They have been mistaken by some observers for vesicles in [Pg 53] which the corpuscles were supposed to be developed, and to escape by the rupture of the walls into the capillary spaces between. This mistake has been clearly pointed out by Klein (loc. cit.).
At the time when these spaces are formed, and especially in the hinder two-thirds of the pellucid area, and in the layer of blood-vessels immediately above the hypoblast, a formation takes place which forms in appearance a secondary investment of the capillaries. Dr Klein was the first to give a correct account of this formation. It results from the cells of the mesoblast in the meshes of the capillary system. Certain of these cells become flattened, and send out fine protoplasmic processes. They arrange themselves so as completely to enclose the spaces between the capillaries, forming in this way vesicles.
Where seen on section (vide fig. 6) at the edge of the vesicles these cells lining the vesicles appear spindle-shaped, and look like a secondary investment of the capillaries. This investment is most noticeable in the hinder two-thirds of the pellucid area; but, though less conspicuous, there is a similar formation in its anterior third, where there would seem to be only veins present. Dr Klein (loc. cit., fig. 12) has also drawn this investment in the anterior third of the pellucid area. He has stated that the vessels in the mesoblast between the splanchnopleure and the somatopleure, and which are enclosed by prolongations from the former, do not possess this secondary investment; he has also stated that the same is true for the sinus terminalis; but I am rather doubtful whether the generalisation will hold, that veins and arteries can from the first be distinguished by the latter possessing this investment. I am also rather doubtful whether the spaces enclosed by the protoplasmic threads between the splanchnopleure and somatopleure are the centres of vessels at all, since I have never seen any blood-corpuscles in them.
It is not easy to learn from sections much about the first stages in the formation of the capillaries, and it is impossible to distinguish between a completely-formed vessel and a mere spherical space. The fine protoplasmic processes which connect the masses of corpuscles can rarely be seen in sections, except when they pass vertically, as they do occasionally (vide Pl. 2, fig. 9) in the opaque area, joining the somatopleure and the [Pg 54] splanchnopleure. Dr Klein considers these latter processes to be the walls of the vessels, but they appear rather to be the processes which will eventually become new capillaries.
From sections, however, it is easy to see that the appearances of the capillaries in the vascular area are similar to the appearances in the pellucid area, from which it is fair to conclude that their mode of formation is the same in both. It is also easy to see that the first formation of vessels occurs in the splanchnopleure, and that even up to the forty-fifth hour but few or no vessels are found in the somatopleure. The mesoblast of the somatopleure is continued into the opaque area as a single layer of spindle-shaped cells.
Sections clearly shew in the case of most of the vessels that the secondary investment of Klein is present, even in the case of those vessels which lie immediately under the somatopleure.
In reference to the origin of particular vessels I have not much to say. Dr Klein's account of the origin of the sinus terminalis is quite correct. It arises by a number of the masses of blood-corpuscles, similar to those described above, becoming connected together by protoplasmic processes. The whole is subsequently converted into a continuous vessel in the usual way.
From the first the sinus terminalis possesses cellular walls, as is clear from its mode of origin. I am inclined to think that Klein is right in saying that the aortæ arise in a similar manner, but I have not worked out their mode of origin very fully.
It will be seen from the account given above that, in reference to the first stages in the development of the blood-vessels, my observations differ very considerably from those of Dr Klein; as to the later stages, however, we are in tolerable agreement. We are in agreement, moreover, as to the fundamental fact that the blood-vessels are formed by a number of cells becoming connected, and by a series of changes converted into a network of vessels, and that they are not in the first instance merely channels between the cells of the mesoblast.
By the forty-fifth hour colourless corpuscles are to be found in the blood whose exact origin I could not determine; probably they come from the walls of the capillaries.
[Pg 55] In the vessels themselves the coloured corpuscles undergo increase by division, as has already been shewn by Remak. Corpuscles in the various stages of division may easily be found. They do not appear to show very active amœboid movements in the vessels, though their movements are sometimes very active when removed from the body.
To recapitulate—some of the cells of the mesoblast of the splanchnopleure send out processes, these processes unite with the processes from other cells, and in this way a network is formed. The nuclei of the original cells divide, and at the points from which the processes start their division is especially rapid. Some of them acquire especially at these points a red colour, and so become converted into blood-corpuscles; the others, together with part of the protoplasm in which they are imbedded, become converted into an endothelium both for the processes and the masses of corpuscles; the remaining protoplasm becomes fluid, and thus the original network of the cells becomes converted into a network of hollow vessels, filled with fluid, in which corpuscles float.
In reference to the development of the heart, my observations are not quite complete. It is, however, easy to prove from sections (vide figs. 10 and 11, Pl. 2) that the cavity of the heart is produced by a splitting or absorption of central cells of the thickened mesoblast of the splanchnopleure, while its muscular walls are formed from the remaining cells of this thickened portion. It is produced in the following way:—When the hypoblast is folded in to form the alimentary canal the mesoblast of the splanchnopleure follows it closely, and where the splanchnopleure turns round to assume its normal direction (fig. 11) its mesoblast becomes thickened. This thickened mass of mesoblast is, as can easily be seen from figs. 10 and 11, Pl. 2, entirely distinct from the mesoblast which forms the outside walls of the alimentary canal. At the point where this thickening occurs an absorption takes place to form the cavity of the heart. The method in which the cavity is formed can easily be seen from figs. 10 and 11. It is in fig. 11 shewn as it takes place in the mesoblast on each side, the folds of the splanchnopleure not having united in the middle line; and hence a pair of cavities are formed, one on each side. It [Pg 56] is, however, probable that, in the very first formation of the heart, the cavity is single, being formed after the two ends of the folded mesoblast have united (vide hz, fig. 10). In some cases the two folds of the mesoblast appear not at first to become completely joined in the middle line; in this case the cavity of the heart is still complete from side to side, but the mesoblast-cells which form its muscular walls are deficient above. By the process of absorption, as I said, a cavity is produced in the thickened part of the mesoblast of the splanchnopleure, a cavity which is single in front, but becomes divided further behind, where the folds of the mesoblast have not united, into two cavities, to form the origin of the omphalomeseraic veins. As the folding proceeds backwards the starting-point of the omphalomeseraic veins is also pushed backwards, and the cavities which were before separated become joined together. From its first formation the heart is lined internally by an endothelium; this is formed of flattened cells, spindle-shaped in section. The exact manner of the origin of this lining I have not been able to determine; it is, however, probable that some of the central mesoblast-cells are directly converted into the cells of the endothelium.
I have obtained no evidence enabling me to determine whether Dr Klein is
correct in stating that the cells of the mesoblast in the interior of the
heart become converted partly into blood-corpuscles and partly into a
cellular lining forming the endothelium of the heart, in the same way that
the blood-vessels in the rest of the blastoderm are formed. But I should be
inclined to think that it is very probable—certainly more probable
than that the cavity of the heart is formed by a process of splitting
taking place. Where I have used the word absorption
in speaking of the
formation of the cavity of the heart, I must be understood as implying that
certain of the interior cells become converted into the endothelium, while
others either form the plasma or become blood-corpuscles.
The originally double formation of the hinder part of the heart probably explains Dr Afanassiev's statement (Bullétin de l'Académ. Impériale de St Pétersb., tom. xiii, pp. 321-335), that he finds the endothelium of the heart originally dividing its interior into two halves; for when the partition of the mesoblast [Pg 57] which separated at first the two halves of the heart became absorbed, the endothelium lining of each of the originally separate vessels would remain complete, dividing the cavity of the heart into two parts. The partition in the central line is, however, soon absorbed.
The account given above chiefly differs from that of Remak by not supposing that the mesoblast-cells which form the heart are in any way split off from the wall of the alimentary canal.
There can be no doubt that His is wrong in supposing that the heart originates from the mesoblast of the splanchnopleure and somatopleure uniting to form its walls, thus leaving a cavity between them in the centre. The heart is undoubtedly formed out of the mesoblast of the splanchnopleure only.
Afanassiev's observations are nearer to the truth, but there are some points in which he has misinterpreted his sections.
Sections Pl. 2, figs. 10 and 11, explain what I have just said about the origin of the heart. Immediately around the notochord the mesoblast is not split, but a very little way outside it is seen to be split into two parts so and sp; the former of these follows the epiblast, and together with it forms the somatopleure, which has hardly begun to be folded at the line where the sections are taken. The latter (sp) forms with the hypoblast (hy) the splanchnopleure, and thus has become folded in to form the walls of the alimentary canal (d). In fig. 11 the folds have not united in the central line, but in fig. 10 they have so united. In fig. 11, where the mesoblast, still following the hypoblast, turns back to assume its normal direction, it is seen to be thickened and to have become split, so that a cavity (of) (of the omphalomeseraic vein) is formed in it on each side, lined by endothelium.
In the section immediately behind section fig. 11 the mesoblast was thickened, but had not become split.
In fig. 10 the hypoblast folds are seen to have united in the centre, so as to form a completely closed digestive canal (d); the folds of the mesoblast have also united, so that there is only a single cavity in the heart (hz), lined, as was the case with the omphalomeseraic veins, by endothelium.
In conclusion, I have to thank Dr Foster for his assistance and suggestions throughout the investigations which have formed [Pg 58] the subject of these three short papers, and which were well carried on in the apartments used by him as a Physiological Laboratory.
EXPLANATION OF PLATE 2.
Fig. 1 is taken from the anterior part of the pellucid area of a thirty hours' chick, with four protovertebræ. At n is a nucleus with two nucleoli.
Figs. 2 and 3 are taken from the posterior end of the pellucid area of a chick with eight protovertebræ. In fig. 3 the nuclei are seen to have considerably increased in number at the points of starting of the protoplasmic processes. At n is seen a nucleus with two nucleoli.
Fig. 4 is taken from the anterior part of the pellucid area of an embryo of thirty-six hours. It shews the narrow processes characteristic of the anterior part of the pellucid area, and the fewer nuclei. Small spaces, which have the appearance of vacuoles, are shewn at v.
Fig. 5 is taken from the posterior part of the pellucid area of a thirty-six hours' embryo. It shews the nuclei, with somewhat irregular nucleoli, which have begun to acquire the red colour of blood-corpuscles; the protoplasmic processes containing the nuclei; the nuclei in the protoplasm surrounding the corpuscles, as shewn at a, a´.
Fig. 6 shews fully formed blood-vessels, in part filled with blood-corpuscles and in part empty. The walls of the capillaries, formed of cells, spindle-shaped in section, are shewn, and also the secondary investment of Klein at k, and at b is seen a narrow protoplasmic process filled with blood-corpuscles.
Fig. 7 is taken from the anterior part of the pellucid area of a thirty-six hours' embryo. It shews a collection of nuclei which are beginning to become blood-corpuscles.
Figs. 1-5 are drawn with an 1/8 object-glass. Fig. 6 is on a much smaller scale. Fig. 7 is intermediate.
Fig. 8.—A transverse section through the dorsal region of a forty-five hours' embryo; ao, aorta with a few blood-corpuscles. v, Blood-vessels, all of them being formed in the splanchnopleure, and all of them provided with the secondary investment of Klein; pe, pellucid area; op, opaque area.
Fig. 9.—Small portion of a section through the opaque area of a thirty-five hours' embryo, showing protoplasmic processes, with nuclei passing from the somatopleure to the splanchnopleure.
Fig. 10.—Section through the heart of a thirty-four hours' embryo. a. Alimentary canal; hb, hind brain; nc, notochord; e, epiblast; so, mesoblast of the somatopleure; sp, mesoblast of the splanchnopleure; hy, hypoblast; hz, cavity of the heart.
[Pg 59] Fig. 11.—Section through the same embryo as fig. 10, and passing through the orifice of the omphalomeseraic vein. of, Omphalomeseraic vein; other references as above.
These two sections shew that the heart is entirely formed from the mesoblast of the splanchnopleure, and that it is formed by the splitting of that part of the mesoblast which has turned to assume its normal direction after being folded in to form the muscular wall of the alimentary canal. In fig. 11 the cavities so formed on each side have not yet united, but in fig. 10 they have united. When the folding becomes more complete the cavities (of, of) in fig. 11 will unite, and in this way the origin of the omphalomeseraic veins will be carried further backwards. In the section immediately behind section 11 the mesoblast had become thickened, but had not split.
[9] From the Quarterly Journal of Microscopical Science, Vol. XIII, 1873.
With Plates 3 and 4.
During the spring of the present year I was studying at the Zoological Station, founded by Dr Dohrn at Naples, and entirely through its agency was supplied with several hundred eggs of various species of Dog-fish (Selachii)—a far larger number than any naturalist has previously had an opportunity of studying. The majority of the eggs belonged to an oviparous species of Mustelus, but in addition to these I had a considerable number of eggs of two or three species of Scyllium, and some of the Torpedo. Moreover, since my return to England, Professor Huxley has most liberally given me several embryos of Scyllium stellare in a more advanced condition than I ever had at Naples, which have enabled me to fill up some lacunæ in my observations.
On many points my investigations are not yet finished, but I have already made out a number of facts which I venture to believe will add to our knowledge of vertebrate embryology; and since it is probable that some time will elapse before I am able to give a complete account of my investigations, I have thought it worth while preparing a preliminary paper in which I have briefly, but I hope in an intelligible manner, described some of the more interesting points in the development of the Elasmobranchii. The first-named species (Mustelus sp.?) was alone used for the early stages, for the later ones I have also employed the other species, whose eggs I have had; but as far as I have [Pg 61] seen at present, the differences between the various species in early embryonic life are of no importance.
Without further preface I will pass on to my investigations.
The Egg-shell.
In the eggs of all the species of Dog-fishes which I have examined the yolk lies nearest that end of the quadrilateral shell which has the shortest pair of strings for attachment. This is probably due to the shape of the cavity of the shell, and is certainly not due to the presence of any structures similar to chalazæ.
The Yolk.
The yolk is not enclosed in any membrane comparable to the vitelline membrane of Birds, but lies freely in a viscid albumen which fills up the egg-capsule. It possesses considerable consistency, so that it can be removed into a basin, in spite of the absence of a vitelline membrane, without falling to pieces. This consistency is not merely a property of the yolk-sphere as a whole, but is shared by every individual part of it.
With the exception of some finely granular matter around the blastoderm, the yolk consists of rather small, elliptical, highly refracting bodies, whose shape is very characteristic and renders them easily recognizable. A number of striæ like those of muscle are generally visible on most of the spherules, which give them the appearance of being in the act of breaking up into a series of discs; but whether these striæ are normal, or produced by the action of water I have not determined.
Position of the Blastoderm.
The blastoderm is always situated, immediately after impregnation, near the pole of the yolk which lies close to the end of the egg-capsule. Its position varies a little in the different species and is not quite constant in different eggs of the same species. But this general situation is quite invariable. It is of about the same proportional size as the blastoderm of a bird.
Segmentation.
In a fresh specimen, in which segmentation has only just commenced, the blastoderm or germinal disc appears as a circular [Pg 62] disc, distinctly marked off by a dark line from the rest of the yolk. This line, as is proved by sections, is the indication of a very shallow groove. The appearance of sharpness of distinction between the germ and the yolk is further intensified by their marked difference of colour, the germ itself being usually of a darker shade than the remainder of the yolk; while around its edge, and apparently sharply separated from it by the groove before mentioned, is a ring of a different shade which graduates at its outer border into the normal shade of the yolk.
These appearances are proved by transverse sections to be deceptive. There is no sharp line either at the sides or below separating the blastoderm from the yolk. In the passage between the fine granular matter of the germ to the coarser yolk-spheres every intermediate size of granule is present; and, though the space between the two is rather narrow, in no sense of the word can there be said to be any break or line between them.
This gradual passage stands in marked contrast with what we shall find to be the case at the close of the segmentation. In the youngest egg which I had, the germinal disc was already divided into four segments by two furrows at right angles. These furrows, however, did not reach its edge; and from my sections I have found that they were not cut off below by any horizontal furrow. So that the four segments were continuous below with the remainder of the germ without a break.
In the next youngest specimen which I had, there were already present eighteen segments, somewhat irregular in size, but which might roughly be divided into an outer ring of larger spheres, separated, as it were, by a circular furrow from an inner series of smaller segments. The furrows in this case reached quite to the edge of the germinal disc.
The remarks I made in reference to the earlier specimen about the separation of the germ from the yolk apply in every particular to the present one. The external limit of the blastoderm was not defined by a true furrow, and the segmentation furrows still ended below without meeting any horizontal furrows, so that the blastoderm was not yet separated by any line from the remainder of the yolk, and the segments of which it was composed were still only circumscribed upon five sides. In [Pg 63] this particular the segmentation in these animals differs materially from that in the Bird, where the horizontal furrows appear very early.
In each segment a nucleus was generally to be seen in sections. I will, however, reserve my remarks upon the nature of the nuclei till I discuss the nuclei of the blastoderm as a whole.
For some little time the peripheral segments continue larger than the more central ones, but this difference of size becomes less and less marked, and before the segments have become too small to be seen with the simple microscope, their size appears to be uniform over the whole surface of the blastoderm.
In the blastoderms somewhat older than the one last described the segments have already become completely separate masses, and each of them already possesses a distinct nucleus. They form a layer one or two segments deep. The limits of the blastoderm are not, however, defined by the already completed segments, but outside these new segments continue to be formed around nuclei which appear in the yolk. At this stage there is, therefore, no line of demarcation between the germ and the yolk, but the yolk is being bored into, so to speak, by a continuous process of fresh segmentation.
The further segmentation of the already existing spheres, and the formation of new ones from the yolk below and to the sides, continues till the central cells acquire their final size, the peripheral ones being still large, and undefined towards the yolk. These also soon reach the final size, and the blastoderm then becomes rounded off towards the yolk and sharply separated from it.
The Nuclei of the Yolk.
Intimately connected with the segmentation is the appearance and history of a number of nuclei which arise in the yolk surrounding the blastoderm.
When the horizontal furrows appear which first separate the blastoderm from the yolk, the separation does not occur along the line of passage from the fine to the coarse yolk, but in the former at some distance from this line.
The blastoderm thus rests upon a mass of finely granular material, from which, however, it is sharply separated. At this [Pg 64] time there appear in this finely granular material a number of nuclei of a rather peculiar character.
They vary immensely in size—from that of an ordinary nucleus to a size greater than the largest blastoderm-cell.
In Pl. 3, fig. 1, n, is shewn their distribution in this finely granular matter and their variation in size. But whatever may be their size, they always possess the same characteristic structure. This is shewn in Pl. 3, figs. 1 and 2, n.
They are rather irregular in shape, with a tendency when small to be roundish, and are divided by a number of lines into distinct areas, in each of which a nucleolus is to be seen. The lines dividing them into these areas have a tendency (in the smaller specimens) to radiate from the centre, as shewn in Pl. 3, fig. 1.
These nuclei colour red with hematoxylin and carmine and brown with osmic acid, while the nucleoli or granules contained in the areas also colour very intensely with all the three above-named reagents.
With such a peculiar structure, in favourable specimens these nuclei are very easily recognised, and their distribution can be determined without difficulty. They are not present alone in the finely granular yolk, but also in the coarsely granular yolk adjoining it. They form very often a special row, sometimes still more markedly than in Pl. 3, fig. 1, along the floor of the segmentation cavity. They are not, however, found alone in the yolk. All the blastoderm-cells in the earlier stages possess precisely similar nuclei! From the appearance of the first nucleus in a segmentation-sphere till a comparatively late period in development, every nucleus which can be distinctly seen is found to be of this character. In Pl. 3, fig. 2, this is very distinctly shewn.
(1) We have, then, nuclei of this very peculiar character scattered through the sub-germinal granular matter, and also universally present in the cells of the blastoderm. (2) These nuclei are distributed in a special manner under the floor of the segmentation cavity on which new cells are continually appearing. Putting these two facts together, there would be the strongest presumption that these nuclei do actually become the nuclei of cells which enter the blastoderm, and such is [Pg 65] actually the case. In my account of the segmentation I have, indeed, already mentioned this, and I will return to it, but before doing so will enter more fully into the distribution of these nuclei in the yolk.
They appear in small numbers around the blastoderm at the close of segmentation, and round each one of them there may at this time be seen in osmic acid specimens, and with high powers, a fine network similar to but finer than that represented in Pl. 3, fig. 2. This network cannot, as a general rule, be traced far into the yolk, but in some exceptionally thin specimens it may be seen in any part of the fine granular yolk around the blastoderm, the meshes of the network being, however, considerably coarser between than around the nuclei. This network may be seen in the fine granular material around the germ till the latest period of which I have yet cut sections of the blastoderm. In the later specimens, indeed, it is very much more distinctly seen than in the earlier, owing to the fact that in parts of the blastoderm, especially under the embryo, the yolk-granules have disappeared partly or entirely, leaving only this fine network with the nuclei in it.
A specimen of this kind is represented in Pl. 3, fig. 2, where the meshes of the network are seen to be finer immediately around the nuclei, and coarser in the intervals. The specimen further shows in the clearest manner that this network is not divided into areas, each representing a cell and each containing a nucleus. I do not know to what extent this network extends into the yolk. I have never yet seen the limits of it, though it is very common to see the coarsest yolk-granules lying in its meshes. Some of these are shewn in Pl. 3, fig. 2, yk.
This network of lines[11] (probably bubbles) is characteristic of many cells, especially ova. We are, therefore, forced to believe that the fine granular and probably coarser granular yolk of this meroblastic egg consists of an active organized basis with [Pg 66] passive yolk-spheres imbedded in it. The organized basis is especially concentrated at the germinal pole of the egg, but becomes less and less in quantity, as compared with the yolk-spheres, the further we depart from this.
Admitting, as I think it is necessary to do, the organized condition of the whole yolk-sphere, there are two possible views as to its nature. We may either take the view that it is one gigantic cell, the ovum, which has grown at the expense of the other cells of the egg-follicle, and that these cells in becoming absorbed have completely lost their individuality; or we may look upon the true formative yolk (as far as we can separate it from the remainder of the food-yolk) as the remains of one cell (the primitive ovum), and the remainder of the yolk as a body formed from the coalescence of the other cells of the egg-follicle, which is adherent to, but has not coalesced with, the primitive ovum, the cells in this case not having completely lost their individuality; and to these cells, the nuclei, I have found, must be supposed to belong.
The former view I think, for many reasons, the most probable. The share of these nuclei in the segmentation, and the presence of similar nuclei in the cells of the germ, both support it, and are at the same time difficulties in the way of the other view. Leaving this question which cannot be discussed fully in a preliminary paper like the present one, I will pass on to another important question, viz.:
How do these nuclei originate? Are they formed by the division of the
pre-existing nuclei, or by an independent formation? It must be admitted
that many specimens are strongly in favour of the view that they increase
by division. In the first place, they are often seen two together;
examples of this will be seen in Pl. 3, fig. 1.
In the second place, I have found several specimens in which five or six
appear close together, which look very much as if there had been an actual
division into six nuclei. It is, however, possible in this case that the
nuclei are really connected below and only appear separate, owing to the
crenate form of the mass. Against this may be put the fact that the
division of a nucleus is by no means so common as has been sometimes
supposed, that in segmentation it has very rarely been observed [Pg 67] that the
nucleus of a sphere first divides[12], and
that then segmentation takes place, but segmentation generally occurs and
then a new nucleus arises in each of the newly formed spheres. Such nuclei
as I have described are rare; they have, however, been observed in the egg
of a Nephelis (one of the Leeches), and have in that case been said
to divide. Dr Kleinenberg, however, by following a single egg through the
whole course of its development, has satisfied himself that this is not the
case, and that, further, these nuclei in Nephelis never form the nuclei of
newly developing cells.
I must leave it an open question, and indeed one which can hardly be solved from sections, whether these nuclei arise freely or increase by division, but I am inclined to believe that both processes may possibly take place. In any case their division does not appear to determine the segmentation or segregation of the protoplasm around them.
As was mentioned in my account of the segmentation, these nuclei first appear during that process, and become the nuclei of the freshly formed segmentation spheres. At the close of segmentation a few of them are still to be seen around the blastoderm, but they are not very numerous.
From this period they rapidly increase in number, up to the commencement of the formation of the embryo as a body distinct from the germ. Though before this period they probably become the nuclei of veritable cells which enter the germ, it is not till this period, when the growth of the blastoderm becomes very rapid and it commences to spread over the yolk, that these new cells are formed in large numbers. I have many specimens of this age which shew the formation of these new cells with great clearness. This is most distinctly to be seen immediately below the embryo, where the yolk-spherules are few in number. At the opposite end of the blastoderm I believe that more of these cells are formed, but, owing to the presence of numerous yolk-spherules, it is much more difficult to make certain of this.
[Pg 68] As to the final destination of these cells, my observations are not yet completed. Probably a large number of them are concerned in the formation of the vascular system, but I will give reasons later on for believing that some of them are concerned in the formation of the walls of the digestive canal and of other parts.
I will conclude my account of these nuclei by briefly summarizing the points I have arrived at in reference to them.
A portion, or more probably the whole, of the yolk of the Dog-fish consists of organized material, in which nuclei appear and increase either by division or by a process of independent formation, and a great number of these subsequently become the nuclei of cells formed around them, frequently at a distance from the germ, which then travel up and enter it.
The formation of cells in the yolk, apart from the general process of
segmentation, has been recognised by many observers. Kupffer (Archiv. für Micr. Anat., Bd. IV. 1868) and
Owsjannikow (Entwicklung der Coregonus,
Bulletin der Akad. St Petersburgh, Vol. XIX.) in osseous fishes[13], Ray
Lankester (Annals and Mag. of Nat. Hist. Vol. IX. 1873, p. 81) in Cephalopoda, Götte (Archiv. für Micr. Anat. Vol.
X.) in the chick, have all described a new
formation of cells from the so-called food-yolk. The organized nature of
the whole or part of this, previous to the formation of the cells from it,
has not, however, as a rule, been distinctly recognised. In the majority of
cases, as, for instance, in Loligo, the nucleus is not the first thing to
be formed, but a plastide is first formed, in which a nucleus subsequently
makes its appearance.
[Pg 69] Formation of the Layers.
Leaving these nuclei, I will now pass on to the formation of the layers.
At the close of segmentation the surface of the blastoderm is composed of cells of a uniform size, which, however, are too small to be seen by the aid of the simple microscope.
The cells of this uppermost layer are somewhat columnar, and can be distinguished from the remainder of the cells of the blastoderm as a separate layer. This layer forms the epiblast; and the Dog-fish agree with Birds, Batrachians, and Osseous fish in the very early differentiation of it.
The remainder of the cells of the blastoderm form a mass, many cells deep, in which it is impossible as yet or till a very considerably later period to distinguish two layers. They may be called the lower layer cells. Some of them near the edge of this mass are still considerably larger than the rest, but they are, as a whole, of a fairly uniform size. Their nuclei are of the same character as the nuclei in the yolk.
There is one point to be noticed in the shape of the blastoderm as a whole. It is unsymmetrical, and a much larger number of its cells are found collected at one end than at the other. This absence of symmetry is found in all sections which are cut parallel to the long axis of the egg-capsule. The thicker end is the region where the embryo will subsequently appear.
This very early appearance of distinction in the blastoderm between the end at which the embryo will appear, and the non-embryonic end is important, especially as it shews the affinity of the modes of development of Osseous fishes and the Elasmobranchii. Oellacher (Zeitschrift für Wiss. Zoologie, Vol. XXXIII. 1873) has shewn, and, though differing from him on many other points, on this point Götte (Arch. für Micr. Anat. Vol. IX. 1873) agrees with him, that a similar absence of symmetry by which the embryonic end of the germ is marked off, occurs almost immediately after the end of segmentation in Osseous fishes. In the early stages of development there are [Pg 70] a number of remarkable points of agreement between the Osseous fish and the Dog-fish, combined with a number of equally remarkable points of difference. Some of these I shall point out as I proceed with my description.
The embryonic end of the germ is always the one which points towards the pole of the yolk farthest removed from the egg-capsule.
The germ grows, but not very rapidly, and without otherwise undergoing any very appreciable change, for some time.
The growth at these early periods appears to be particularly slow, especially when compared with the rapid manner in which some of the later stages of the development are passed through.
The next important change which occurs is the formation of the so-called
segmentation cavity.
This forms a very marked feature throughout the early stages. It
appears, however, to have somewhat different relations to the blastoderm
than the homologous structure in other vertebrates. In its earliest stage
which I have observed, it appears as a small cavity in the centre of the
lower layer cells. This grows rapidly, and its roof becomes composed of
epiblast and only a thin lining of lower layer
cells, while
its floor is formed by the yolk (Pl. 3, fig. 3,
sg). In the next and third stage (Pl. 3, fig. 4, sg)
its floor is formed by a thin layer of cells, its roof remaining as before.
It has, however, become a less conspicuous formation than it was; and in
the last (fourth) stage in which it can be distinguished it is very
inconspicuous, and almost filled up by cells.
What I have called the second stage corresponds to a period in which no
trace of the embryo is to be seen. In the third stage the embryonic end of
the blastoderm projects outwards to form a structure which I shall speak of
as the embryonic rim,
and in the fourth and last stage a distinct
medullary groove is formed. For a considerable period during the second
stage the segmentation cavity remains of about the same size; during the
third stage it begins to be encroached upon, and becomes smaller both
absolutely, and relatively to the increased size of the germ.
[Pg 71] The segmentation cavity of the Dog-fish most nearly agrees with that of Osseous fishes in its mode of formation and relation to the embryo.
Dog-fish resemble Osseous fish in the fact that their embryos are entirely formed from a portion of the germ which does not form part of the roof of the segmentation cavity, so that the cells forming the roof of the segmentation cavity take no share at any time in the formation of their embryos. They further agree with Osseous fish (always supposing that the descriptions of Oellacher, loc. cit., and Götte, Archiv. für Micr. Anat. Bd. IX. are correct) in the floor of the segmentation cavity being formed at one period by yolk. Together with these points of similarity there are some important differences.
(1) The segmentation cavity in the Osseous fish from the first arises as a cavity between the yolk and the blastoderm, and its floor is never at any period covered with cells. In the Dog-fish, as we have said above, both in the earlier and later periods the floor is covered with cells.
(2) The roof in the Dog-fish is invariably formed by the epiblast and a row of flattened lower layer cells.
According to both Götte and Oellacher the roof of the segmentation cavity in Osseous fishes is in the earlier stages formed alone of the two layers which correspond with the single layer forming the epiblast in the Dog-fish. In Osseous fishes it is very difficult to distinguish the various layers, owing to the similarity of their component cells. In Dog-fish this is very easy, owing to the great distinctness of the epiblast, and it appears to me, on this account, very probable that the two above-named observers may be in error as to the constitution of its roof in the Osseous fish. With both the Bird and the Frog the segmentation cavity of the Dog-fish has some points of agreement, and some points of difference, but it would take me too far from my present subject to discuss them.
When the segmentation cavity is first formed, no great changes have taken place in the cells forming the blastoderm. The upper layer—the epiblast—is composed of a single layer of columnar cells, and the remainder of the cells of blastoderm, [Pg 72] forming the lower layer, are of a fairly uniform size, and polygonal from mutual pressure. The whole edge of the blastoderm is thickened, but this thickening is especially marked at its embryonic end.
This thickened edge of the blastoderm is still more conspicuous in the next and second stage (Pl. 3, fig. 3).
In the second stage the chief points of progress, in addition to the increased thickness of the edge of the blastoderm, are—
(1) The increased thickness and distinctness of the epiblast, caused by its cells becoming more columnar, though it remains as a one-cell-thick layer.
(2) The disappearance of the cells from the floor of the segmentation cavity.
The lower layer cells have undergone no important changes, and the blastoderm has increased very little if at all in size.
From Pl. 3, fig. 3, it is seen that there is a far larger collection of cells at the embryonic than at the opposite end.
Passing over some rather unimportant stages, I will come to the next important one.
The general features of this (the third) stage in a surface view are—
(1) The increase in size of the blastoderm.
(2) The diminution in size of the segmentation cavity, both relatively and absolutely.
(3) The appearance of a portion of the blastoderm projecting beyond the
rest over the yolk. This projecting rim extends for nearly half the
circumference of the yolk, but is most marked at the point where the embryo
will shortly appear. I will call it the embryonic rim.
These points are still better seen from sections than from surface views, and will be gathered at once from an inspection of Pl. 3, fig. 4.
The epiblast has become still more columnar, and is markedly thicker in
the region where the embryo will appear. But its most remarkable feature is
that at the outer edge of the embryonic rim
(er) it turns round and becomes continuous with the
lower layer cells. This feature is most important, and involves some
peculiar modifications in the development. [Pg 73] I will, however,
reserve a discussion of its meaning till the next stage.
The only other important feature of this stage is the appearance of a layer of cells on the floor of the segmentation cavity.
Does this layer come from an ingrowth from the thickened edge of the blastoderm, or does it arise from the formation of new cells in the yolk?
It is almost impossible to answer this question with certainty. The following facts, however, make me believe that the newly formed cells do play an important part in the formation of this layer.
(1) The presence at an earlier date of almost a row of nuclei under the floor of the segmentation cavity (Pl. 3, fig. 1).
(2) The presence on the floor of the cavity of such large cells as those represented in fig. 1, bd, cells which are very different, as far as the size and granules are concerned, from the remainder of the cells of the blastoderm.
On the other hand, from this as well as other sections, I have satisfied myself that there is a distinct ingrowth of cells from the embryonic swelling. It is therefore most probable that both these processes, viz. a fresh formation and an ingrowth, have a share in the formation of the layer of cells on the floor of the segmentation cavity.
In the next stage we find the embryo rising up as a distinct body from
the blastoderm, and I shall in future speak of the body, which now becomes
distinct as the embryo. It corresponds with what Kupffer (loc.
cit.) in his paper on the Osseous Fishes
has called the
embryonic keel.
This starting-point for speaking of the embryo as a
distinct body is purely arbitrary and one merely of convenience. If I
wished to fix more correctly upon a period which could be spoken of as
marking the commencing formation of the embryo, I should select the time
when structures first appear to mark out the portion of the germ from which
the embryo becomes formed; this period would be in the Elasmobranchii, as
in the Osseous fish, at the termination of segmentation, when the want of
symmetry between the embryonic end of the germ and the opposite end first
appears.
[Pg 74]
I described in the last stage the appearance of the embryonic rim.
It is in the middle point of this, where it projects most, that the
formation of the embryo takes place. There appear two parallel folds
extending from the edge of the blastoderm towards the centre, and cut off
at their central end by another transverse fold. These three folds raise
up, between them, a flat broadish ridge, the embryo
(Pl. 3, fig. 5). The head end of the embryo is the end
nearest the centre of the blastoderm, the tail end being the one formed by
its (the blastoderm's) edge.
Almost from its first appearance this ridge acquires a shallow groove—the medullary groove (Pl. 3, fig. 5, mg)—along its middle line, where the epiblast and hypoblast are in absolute contact (vide fig. 6a, 7a, 7b, &c.) and where the mesoblast (which is already formed by this stage) is totally absent. This groove ends abruptly a little before the front end of the embryo, and is deepest in the middle and wide and shallow behind.
On each side of it is a plate of mesoblast equivalent to the combined vertebral and lateral plates of the Chick. These, though they cannot be considered as entirely the cause of the medullary groove, may perhaps help to make it deeper. In the parts of the germ outside the embryo the mesoblast is again totally absent, or, more correctly, we might say that outside the embryo the lower layer cells do not become differentiated into hypoblast and mesoblast, and remain continuous only with the lower of the two layers into which the lower layer cells become differentiated in the body of embryo. This state of things is not really very different from what we find in the Chick. Here outside the embryo (i.e. in the opaque area) there is a layer of cells in which no differentiation into hypoblast and mesoblast takes place, but the layer remains continuous rather with the hypoblast than the mesoblast.
There is one peculiarity in the formation of the mesoblast which I wish
to call attention to, i.e. its formation as two lateral masses, one
on each side of the middle line, but not continuous across this line (vide figs. 6a and 6b, and 7a and
7b). Whether this remarkable condition is the most primitive, [Pg 75]
i.e. whether, when in the stage before this the mesoblast is first
formed, it is only on each side of the middle line that the differentiation
of the lower layer cells into hypoblast and mesoblast takes place, I do not
certainly know, but it is undoubtedly a very early condition of the
mesoblast. The condition of the mesoblast as two plates, one on each side
of the neural canal, is precisely similar to its embryonic condition in
many of the Vermes, e.g. Euaxes and Lumbricus. In these there
are two plates of mesoblast, one on each side of the nervous cord, which
are known as the Germinal streaks (Keimstreifen) (vide Kowalevsky Würmern u. Arthropoden
;
Mém. de l'Acad. Imp. St Pétersbourg, 1871).
From longitudinal sections I have found that the segmentation cavity has ceased by this stage to have any distinct existence, but that the whole space between the epiblast and the yolk is filled up with a mass of elongated cells, which probably are solely concerned in the formation of the vascular system. The thickened posterior edge of the blastoderm is still visible.
At the embryonic end of the blastoderm, as I pointed out in an earlier stage, the epiblast and the lower layer cells are perfectly continuous.
Where they join the epiblast, the lower layer cells become distinctly divided, and this division commenced even in the earlier stage, into two layers; a lower one, more directly continuous with the epiblast, consisting of cells somewhat resembling the epiblast-cells, and an upper one of more flattened cells (Pl. 3, fig. 4, m). The first of these forms the hypoblast, and the latter the mesoblast. They are indicated by hy and m in the figures. The hypoblast, as I said before, remains continuous with the whole of the rest of lower layer cells of the blastoderm (vide fig. 7b). This division into hypoblast and mesoblast commences at the earlier stage, but becomes much more marked during this one.
In describing the formation of the hypoblast and mesoblast in this way I have assumed that they are formed out of the large mass of lower layer cells which underlie the epiblast at the embryonic end of the blastoderm. But there is another and, in some ways, rather a tempting view, viz. [Pg 76] to suppose that the epiblast, where it becomes continuous with the hypoblast, in reality becomes involuted, and that from this involuted epiblast are formed the whole mesoblast and hypoblast.
In this case we would be compelled to suppose that the mass of lower layer cells which forms the embryonic swelling is used as food for the growth of the involuted epiblast, or else employed solely in the growth over the yolk of the non-embryonic portion of the blastoderm; but the latter possibility does not seem compatible with my sections.
I do not believe that it is possible, from the examination of sections alone, to decide which of these two views (viz. whether the epiblast is involuted, or whether it becomes merely continuous with the lower layer cells) is the true one. The question must be decided from other considerations.
The following ones have induced me to take the view that there is no involution, but that the mesoblast and hypoblast are formed from the lower layer cells.
(1) That it would be rather surprising to find the mass of lower layer
cells which forms the embryo swelling
playing no part in the
formation of embryo.
(2) That the view that it is the lower layer cells from which the hypoblast and mesoblast are derived agrees with the mode of formation of these two layers in the Bird, and also in the Frog; since although, in the latter animal, there is an involution, this is not of the epiblast, but of the larger cells of the lower pole of the yolk, which in part correspond with what I have called the lower layer cells in the Dog-fish.
If the view be accepted that it is from the lower layer cells that the hypoblast and mesoblast are formed, it becomes necessary to explain what the continuity of the hypoblast with the epiblast means.
The explanation of this is, I believe, the keystone to the whole
position. The vertebrates may be divided as to their early development into
two classes, viz. those with holoblastic
ova, in which the digestive canal is formed by an involution
with the presence of an anus of Rusconi.
This class includes Amphioxus,
the Lamprey,
the
Sturgeon,
and Batrachians.
[Pg 77] The second class are those with meroblastic ova and no anus of Rusconi, and with an alimentary canal formed by the infolding of the sheet of hypoblast, the digestive canal remaining in communication with the food-yolk for the greater part of embryonic life by an umbilical canal.
This class includes the Elasmobranchii,
Osseous fish,
Reptiles,
and Aves.
The mode of formation of the alimentary canal in the first class is clearly the more primitive; and it is equally clear that its mode of formation in the second class is an adaptation due to the presence of the large quantity of food-yolk.
In the Dog-fish I believe that we can see, to a certain extent, how the change from the one to the other of these modes of development of the alimentary canal took place.
In all the members of the first class, viz.
Amphioxus,
the Lamprey,
the Sturgeon,
and the
Batrachians,
the epiblast becomes continuous with the hypoblast at
the so-called anus of Rusconi,
and the alimentary canal, potentially
in all and actually in the Sturgeon (vide
Kowalevsky, Owsjannikow, and Wagner, Bulletin der Acad. d.
St Petersbourg, Vol. XIV. 1870, "Entwicklung der Störe"), communicates freely at its
extreme hind end with the neural canal. The same is the case in the
Dog-fish. In these, when the folding in to form the alimentary canal on the
one hand, and the neural on the other, takes place, the two foldings unite
at the corner, where the epiblast and hypoblast are in continuity, and
place the two tubes, the neural and alimentary, in free communication with
each other[14].
There is, however, nothing corresponding with the anus of
Rusconi,
which merely indicates the position of the involution of the
digestive canal, and subsequently completely closes up, though it nearly
coincides in position with the true anus in the Batrachians, &c.
This remarkable point of similarity between the Dog-fish's development and the normal mode of development in the first class (the holoblastic) of vertebrates, renders it quite clear that the continuity of the epiblast and hypoblast in the Dogfish [Pg 78] is really the remnant of a more primitive condition, when the alimentary canal was formed by an involution. Besides the continuity between neural and alimentary canals, we have other remnants of the primitive involution. Amongst these the most marked is the formation of the embryonic rim, which is nothing less than the commencement of an involution. Its form is due to the flattened, sheet-like condition of the germ. In the mode in which the alimentary canal is closed in front I shall shew there are indications of the primitive mode of formation of the alimentary canal; and in certain peculiarities of the anus, which I shall speak of later, we have indications of the primitive anus of Rusconi; and finally, in the general growth of the epiblast (small cells of the upper pole of the Batrachian egg) over the yolk (lower pole of the Batrachian egg), we have an example of the manner in which the primitive involution, to form the alimentary canal, invariably disappears when the quantity of yolk in an egg becomes very great.
I believe that in the Dog-fish we have before our eyes one of the steps by which a direct mode of formation comes to be substituted for an indirect one by involution. We find, in fact, in the Dog-fish, that the cells from which are derived the mesoblast and hypoblast come to occupy their final position in the primitive arrangement of the cells during segmentation, and not by a subsequent and secondary involution.
This change in the mode of formation of the alimentary canal is clearly a result of change of mechanical conditions from the presence of the large food-yolk.
Excellent parallels to it will be found amongst the Mollusca. In this class the presence or absence of food-yolk produces not very dissimilar changes to those which are produced amongst vertebrates from the same cause.
The continuity of the hypoblast and epiblast at the embryonic rim is a remnant which, having no meaning or function, except in reference to the earlier mode of development, is likely to become lost, and in Birds no trace of it is any longer to be found.
I will not in the present preliminary paper attempt hypothetically to trace the steps by which the involution gradually [Pg 79] disappeared, though I do not think it would be very difficult to do so. Nor will I attempt to discuss the question whether the condition with a large amount of food-yolk (as seems more probable) was twice acquired—once by the Elasmobranchii and Osseous fishes, and once by Reptiles and Birds—or whether only once, the Reptiles and Birds being lineal descendants of the Dog-fish.
In reference to the former point, however, I may mention that the Batrachians and Lampreys are to a certain extent intermediate in condition between the Amphioxus and the Dog-fishes, since in them the yolk becomes divided during segmentation into lower layer cells and epiblast, but a modified involution is still retained, while the Dog-fish may be looked upon as intermediate between Birds and Batrachians, the continuity at the hind end between the epiblast and hypoblast being retained by them, though not the involution.
It may be convenient here to call attention to some of the similarities and some of the differences which I have not yet spoken of between the development of Osseous fish and the Dog-fish in the early stages. The points of similarity are—(1) The swollen edge of the blastoderm. (2) The embryo-swelling. (3) The embryo-keel. (4) The spreading of the blastoderm over the yolk-sac from a point corresponding with the position of the embryo, and not with the centre of the germ. The growth is almost nothing at that point, and most rapid at the opposite pole of the blastoderm, being less and less rapid along points of the circumference in proportion to their proximity to the embryonic swelling. (5) The medullary groove.
In external appearance the early embryos of Dog-fish and Teleostei are very similar; some of my drawings could almost be substituted for those given by Oellacher. This similarity is especially marked at the first appearance of the medullary groove. In the Dog-fish the medullary groove becomes converted into the medullary canal in the same way as in Birds and all other vertebrates, except Osseous fishes, where it comes to nothing, and is, in fact, a rudimentary structure. But in spite of Oellacher's assertions to the contrary, I am convinced from the similarity of its position and appearance to the true medullary groove in the Dog-fish, that the groove which appears [Pg 80] in Osseous fishes is the true medullary groove; although Oellacher and Kuppfer appear to have conclusively proved that it does not become converted into the medullary canal. The chief difference between the Dog-fish and Osseous fish, in addition to the point of difference about the medullary groove, is that the epiblast is in the Dog-fish a single layer, and not divided into nervous and epidermic layers as in Osseous fish, and this difference is the more important, since, throughout the whole period of development till after the commencement of the formation of the neural canal, the epiblast remains in Dog-fish as a one-cell-deep layer of cells, and thus the possibility is excluded of any concealed division into a neural and epidermic layer, as has been supposed to be the case by Stricker and others in Birds.
Development of the Embryo.
After the embryo has become definitely established, for some time it grows rapidly in length, without externally undergoing other important changes, with the exception of the appearance of two swellings, one on each side of its tail.
These swellings, which I will call the Caudal lobes (figs. 8 and 9, ts), are also found in Osseous fishes, and have been called by Oellacher the Embryonal saum. They are caused by a thickening of mesoblast on each side of the hind end of the embryo, at the edge of the embryonic rim, and form a very conspicuous feature throughout the early stages of the development of the Dog-fish, and are still more marked in the Torpedo (Pl. 3, fig. 9). Although from the surface the other changes which are visible are very insignificant, sections shew that the notochord is commencing to be formed.
I pointed out that beneath the medullary groove the epiblast and
hypoblast were not separated by any interposed mesoblast. Along the line
(where the mesoblast is deficient) which forms the long axis of the embryo,
a rod-like thickening of the hypoblast appears (Pl. 3, figs. 7a and 7b, ch and ch´), first
at the head end of the embryo, and gradually extends backwards. This is the
rudiment of the notochord; it remains attached for some time to the
hypoblast, and becomes separated from it first at [Pg 81] the head end of the
embryo, and the separation is then carried backwards. This thickening of
the hypoblast projects up and comes in contact with the epiblast, and in
the later stages with bad (especially chromic-acid) specimens the line of
separation between the epiblast and the thickening may become a little
obscured, and might possibly lead to the supposition that a structure
similar to that which has been called the axis
cord
was present. In all my best (osmic-acid) specimens the line of
junction is quite clear; and any one who is aware how easily two separate
masses of cells may be made indistinguishably to fuse together from simple
pressure will not be surprised to find the occasional obscurity of the line
of junction between the epiblast and hypoblast. In the earlier stage of the
thickening there is never in the osmic-acid preparations any appearance of
fusion except in very badly prepared ones. Its mode of formation will be
quite clear without further description from an inspection of Pl. 3, figs. 7a and 7b, ch and ch´. Both
are taken from one embryo. In fig. 7b, the most anterior of the two,
the notochord has become quite separated from the hypoblast. In fig.
7a, ch, there is only a very
marked thickening of hypoblast, which reaches up to the epiblast, but the
thickening is still attached to the hypoblast. Had I had space to insert a
drawing of a third section of the same embryo there would only have been a
slight thickening of the hypoblast. In the earlier stage it will be seen,
by referring to figs. 6a and 6b, that there is no sign of a
thickening of the hypoblast. My numerous sections (all made from embryos
hardened in osmic acid) shewing these points are so clear that I do not
think there can be any doubt whatever of the notochord being formed as a
thickening of the hypoblast. Two interpretations of this seem possible.
I mentioned that the mesoblast appeared to be primitively formed as two independent sheets, split off, so to speak, from the hypoblast, one on each side of the middle line of the embryo. If we looked upon the notochord as a third median sheet of mesoblast, split off from the hypoblast somewhat later than the other two, we should avoid having to admit its hypoblastic origin.
Professor Huxley, to whom I have shewn my specimens, strongly advocates this view.
[Pg 82] The other possibility is that the notochord is primitively a true hypoblastic structure which has only by adaptation become an apparently mesoblastic one in the higher vertebrates. In favour of this view are the following considerations:
(1) That this is the undoubtedly natural interpretation of the sections. (2) That the notochord becomes separated from the hypoblast after the latter has acquired its typical structure, and differs in that respect from the two lateral sheets of mesoblast, which are formed coincidently with the hypoblast by a homogeneous mass of cells becoming differentiated into two distinct layers. (3) That the first mode of looking at the matter really proves too much, since it is clear that by the same method of reasoning we could prove the mesoblastic origin of any organ derived from the hypoblast and budded off into the mesoblast. We would merely have to assert that it was really a mass of mesoblast budded off from the hypoblast rather later than the remainder of the mesoblast. Still, it must be admitted that the first view I have suggested is a possible, not to say a probable one, though the mode of arguing by which it can be upheld may be rather dangerous if generally applied. We ought not, however, for that reason necessarily to reject it in the present case. As Mr Ray Lankester pointed out to me, if we accept the hypoblastic origin of the notochord, we should find a partial parallel to it in the endostyle of Tunicates, and it is perhaps interesting to note in reference to it that the notochord is the only unsegmented portion of the axial skeleton.
Whether the strong à priori difficulties of the hypoblastic origin of the notochord are sufficient to counterbalance the natural interpretation of my sections, cannot, I think, be decided from the single case of the Dog-fish. It is to be hoped that more complete investigations of the Lamprey, &c., may throw further light upon the question.
Whichever view of the primitive origin of the notochord is the true one, its apparent origin is very instructive as illustrating the possible way in which an organ might come to change the layer to which it primarily belonged.
If the notochord is a true mesoblastic structure, it is easy to be seen how, by becoming separated from the hypoblast a little later than is the case with the Dog-fish, its mesoblastic [Pg 83] origin would become lost; while if, on the other hand, it is primitively a hypoblastic structure, we see from higher vertebrates how, by becoming separated from the hypoblast rather earlier than in the Dog-fish, viz. at the same time as the rest of the mesoblast, its primitive derivation from the hypoblast has become concealed.
The view seemingly held by many embryologists of the present day, that an organ, when it was primitively derived from one layer, can never be apparently formed in another layer, appears to me both unreasonable on à priori grounds, and also unsupported by facts.
I see no reason for doubting that the embryo in the earliest periods of development is as subject to the laws of natural selection as is the animal at any other period. Indeed, there appear to me grounds for the thinking that it is more so. The remarkable differences in allied species as to the amount of food-yolk, which always entail corresponding alterations in the development—the different modes of segmentation in allied species, such as are found in the Amphipoda and Isopoda—the suppression of many stages in freshwater species, which are retained in the allied marine species—are all instances of modifications due to natural selection affecting the earliest stages of development. If such points as these can be affected by natural selection I see no reason why the arrangement of individual cells (or rather primitive elements) should not also be modified; why, in fact, a mass of cells which was originally derived from one layer, but in the course of development became budded off from that layer and entered another layer, should not by a series of small steps cease ever to be attached to the original layer, but from the first moment it can be distinguished should be found as a separate mass in the second layer.
The change of layers will, of course, only take place where some economy is effected by it. The variations in the mode of development of the nervous system may probably be explained in this way.
If we admit that organs can undergo changes, as to the primitive layer from which they are derived, important consequences must follow.
It will, for instance, by no means be sufficient evidence of [Pg 84] two organs not being homologous that they are not developed from the same layer. It renders the task of tracing out the homologies from development much more difficult than if the ordinary view of the invariable correspondence of the three layers throughout the animal kingdom be accepted. Although I do not believe that this correspondence is invariable or exact, I think that we both find and should expect to find that it is, roughly speaking, fairly so.
Thus, the muscles, internal skeleton, and connective tissue are always placed in the adult between the skin (epidermis) and the epithelium of the alimentary canal.
We should therefore expect to find them, and, as a matter of fact, we always do find them, developed from a middle layer when this is present.
The upper layer must always and does always form the epidermis, and similarly the lower layer or hypoblast must form a part of the epithelium of the alimentary canal. A full discussion of this question would, however, lead me too far away from my present subject.
The only other point of interest which I can touch on in this stage is the commencing closure of the alimentary canal in the region of the head. This is shewn in Pl. 3, figs. 6a, 6b, 7b, n.a. From these figures it can be seen that the closing does not take place as much by an infolding as by an ingrowth from the side walls of the alimentary canal towards the middle line. In this abnormal mode of closing of the alimentary canal we have again, I believe, an intermediate stage between the mode of formation of the alimentary canal in the Frog and the typical folding in which occurs in Birds. There is, however, another point in reference to it which is still more interesting. The cells to form the ingrowth from the bottom (ventral) wall of the alimentary canal are derived by a continuous fresh formation from the yolk, being formed around the nuclei spoken of above (vide p. 63 et seq.). All my sections shew this with more or less clearness, especially those a little later than fig. 6b, in which the lower wall of the alimentary canal is nearly completed. This is the more interesting since, from the mode of formation of the alimentary canal in the Batrachians, &c., we might expect that the cells from the yolk would take [Pg 85] a share in its formation in the Dog-fish. I have not as yet made out for certain the share which is taken by these freshly formed cells of the yolk in the formation of any other organ.
By the completion of its lower wall in the way described, the throat early becomes a closed tube, its closing taking place before any other important changes are visible in the embryo from surface views.
A considerable increase in length is attained before other changes than an increase in depth of the medullary groove and a more complete folding off of the embryo from the blastoderm take place. The first important change is the formation of the protovertebræ.
These are formed by the lateral plates of mesoblast, which I said were equivalent at once to the vertebral and lateral plates in the Bird, becoming split by transverse divisions into cubical masses.
At the time when this occurs, and, indeed, up till a considerably later period, the mesoblast is not split into somatopleure and splanchnopleure, and it is not divided into vertebral and lateral plates. The transverse lines of division of the protovertebræ do not, however, extend to the outer edge of the undivided lateral plates.
The differences between this mode of formation of the protovertebræ and that occurring in Birds are too obvious to require pointing out. I will speak of them more fully when I have given the whole history of the protovertebræ of the Dog-fish.
I will only now say that I have had in the early stages to investigate the formation of the protovertebræ entirely by means of sections, the objects being too opaque to be otherwise studied.
The next change of any importance is the commencement of the formation of the head. The region of the head first becomes distinguishable by the flattening out of the germ at its front end.
The flattened-out portion of the germ grows rapidly, and forms a spatula-like termination to the embryo (Pl. 3, fig. 8).
In the region of the head the medullary groove is at first totally absent (vide section, Pl. 3, fig. 8a).
Indeed, as can be seen from fig. 8b, the laminæ dorsales, so [Pg 86] far from bending up at this stage, actually bend down in the opposite direction.
I am at present quite unable even to form a guess what this peculiar feature of the brain means. It, no doubt, has some meaning in reference to the vertebrate ancestry if we could only discover it. The peculiar spatula-like flattened condition of the head is also (vide loc. ant. cit.) apparently found in the Sturgeons; it must therefore almost undoubtedly be looked upon as not merely an accidental peculiarity.
While these changes have been taking place in the head not less important changes have occurred in the remainder of the body. In the first place the two caudal lobes have increased in size, and have become, as it were, pushed in together, leaving a groove between them (fig. 8, ts). They are very conspicuous objects, and, together with the spatula-like head, give the whole embryo an almost comical appearance. The medullary canal has by this time become completely closed in the region of the tail (figs. 8 and 8b).
It is still widely open in the region of the back, and, though more nearly closed again in the neck, is, as I have said, flattened out to nothing in the head.
The groove[15] between the two caudal lobes must not be confused (as may easily be done) with the medullary groove, which by the time the former groove has become conspicuous is a completely closed canal.
The vertebral plates are not divided (vide fig. 7) into a somatopleuric and splanchnopleuric layer by this stage, except in the region of the head (vide fig. 8b, pp), where there is a distinct space between the two layers, which is undoubtedly homologous with the pleuro-peritoneal cavity of the hinder portion of the body.
It is probably the same cavity which Oellacher (loc. cit.) calls in Osseous fishes the pericardial cavity. In the Dog-fish, at least, it has no connection with the pericardium. Of its subsequent history I shall say a few words when I come to speak of the later stages.
[Pg 87] The embryo does not take more than twenty-four hours in passing from this stage, when the head is a flat plate, to the stage when the whole neural canal (including the region of the head) is closed in. The other changes, in addition to the closing in of the neural canal, are therefore somewhat insignificant. The folding off of the embryo from the germ has, however, progressed considerably, and a portion of the hind gut is closed in below. This is accomplished, not by a tail-fold, as in Birds, but by two lateral folds, which cause the sides of the body to meet and coalesce below. At the extreme hind end, where the epiblast is continuous with the hypoblast, the lateral folds turn round, so to speak, and become continuous with the medullary folds, so that when the various folds meet each other an uninterrupted canal is found passing round from the neural into the alimentary canal, and placing these two in communication at the tail end of the body. Since I have already mentioned this, and spoken of its significance, I will not dwell on it further here.
The cranial flexure commences coincidently with the closing in of the neural canal in the region of the brain, and the division into fore, mid, and hind brain becomes visible at the same time as or even before the closing of the canal occurs. The embryo has now become more or less transparent, and protovertebræ, of which about twenty are present, can now be seen in the fresh specimens. The heart, however, is not yet formed.
Up to this period, a period at which the embryo becomes very similar in external appearance to any other vertebrate embryo, I have followed in my description a chronological order. I shall now cease to do so, since it would be too long for a preliminary notice of this kind, but shall confine myself to the history of a few organs whose development is either more important or more peculiar than that of the others.
The Protovertebræ.
I have thought it worth while to give a short history of the development of the protovertebræ, firstly, because it is very easy to follow this in the Dog-fish, and, secondly, because [Pg 88] I believe that the Dog-fish have more nearly retained the primitive condition of the protovertebræ than any other vertebrate whose embryology has hitherto been described with sufficient detail.
I intend to describe, at the same time, the development of the spinal nerves.
I left each lateral mass of mesoblast in my last stage as a plate which had not yet become split into a somatic and a splanchnic sheet (Pl. 3, fig. 8a, vp), but which had become cut by transverse lines (not, indeed, extending to the outer limit of the sheet, but as yet not cut off by longitudinal lines of cleavage) into segments, which I called protovertebræ.
This sheet of mesoblast is fairly thick at its proximal (upper) end, but thins off laterally to a sheet two cells deep, and its cells are so arranged as to foreshadow its subsequent splitting into somatic and splanchnic sheets. Its upper (proximal) end is at this stage level with the bottom of the neural canal, but soon begins to grow upwards, and at the same time the splitting into somatopleure and splanchnopleure commences (Pl. 3, fig. 10, so and sp).
The separation between the two sheets is first visible in its uppermost part, and thence extends outwards. By this means each of the protovertebræ becomes divided into two sheets, which are only connected at their upper ends and outside the region of the body. I speak of the whole lateral sheet as being composed of protovertebræ, because at this time no separation into vertebral and lateral plates can be seen; but I may anticipate matters by saying that only the upper portion of the sheet from the level of the top of the digestive canal, becomes subsequently the true protovertebræ. From this it is clear that the pleuro-peritoneal cavity extends primitively quite up to the top of the protovertebræ; and that thus a portion of a sheet of mesoblast, at first perfectly continuous with the splanchnic sheet from which is derived the muscular wall of the alimentary canal, is converted into a part of the voluntary muscular system of the body, having no connection whatever with the involuntary muscular system of the digestive tract.
The pleuro-peritoneal cavity is first distinctly formed at a [Pg 89] time when only two visceral clefts are present. Before the appearance of a third visceral cleft in a part of the innermost layer of each protovertebræ (which may be called the splanchnic layer, from its being continuous with the mesoblast of the splanchnopleure), opposite the bottom of the neural tube, some of the cells commence to become distinguishable from the rest, and to form a separate mass. This mass becomes much more distinct a little later, its cells being characterised by being spindle-shaped, and having an elongated nucleus which becomes deeply stained by reagents (Pl. 4, fig. 11, mp´). Coincidently with its appearance the young Dog-fish commences spontaneously to move rapidly from side to side with a kind of serpentine motion, so that, even if I had not traced the development of this differentiated mass of cells till it becomes a band of muscles close to the notochord, I should have had little doubt of its muscular nature. It is indicated in figs. 11, 12, 13, by the letters mp´. Its early appearance is most probably to be looked upon as an adaptation consequent upon the respiratory requirements of the young Dog-fish necessitating movements within the egg.
Shortly after this date, at a period when three visceral clefts are present, I have detected the first traces of the spinal nerves.
At this time they appear in sections as small elliptical masses of cells, entirely independent of the protovertebræ, and closely applied to the upper and outer corners of the involuted epiblast of the neural canal (Pl. 4, fig. 11, spn). These bodies are far removed from any mesoblastic structures, and at the same time the cells composing them are not similar to the cells composing the walls of the neural canal, and are not attached to these, though lying in contact with them. I have not, therefore, sufficient evidence at present to enable me to say with any certainty where the spinal nerves are derived from in the Dog-fish. They may be derived from the involuted epiblast of the neural canal, and, indeed, this is the most natural interpretation of their position.
On the other hand, it is possible that they are formed from wandering cells of the mesoblast—a possibility which, with our present knowledge of wandering cells, must not be thrown aside as altogether improbable.
[Pg 90] In any case, it is clear that the condition in the Bird, where the spinal nerves are derived from tissue of the protovertebræ, is not the primitive one. Of this, however, I will speak again when I have concluded my account of the development of the protovertebræ.
About the same time that the first rudiments of the nerves appear, the division of the mesoblast of the sides of the body into a vertebral and a lateral portion occurs. This division first appears in the region where the oviduct (Müller's duct) is formed (Pl. 4, fig. 11, ov).
At this part opposite the level of the dorsal aorta the two sheets, viz. the splanchnic and the somatic, unite together, and thus each lateral sheet of mesoblast becomes divided into an upper portion (fig. 11, mp), split up by transverse partitions into protovertebræ, and a lower portion not so split, but consisting of an outer layer, the true somatopleure, and an inner layer, the true splanchnopleure. These two divisions of the primitive plate are thus separated by the line at which a fusion between the mesoblast of the somatopleure and splanchnopleure takes place. The mass of cells resulting from the fusion at this point corresponds with the intermediate cell-mass of Birds (vide Waldeyer, Eierstock und Ei).
At the same time, in the upper of these two sheets (the protovertebræ), the splanchnic layer sends a growth of cells inwards towards the notochord and the neural canal. This growth is the commencement of the large quantity of mesoblastic tissue around the notochord, which is in part converted into the axial skeleton, and in part into the connective tissue adjoining this.
This mass of cells is at first quite continuous with the splanchnic layer of the protovertebræ, and I see no reason for supposing that it is not derived from the growth of the cells of this layer. The ingrowth to form it first appears a little after the formation of the dorsal aorta; but, as far as I have been able to see, its cells have no connection with the walls of the aorta.
What I have said as to the development of the skeleton-forming layer will be quite clear from figs. 11 and 12a; and from these it will also be clear, especially from fig. 11a, that [Pg 91] the outermost layer of this mass of cells, which was the primitive splanchnic layer of the protovertebræ, still retains its epithelial character, and so can easily be distinguished from those cells which will form the skeleton. In the next stage which I have figured (fig. 12a), this outer portion of the splanchnic layer is completely separated from the skeleton-forming cells, and at the same time, having united below as well as above with the outer (somatic) layer of the two layers of which the protovertebræ are formed, the two together form an independent mass (fig. 12, mp), similar in appearance and in every way homologous with the muscle-plate of Birds.
On the inner side of this, which we may now call the muscle-plate, is seen the bundle of earlier-developed muscles (fig. 12, mp´) which I spoke of before.
The section represented in fig. 12 is from a very considerably later embryo than that represented in fig. 11, so that the skeleton-forming cells, few in number in the earlier section, have become very numerous in the later one, and have grown up above the neural canal, and also below the notochord, between the digestive canal and the aorta. They have, moreover, changed their character; they were round before, now they have become stellate. As to their further history, it need only be said that the layer of them immediately around the notochord and neural canal forms the cartilaginous centra and arches of the vertebræ, and that the remaining portion of them, which becomes much more insignificant in size as compared with the muscles, forms the connective tissue of the skeleton and of the parts around and between the muscles.
A muscle-plate itself is at this stage (shewn in fig. 12) composed of an inner and an outer layer of columnar cells (splanchnic and somatic) united at the upper and lower ends of the plate, and on the inner of the two lies the more developed mass of muscles before spoken of (mp´).
Each of these plates now grows both upwards and downwards; and at the same time connective-tissue cells appear between the plates and epidermis; but from where they come I do not know for certain; very probably they are derived from the somatic layer of the muscle-plate.
While the muscle-plates continue to grow both upwards and [Pg 92] downwards, the cells of which they are composed commence to become elongated and soon acquire an unmistakably muscular character (Pl. 4, fig. 13, mp).
Before this has occurred the inner mass of muscles has also undergone further development and become a large and conspicuous band of muscles close to the notochord (fig. 13, mp´).
At the same time that the muscle-plates acquire the true histological character of muscle, septa of connective tissue grow in and divide them into a number of distinct segments which subsequently form separate bands of muscle. I will not say more in reference to the development of the muscular system than that the whole of the muscles of the body (apart from the limbs, the origin of whose muscular system I have not yet investigated) are derived from the muscle-plates which grow upwards above the neural canal and downwards to the ventral surface of the body.
During the time the muscle-plates have been undergoing these changes the nerve masses have also undergone developmental changes.
They become more elongated and fibrous, their main attachment to the neural tube being still at its posterior (dorsal) surface, near which they first appeared. Later still they become applied closely to the sides of the neural tube and send fibres to it below as well as above. Below (ventral to) the neural tube a ganglion appears, forming only a slight swelling, but containing a number of characteristic nerve-cells. The ganglion is apparently formed just below the junction of the anterior and posterior roots, though probably the fibres of the two roots do not mix till below it.
The main points which deserve notice in the development of the protovertebræ are—
(1) That at the time when the mesoblast becomes split horizontally into somatopleure and splanchnopleure the vertebral and lateral plates are one, and the splitting extends to the very top of the vertebral or muscle-plate, so that the future muscle-plates are divided into a splanchnic and somatic layer, the space between which is at first continuous with the pleuro-peritoneal cavity.
[Pg 93] (2) That the following parts are respectively formed by the vertebral and lateral plates:
(a) Vertebral plate. From the splanchnic layer of this, or from cells which appear close to and continuous with it, the skeleton, and connective tissue of the upper part of the body, are derived.
The remainder of the plate, consisting of a splanchnic and somatic layer, is entirely converted into the muscles of the trunk, all of which are derived from it.
(b) Between the vertebral plate and the lateral plate is a mass of cells where, as I mentioned above, the mesoblast of the somatopleure and splanchnopleure fuse together. This mass of cells is the equivalent of the intermediate cell mass of Birds (vide Waldeyer, Eierstock und Ei).
From it are derived the Wolffian bodies and duct, the oviduct, the ovaries and the testis, and the connective tissue of the parts adjoining these.
(c) The lateral plate. From the somatic layer of this is derived the connective tissue of the ventral half of the body; the mesoblast of the limbs, including probably the muscles, and certainly the skeleton. From its splanchnic layer are derived the muscles and connective tissue of the alimentary canal.
(3) The spinal nerves are developed independently of the protovertebræ, so that the protovertebræ of the Elasmobranchii do not appear to be of such a complicated structure as the protovertebræ of Birds.
The Digestive Canal.
I do not intend to enter into the whole history of the digestive canal, but to confine myself to one or two points of interest connected with it. These fall under two heads:
(1) The history of the portion of the digestive canal between the anus and the end of the tail where the digestive canal opens into the neural canal.
(2) Certain less well-known organs derived from the digestive canal.
[Pg 94] The anus is a rather late formation, but its position becomes very early marked out by the hypoblast of the digestive canal approaching at that point close to the surface, whilst receding to some little distance from it on either side. The portion of the digestive tract I propose at present dealing with is that between this point, which I will call, for the sake of brevity, the anus and the hind end of the body. This portion of the canal is at first very short; it is elliptical in section, and of rather a larger bore than the remainder of the canal. Its diameter becomes, however, slightly less as it approaches the tail, dilating again somewhat at its extreme end. It is lined by a markedly columnar epithelium. Though at first very short, its length increases with the growth of the tail, but at the same time its calibre continually becomes smaller as compared with the remainder[TN1] of the alimentary canal.
It commences to become smaller, first of all, near, though not quite, at its extreme hind end, and thus becomes of a conical shape; the base of the cone being just behind the anus, while the apex of the cone is situated a short distance from the hind end of the embryo. The extreme hind end, however, at the same time does not diminish in size, and becomes relatively (if not also absolutely) much larger in diameter than it was at first, as compared with the remainder of the digestive canal. It becomes, in fact, a vesicle or vesicular dilatation at the end of a conical canal.
Just before the appearance of the external gills this part of the digestive canal commences to atrophy. It begins to do so close to the terminal vesicle, which, however, still remains as or more conspicuous than it was before. The lumen of the canal becomes smaller and smaller, and finally it becomes a solid string of cells, and these also soon become indistinguishable and not a trace of the canal is left.
Almost the whole of it has disappeared before the vesicle begins to atrophy, but very shortly after all trace of the rest of the canal has vanished the terminal vesicle also vanishes. This occurs just about the time or shortly after the appearance of the external gills—there being slight differences probably in this respect in the different species.
In this history there are two points of especial interest:
[Pg 95] (1) The terminal vesicle.
(2) The disappearance of a large and well-developed portion of the alimentary canal.
The interest in the terminal vesicle lies in the possibility of its being some rudimentary structure.
In Osseous fishes Kupffer has described the very early appearance of a
vesicle near the tail end, which he doubtfully speaks of as the
allantois.
The figure he gives of it in his earlier paper (Archiv. für Micro. Anat. Vol.
II. pl. xxiv,
fig. 2) bears a very strong resemblance to my figures of this vesicle at
the time when the hind end of the alimentary canal is commencing to
disappear; and I feel fairly confident that it is the same structure as I
have found in the Dog-fish: but until the relations of the Kupffer's
vesicle to the alimentary canal are known, any comparison between it and
the terminal vesicle in the Dog-fish must be to a certain extent
guess-work.
I have, however, been quite unsuccessful in finding any other vesicular structure which can possibly correspond to the so-called allantoic vesicle of Osseous fish.
The disappearance of a large portion of the alimentary canal behind the anus is very peculiar. In order, however, to understand the whole difficulties of the case I shall be obliged to speak of the relations of the anus of the Dog-fish to the anus of Rusconi in the Lamprey, &c.
In those vertebrates whose alimentary canal is formed by an involution,
the anus of Rusconi represents the opening of this involution, and
therefore the point where the alimentary canal primitively communicates
with the exterior. When, however, the anus of Rusconi
becomes
closed, the wall of the alimentary canal still remains at that
point in close juxtaposition to the surface, and the new and final anus is
formed at or close to that point. In the Dog-fish, although the anus of
Rusconi is not present, still, during the closing of the alimentary canal,
the point which would correspond with this becomes marked out by the
alimentary canal there approaching the surface, and it is at this point
that the involution to form the true anus subsequently appears.
The anus in the Dog-fish has thus, more than a mere secondary significance. It corresponds with the point of closing of [Pg 96] the primitive involution. If it was not for this peculiarity of the vertebrate anus we would naturally suppose, from the disappearance of a considerable portion of the alimentary canal lying behind its present termination, that in the adult the alimentary canal once extended much farther back than at present, and that the anus we now find was only a secondary anus, and not the primitive one. It is perhaps possible that this hinder portion of the alimentary canal is a result of the combined growth of the tail and the persisting continuity (at the end of the body) of the epiblast with the hypoblast.
Whichever view is correct, it may be well to mention, in order to shew
that the difficulty about the anus of Rusconi is no mere visionary one,
that Götte (Untersuchung über die Entwicklung der Bombinator
igneus,
Archiv. für Micro. Anat., vol. V. 1869) has also
described the disappearance of the hind portion of the alimentary canal in
Batrachians, a rudiment (according to him) remaining in the shape of a
lymphatic trunk.
It is, perhaps, possible that we have a further remnant of this hind
portion
of the alimentary canal amongst the higher vertebrates in the
allantois.
Organs developed from the Digestive Canal.
In reference to the development of the liver, pancreas, &c., as far as my observations have at present gone, the Dog-fish presents no features of peculiar interest. The liver is developed as in the Bird, and independently of the yolk.
There are, however, two organs derived from the hypoblast which deserve more attention. Immediately under the notochord, and in contact with it (vide Pl. 3, fig. 10; Pl. 4, figs. 11 and 12, x), a small roundish (in section) mass of cells is to be seen in most of the sections.
Its mode of development is shewn in fig. 10, x. That section shows a mass of cells becoming pinched off from the top of the alimentary canal. By this process of pinching off from the alimentary canal a small rod-like body close under the notochord is formed. It persists till after the appearance of the external gills, but later than that I have not hitherto succeeded in finding any trace of it.
[Pg 97] It was first seen by Götte (loc. cit.) in the Batrachians, and he gave a correct account of its development, and added that it became the thoracic duct.
I have not myself worked out the later stages in the development of this body with sufficient care to be in a position to judge of the correctness of Götte's statements as to its final fate. If it is true that it becomes the thoracic duct it is very remarkable, and ought to throw some light upon the homologies of the lymphatic system.
Some time before the appearance of the external gills another mass of cells becomes, I believe, constricted off from the part of the alimentary canal in the neighbourhood of the anus, and forms a solid rod composed at first of dark granular cells lying between the Wolffian ducts. I have not followed out its development quite completely, but I have very little doubt that it is really constricted off from a portion of the alimentary canal chiefly in front of the point where the anus appears, but also, I believe, from a small portion behind this.
Though the cells of which it is composed are at first columnar and granular (fig. 12, su, r), they soon begin to become altered, and in the latter stage of its development the body forms a conspicuous rounded mass of cells with clear protoplasm, and each provided with a large nucleus. Later still it becomes divided into a number of separate areas of cells by septa of connective tissue, in which (the septa) capillaries are also present. Since I have not followed it to its condition in the adult, I cannot make any definite statements as to the fate of this body; but I think that it possibly becomes the so-called suprarenal organ, which in the Dog-fish forms a yellowish elongated body lying between the two kidneys.
The development of the Wolffian Duct and Body and of the Oviduct.
The development of the Wolffian duct and the Oviduct in the various classes of vertebrates is at present involved in some obscurity, owing to the very different accounts given by different observers.
[Pg 98] The manner of development of these parts in the Dog-fish is different from anything that previous investigators have met with in other classes, but I believe that it gives a clearer insight into the true constitution of these parts than vertebrate embryology has hitherto supplied, and at the same time renders easier the task of understanding the differences in the modes of development in the different classes.
I shall commence with a simple description of the observed facts, and then give my view as to their meaning. At about the time of the appearance of the third visceral cleft, and a short way behind the point up to which the alimentary canal is closed in front, the splanchnopleure and somatopleure fuse together opposite the level of the dorsal aorta.
From the mass of cells formed by this fusion a solid knob rises up towards the epiblast (Pl. 4, fig. 11b, ov), and from this knob a solid rod of cells grows backwards towards the tail (fig. 11c, ov) very closely applied to the epiblast. This description will be rendered clear by referring to figs. 11b and c. Fig. 11b is a section at the level of the knob, and fig. 11c is a section of the same embryo a short way behind this point. So closely does the rod of cells apply itself to the epiblast that it might very easily be supposed to be derived from it. Such, indeed, was at first my view till I cut a section passing through the knob. In order, however, to avoid all possibility of mistake I made sections of a large number of embryos of about the age at which this appears, and invariably found the large knob in front, and from it the solid string growing backwards.
This string is the commencement of the Oviduct or Müller's duct, which in the Dog-fish as in the Batrachians is the first portion of the genito-urinary system to appear, and is in the Dog-fish undoubtedly at first solid. All my specimens have been hardened with osmic acid, and with specimens hardened with this reagent it is quite easy to detect even the very smallest hole in a mass of cells.
As a solid string or rod of cells the Oviduct remains for some time; it grows, indeed, rapidly in length, the extreme hind end of the rod being very small and the front end continuing to remain attached to the knob. The knob, however, travels inwards and approaches nearer and nearer to the true pleuro-peritoneal [Pg 99] cavity, always remaining attached to the intermediate cell mass.
At about the time when five visceral clefts are present the Oviduct first begins to get a lumen and to open at its front end into the pleuro-peritoneal cavity. The cells of the rod are first of all arranged in an irregular manner, but gradually become columnar and acquire a radiating arrangement around a central point. At this point, where the ends of all the cells meet, a very small hole appears, which gradually grows larger and becomes the cavity of the duct (fig. 12, ov). The hole first makes its appearance at the anterior end of the duct, and then gradually extends backwards, so that the hind end is still without a lumen, when the lumen of the front end is of a considerable size.
At the front knob the same alteration in the cells takes place as in the rest of the duct, but the cells become deficient on the side adjoining the pleuro-peritoneal cavity, so that an opening is formed into the pleuro-peritoneal cavity, which soon becomes of a considerable size. Soon after its first formation, indeed, the opening becomes so large that it may be met in from two to three consecutive sections if these are very thin.
Thus is formed the lumen of the Oviduct. The duct still, at this age, ends behind without having become attached to the cloaca, so that at this time the Oviduct is a canal closed behind, but communicating in front by a large opening with the pleuro-peritoneal cavity.
It has during this time been travelling downwards, and is now much nearer the pleuro-peritoneal cavity than the epiblast.
It may be well to point out that the mode of development which I have described is really not very different from an involution, and must, in fact, be only looked upon as a modification of an involution. Many examples from all classes in the animal kingdom could be selected to exemplify how an involution may become simply a solid thickening. In the Osseous fish nearly all the organs which are usually formed by an involution have undergone this change in their mode of development. I shall attempt to give reasons later on for the solid form having been acquired in this particular case of the Oviduct.
[Pg 100] At about the time when a lumen appears in the Oviduct the first traces of the Wolffian duct become visible.
At intervals along the whole length, between the front and hind ends of the Oviduct, involutions arise from the pleuro-peritoneal cavity (fig. 12a, pwd) on the inside (nearer the middle line) of the Oviduct. The upper ends of these numerous involutions unite together and form a string of cells, at first solid, but very soon acquiring a lumen, and becoming a duct which communicates (as it clearly must from its mode of formation), at numerous points with the pleuro-peritoneal cavity. It is very probable that there is one involution to each segment of the body between the front and hind ends of the Oviduct. This duct is the Wolffian duct, which thus, together with the Oviduct, is formed before the appearance of the external gills.
For a considerable period the front end of the Oviduct does not undergo important changes; the hind end, however, comes into connection with the extreme end of the alimentary canal. The two Oviducts do not open together into the cloaca, though, as my sections prove, their openings are very close together. The whole Oviduct, as might be expected, shares in the general growth, and its lumen becomes in both sexes very considerably greater than it was before.
It is difficult to define the period at which I find these changes accomplished without giving drawings of the whole embryo. The stage is one considerably after the external gills have appeared, but before the period at which the growth of the olfactory bulbs renders the head of an elongated shape.
During the same period the Wolffian duct has undergone most important changes. It has commenced to bud off diverticula, which subsequently become the tubules of the Wolffian body (vide fig. 13, wd). I am fairly satisfied that the tubules are really budded off, and are not formed independently in the mesoblast. The Dog-fish agrees so far with Birds, where I have also no doubt the tubules of the Wolffian body are formed as diverticula from the Wolffian duct.
The Wolffian ducts have also become much longer than the Oviduct, and are now found behind the anus, though they do not extend as far forward as does the Oviduct.
[Pg 101] They have further acquired a communication with the Oviduct, in the form of a narrow duct passing from each of them into an Oviduct a short way before the latter opens into the cloacal dilatation of the alimentary canal.
The canals formed by the primitive involution leading from the pleuro-peritoneal cavity into the Wolffian duct have become much more elongated, and at the same time narrower. One of these is shewn in fig. 13, pwd.
Any doubt which could possibly be entertained as to the true character of the ducts whose development I have described is entirely removed by the development of the tubules of the Wolffian body. In the still later stage than this further proofs are furnished involving the function of the Oviduct. At the period when the olfactory lobes have become so developed as to render the head of the typical elongated shape of the adult, I find that the males and females can be distinguished by the presence in the former of the clasping appendages[16]. I find at this stage that in the female the front ends of the Oviducts have approached the middle line, dilated considerably, and commenced to exhibit at their front ends the peculiarities of the adult. In the male they are much less conspicuous, though still present.
At the same time the tubules of the Wolffian body become much more numerous, the Malpighian tufts appear, and the ducts cease almost, if not entirely, to communicate with the pleuro-peritoneal cavity. I have not made out anything very definitely as to the development of the Malpighian tufts, but I am inclined to believe that they arise independently in the mesoblast of the intermediate cell mass.
The facts which I have made out in reference to the development of the Wolffian duct, especially of its arising as a series of involutions from the pleuro-peritoneal cavity, will be found, I believe, of the greatest importance in understanding the true constitution of the Wolffian body. To this I will return directly, but first wish to clear the ground by insisting upon one preliminary point.
From their development the Oviduct and Wolffian body appear to stand to each other in the relation of the Wolffian [Pg 102] duct being the equivalent to a series, so to speak, of Oviducts.
I pointed out before that the mode of development of the Oviduct could only be considered as a modification of a simple involution from the pleuro-peritoneal cavity. Its development, both in the Birds and in the Batrachians as an involution, still more conclusively proves the truth of this view.
The explanation of its first appearing as a solid rod of cells which keeps close to the epiblast is, I am inclined to think, the following. Since the Oviduct had to grow a long way backwards from its primitive point of involution, it was clearly advantageous for it not to bore its way through the mesoblast of the intermediate cell mass, but to pass between this and the epiblast. This modification having been adopted, was followed by the knob forming the origin of the duct coming to be placed at the outside of the intermediate cell mass rather than close to the pleuro-peritoneal cavity, a change which necessitated the mode of development by an involution being dropped and the solid mode of development substituted for it, a lumen being only subsequently acquired.
In support of the modification in the development being due to this cause is the fact that in Birds a similar modification has taken place with the Wolffian duct. The Wolffian duct there arises differently from its mode of development in all the lower vertebrates as a solid rod close to the epiblast[17], instead of as an involution.
If the above explanation about the Oviduct be correct, then it is clear that similar causes have produced a similar modification in development (only with a different organ) in Birds; while, at the same time, the primitive mode of origin of the Oviduct (Müller's duct) has been retained by them.
The Oviduct, then, may be considered as arising by an involution from the pleuro-peritoneal cavity.
The Wolffian duct arises by a series of such involutions, all of which are behind (nearer the tail) the involution to form the Oviduct.
[Pg 103] The natural interpretation of these facts is that in the place of the Oviduct and Wolffian body there were primitively a series of similar bodies (probably corresponding in number with the vertebral segments), each arising by an involution from the pleuro-peritoneal cavity; and that the first of these subsequently became modified to carry eggs, while the rest coalesced to form the Wolffian duct.
If we admit that the Wolffian duct is formed by the coalescence of a series of similar organs, we shall only have to extend the suggestion of Gegenbaur as to the homology of the Wolffian body in order to see its true nature. Gegenbaur looks upon the whole urinogenital system as homologous with a pair of segmental organs. Accepting its homology with the segmental organs, its development in Elasmobranchii proves that it is not one pair, but a series of pairs of segmental organs with which the urinogenital system is homologous. The first of these have become modified so as to form the Oviducts, and the remainder have coalesced to form the Wolffian ducts.
The part of a segmental organ which opens to the exterior appears to be lost in the case of all but the last one, where this part is still retained, and serves as the external opening for all.
Whether the external opening of the first segmental organ (Oviduct) is retained or not is doubtful. Supposing it has been lost, we must look upon the external opening for the Wolffian body as serving also for the Oviduct. In the case of all other vertebrates whose development has been investigated (but the Elasmobranchii), the Wolffian duct arises by a single involution, or, what is equivalent to it, the other involutions having disappeared. This even appears to be the case in the Marsipobranchii. In the adult Lamprey the Wolffian duct terminates at its anterior end by a large ciliated opening into the pleuro-peritoneal cavity. It will, perhaps, be found, when the development of the Marsipobranchii is more carefully studied, that there are primitively a number of such openings[18]. The Oviduct, when present, arises in other vertebrates [Pg 104] as a single involution, strongly supporting the view that its mode of formation in the Dog-fish is fundamentally merely an involution.
The duct of the testes is, I have little doubt, derived from the anterior part of the Wolffian body; if so, it must be looked upon as not precisely equivalent to the Oviduct, but rather to a series of coalesced organs, each equivalent to the Oviduct. The Oviduct is in the Elasmobranchii, as in other vertebrates, primitively developed in both sexes. In the male, however, it atrophies. I found it still visible in the male Torpedos, though much smaller than in the females near the close of intra-uterine life.
Whether or not these theoretical considerations as to the nature of the Wolffian body and Oviduct are correct, I believe that the facts I have brought to light in reference to the development of these parts in the Dog-fish will be found of service to every one who is anxious to discover the true relations of these parts.
Before leaving the subject I will say one or[TN2] two words
about the development of the Ovary. In both sexes the germinal epithelium
(fig. 13) becomes thickened below the Oviduct, and in both sexes a knob (in
section but really a ridge) comes to project into the pleuro-peritoneal
cavity on each side of the mesentery (fig. 13, pov). In both sexes, but especially the females, the
epithelium on the upper surface of this ridge becomes very much thickened,
whilst subsequently it elsewhere atrophies. In the females, however, the
thickened epithelium on the knob grows more and more conspicuous, and
develops a number of especially large cells with large nuclei, precisely
similar to Waldeyer's (loc. cit.) primitive ova
of
the Bird. In the male the epithelium on the ridge, though containing
primitive ova, is not as conspicuous as in the female. Though I have not
worked out the matter further than this at present, I still have no doubt
that these projecting ridges become the Ovaries.
The study of the development of the parts of the head, on account of the crowding of organs which occurs there, always presents greater difficulties to the investigator than that of the remainder of the body. My observations upon it are correspondingly incomplete. I have, however, made out a few points connected with it in reference to some less well-known organs, which I have thought it worth while calling attention to in this preliminary account.
The continuation of the Pleuro-peritoneal Cavity into the Head.
In the earlier part of this paper (p. 86) I called attention to the extension of the separation between somatopleure and splanchnopleure into the head, forming a space continuous with the pleuro-peritoneal cavity (Pl. 3, fig. 8a, pp); this becomes more marked in the next stage, and, indeed, the pleuro-peritoneal cavity is present for a considerable time in the head before it becomes visible elsewhere. At the time of the appearance of the second visceral cleft it has become for the most part atrophied, but there persist two separated portions of it in front of the first cleft, and also remnants of it less well marked between and behind the two clefts. The visceral clefts necessarily divide it into separate parts.
The two portions in front of the first visceral cleft remain very conspicuous till the appearance of the external gills, and above the hinder one of the two the fifth nerve bifurcates.
These two are shewn as they appear in a surface view in fig. 14, pp. They are in reality somewhat flattened spaces, lined by a mesoblastic epithelium; the epithelium on the inner surface of the space corresponding to the splanchnopleure, and that on the outer to the somatopleure.
I have not followed the history of these later than the time of the appearance of the external gills.
The presence of the pleuro-peritoneal cavity in the head is interesting, as shewing the fundamental similarity between the head and the remainder of the body.
All my sections seem to prove that it is a portion of the epiblastic involution to form the mouth which is pinched off to form the pituitary body, and not a portion of the hypoblast of the throat. Since Götte (Archiv. für Micr. Anat. Bd. IX.) has also found that the same is the case with the Batrachians and Mammalia, I have little doubt it will be found to be universally the case amongst vertebrates.
Probably the observations which lead to the supposition that it was the throat which was pinched off to form the pituitary body were made after the opening between the mouth and throat was completed, when it would naturally be impossible to tell whether the pinching off was from the epiblast of the mouth involution or the hypoblast of the throat.
The Cranial Nerves.
The cranial nerves in their early condition are so clearly visible that I have thought it worth while giving a figure of them, and calling attention to some points about their embryonic peculiarities.
From my figure (14) it will be seen that there is behind the auditory vesicle a nervous tract, from which four nerves descend, and that each of these nerves is distributed to the front portion of a visceral arch. When the next and last arch (in this species) is developed, a branch from this nervous mass will also pass down to it. That each of these is of an equal morphological value can hardly be doubted.
The nerve to the third arch becomes the glosso-pharyngeal (fig. 14, gl), the nerves to the other arches become the branchial branches of the vagus nerve (fig. 14, vg). Thus the study of their development strongly supports Gegenbaur's view of the nature of the vagus and glosso-pharyngeal, viz. that the vagus is a compound nerve, each component part of it which goes to an arch being equivalent to one nerve, such as the glosso-pharyngeal.
Of the nerves in front of the auditory sac the posterior is the seventh nerve (fig. 14, VII). Its mode of distribution to [Pg 107] the second arch leaves hardly a doubt that it is equivalent to one such nerve as those distributed to the posterior arches. Subsequently it acquires another branch, passing forwards towards the arch in front.
The most anterior nerve is the fifth (fig. 14, V), of which two branches are at this stage developed. The natural interpretation of its present condition is, that it is equivalent to two nerves, but the absence of relation in its branches to any visceral clefts renders it more difficult to determine the morphology of the fifth nerve than of the other nerves. The front branch of the two is the ophthalmic branch of the adult, and the hind branch the inferior maxillary branch. The latter branch subsequently gives off low down, i.e. near its distal extremity, another branch, the superior maxillary branch.
In its embryonic condition this latter branch does not appear like a third branch of the fifth, equivalent to the seventh or the glosso-pharyngeal nerves, but rather resembles the branch of the seventh nerve which passes to the arch in front, which also is present in all the other cranial nerves.
Modes of Preparation.
Before concluding I will say one or two words as to my modes of preparation.
I have used picric and chromic acids, both applied in the usual way; but for the early stages I have found osmic acid by far the most useful reagent. I placed the object to be hardened, in osmic acid (half per cent.) for two hours and a half, and then for twenty four in absolute alcohol.
I then embedded and cut sections of it in the usual way, without staining further.
I found it advantageous to cut sections of these embryos immediately after hardening, since if kept for long in the absolute alcohol the osmic acid specimens are apt to become brittle.
[Pg 108] LIST OF WORKS REFERRED TO.
Gegenbaur. Anat. der Wirbelthiere, III Heft, Leipzig, 1873.
A. Götte. Archiv. für Micr. Anat., Vol.
X. 1873. Der Keim der
Forelleneies,
Archiv. Für. Micr. Anat., Vol. IX. 1873. Untersuchung über die
Entwicklung der Bombinator igneus,
Archiv. für Micr.
Anat., Vol. V. 1869. Kurze Mittheilungen aus
der Entwicklungsgeschichte der Unke,
Archiv. für Micr.
Anat., Vol. IX. 1873.
Kupffer. Archiv. für Micr. Anat., Vol. II. 1866, p. 473. Ibid. Vol. IV. 1868, p. 209.
Kowalevsky. Entwicklungsgeschichte der Holothurien,
Mémoires de l'Acad. Impér. des Sciences de St
Petersbourg, vii ser. Vol. XI. 1867.
Kowalevsky,
Owsjannikow, und Wagner. Entwicklung der Störe,
Bulletin der K. Acad. St Petersbourg, Vol. XIV. 1873.
Kowalevsky. Embryologische Studien an Würmern und Arthropoden,
Mémoirs de l'Acad.
Impér. des Sciences de St
Petersbourg, Vol. XIV. 1871.
E. Ray Lankester. Annals and Mag. of Nat. History, Vol. XI. 1873, p. 81.
W. Müller. Ueber die Persistenz der Urniere bei Myxine Glutinosa,
Jenaische Zeitschrift, Vol. VII. 1873.
Oellacher. Zeitschrift für Wiss. Zoologie, Vol. XXIII. 1873.
Owsjannikow. Entwicklung der Coregonus,
Bul. der K. Akad. St
Petersbourg, Vol. XIX.
Romiti. Archiv. für Micr. Anat., Vol. IX. 1873.
Waldeyer. Eierstock u. Eie.
EXPLANATION OF PLATES 3 AND 4.
COMPLETE LIST OF REFERENCE LETTERS.
al. Alimentary canal. ao. Dorsal aorta. auv. Auditory vesicle. bd. Formative cell probably derived from the yolk. cav. Cardinal vein. ch. Notochord. ch´. Thickening of hypoblast to form the notochord. Eb. Line indicating the edge of the blastoderm. ep. Epiblast. ep´. Epidermis. er. Embryonic rim. es. Embryonic swelling. gl. Glosso-pharyngeal nerve. h. Head. ht. Heart. hy. Hypoblast. ll. Lower layer cells. ly. Line of separation between the blastoderm and the yolk. m. Mesoblast. mc. Medullary canal. mg. Medullary groove. mp. Muscle-plate. mp´. Early formed mass of muscles. n. Peculiar nuclei formed in the yolk. n´. Similar nuclei in the cells of the blastoderm. na. Cells which help to close in the alimentary canal, and which are derived from the yolk. ny. Network of lines present in the food-yolk. ol. Olfactory pit. op. Eye. ov. Oviduct. pn. Pineal gland. pov. Projection which becomes the ovary. pp. Pleuro-peritoneal cavity. pp´. Remains of pleuro-peritoneal cavity in the head. prv. Protovertebræ. pwd. Primary points of involution from the pleuro-peritoneal cavity by the coalescence of which the Wolffian duct is formed. sg. Segmentation cavity. so. Somatopleure. sos. Stalk connecting embryo with yolk-sac. sp. Splanchnopleure. spn. [Pg 109] Spinal nerve. sur. Suprarenal body. ts. Caudal lobes. v. Blood-vessel. vg. Vagus nerve. V. Fifth nerve. VII. Seventh nerve. vc, 1, 2, 3, &c. 1st, 2nd and 3rd &c. visceral clefts. vp. Vertebral plates. wd. Wolffian duct. x. Peculiar body underlying the notochord derived from the hypoblast. yk. Yolk spherules.
All the figures were drawn with the Camera Lucida.
Plate 3.
Fig. 1. Section parallel with the long axis of the embryo through a blastoderm, in which the floor of the segmentation cavity (sg) is not yet completely lined by cells. The roof of the segmentation cavity is broken. (Magnified 60 diam.) The section is intended chiefly to illustrate the distribution of nuclei (n) in the yolk under the blastoderm. One of the chief points to be noticed in their distribution is the fact that they form almost a complete layer under the floor of the segmentation cavity. This probably indicates that the cells whose nuclei they become take some share in forming the layer of cells which subsequently (vide fig. 4) forms the floor of the cavity.
Fig. 2. Small portion of blastoderm and subjacent yolk of an embryo at the time of the first appearance of the medullary groove. (Magnified 300 diam.)
The specimen is taken from a portion of the blastoderm which will form part of the embryo. It shews two large nuclei of the yolk (n) and the network in the yolk between them; this network is seen to be closer around the nuclei than in the intervening space. The specimen further shews that there are no areas representing cells around the nuclei.
Fig. 3. Section parallel with the long axis of the embryo through a blastoderm, in which the floor of the segmentation cavity is not yet covered by a complete layer of cells. (Magnified 60 diam.)
It illustrates (1) the characters of the epiblast, (2) the embryonic swelling (es), (3) the segmentation cavity (sg). It should have been drawn upon the same scale as fig. 4; the line above it represents its true length upon this scale.
Fig. 4. Longitudinal section through a blastoderm at the time of the first appearance of the embryonic rim, and before the formation of the medullary groove. (Magnified 45 diam.)
It illustrates (1) the embryonic rim, (2) the continuity of epiblast and hypoblast at edge of this, (3) the continual differentiation of the lower layer cells, to form, on the one hand, the hypoblast, which is continuous with the epiblast, and on the other the mesoblast, between this and the epiblast; (4) the segmentation cavity, whose floor of cells is now completed.
N.B. The cells at the embryonic end of the blastoderm have been made rather too large.
Fig. 5. Surface view of the blastoderm shortly after the appearance of the medullary groove. To shew the relation of the embryo to the blastoderm.
Fig. 6a and b. Two transverse sections of the same embryo, shortly after the appearance of the medullary groove. (Magnified 96 diam.)
a. In the region of the groove. It shews (1) the two masses of mesoblast on each side, and the deficiency of the mesoblast underneath the medullary groove; (2) the commencement of the closing in of the alimentary canal below, chiefly from cells (na) derived from the yolk.
b. Section in the region of the head where the medullary groove is deficient, other points as above.
[Pg 110] Fig. 7a and b. Two transverse sections of an embryo about the age or rather younger than that represented in fig. 5. (Magnified 96 diam.)
a. Section nearer the tail; it shews the thickening of the hypoblast to form the notochord (ch´).
In b the thickening has become completely separated from the hypoblast as the notochord. In a the epiblast and hypoblast are continuous at the edge of the section, owing to the section passing through the embryonic rim.
Fig. 8. Surface view of a spatula-shaped embryo. The figure shews (1) the flattened head (h) where the medullary groove is deficient, (2) the caudal lobes, with a groove between them; it also shews that at this point, the medullary groove has become roofed over and converted into a canal.
Fig. 8a. Transverse section of fig. 8, passing through the line a. (Magnified 90 diam.) The section shews (1) the absence of the medullary groove in the head and the medullary folds turning down at this time instead of upwards; (2) the presence of the pleuro-peritoneal cavity in the head (pp); (3) the completely closed alimentary canal (al).
Fig. 8b. Transverse section of fig. 8, through the line b. (Magnified 90 diam.) It shews (1) the neural canal completely formed; (2) the vertebral plates of mesoblast not yet split up into somatopleure and splanchnopleure.
Fig. 9. Side view of an embryo of the Torpedo, seen as a transparent object a little older than the embryo represented in fig. 8. (Magnified 20 diam.) The internal anatomy has hardly altered, with the exception of the medullary folds having closed over above the head and the whole embryo having become more folded off from the germ.
The two caudal lobes, and the very marked groove between them, are seen at ts. The front end of the notochord became indistinct, and I could not see its exact termination. The epithelium of the alimentary canal (al) is seen closely underlying the notochord and becoming continuous with the epiblast at the hind end of the notochord.
The first visceral cleft (1vc) and eye (op) are just commencing to be formed, and the cranial flexure has just appeared.
Fig. 10. Section through the dorsal region of an embryo somewhat older than the one represented in fig. 9. (Magnified 96 diam.)
It shews (1) the formation by a pinching off from the top of the alimentary canal of a peculiar body which underlies the notochord (x); (2) the primitive extension of the pleuro-peritoneal cavity up to the top of the vertebral plates.
Plate 4.
Fig. 11a, b, and c. Three sections closely following each other from an embryo in which three visceral clefts are present; a is the most anterior of the three. (Magnified 96 diam.) In all of these the muscle-plates are shewn at mp. They have become separated from the lateral plates in b and c, but are still continuous with them in a. The early formed mass of muscles is also shewn in all the figures (mp´).
The figures further shew (1) the formation of the spinal nerves (spn) as small bodies of cells closely applied to the upper and outer edge of the neural canal.
(2) The commencing formation of the cells which form the axial skeleton from the inner (splanchnopleuric) layer of the muscle-plate. Sections b and c are given more especially to shew the mode of formation of the oviduct (ov).
[Pg 111] In b it is seen as a solid knob (ov), arising from the point where the somatopleure and splanchnopleure[TN3] unite, and in c (the section behind b) as a solid rod (ov) closely applied to the epiblast, which has grown backwards from the knob seen in b.
N.B. In all three sections only one side is completed.
Fig. 12a and b. Two transverse sections of an embryo just before the appearance of the external gills. (Magnified 96 diam.)
In a there is seen to be an involution on each side (pwd), while b is a section from the space between two involutions from the pleuro-peritoneal cavity, so that the Wolffian duct (at first solid) (wd) is not connected as in a with the pleuro-peritoneal cavity. The further points shewn in the sections are—
(1) The commencing formation of the spiral valve (al).
(2) The suprarenal body (sur).
(3) The oviduct (ov), which has acquired a lumen.
(4) The increase in length of the muscle-plates, the spinal nerves, &c.
Fig. 13. Section through the dorsal region of an embryo in which the external gills are of considerable length. (Magnified 40 diam.) The chief points to be noticed:
(1) The formation of the Wolffian body by outgrowths from the Wolffian duct (wd).
(2) One of the still continuing connections (primitive involutions) between the Wolffian duct and the pleuro-peritoneal cavity (pwd).
(3) The oviduct largely increased in size (ov).
N.B. On the left side the oviduct has been accidentally made too small.
(4) The growth downwards of the muscle-plate to form the muscles of the abdomen.
(5) The formation of an outgrowth on each side of the mesentery (pov), which will become the ovary.
(6) The spiral valve (al).
Fig. 14. Transparent view of the head of an embryo shortly before the appearance of the external gills. (Magnified 20 diam.) The chief points to be noticed are—
(1) The relation of the cranial nerves to the visceral clefts and the manner in which the glosso-pharyngeal (gl) and vagus (vg) are united.
(2) The remnants of the pleuro-peritoneal cavity in the head (pp).
(3) The eye (op). The stalk, as well as the bulb of the eye, are supposed to be in focus, so that the whole eye has a somewhat peculiar appearance.
[10] From the Quarterly Journal of Microscopical Science, Vol. XIV. 1874. Read in Section D, at the Meeting of the British Association at Belfast.
[11] The interpretation of this network is entirely due to Dr Kleinenberg, who suggested it to me on my shewing him a number of specimens exhibiting the nuclei and network.
[12]
Kowalevsky (Beiträge zur Entwicklungsgeschichte der
Holothurien,
Mémoirs de l'Ac. Imp. de St
Petersbourg, vii ser., Vol. XI. 1867) describes the division of nuclei during
segmentation in the Holothurians, and other observers have described it
elsewhere.
[13]
Götte, at the end of a paper on The Development of the Layers in the
Chick
(Archiv. für Micr. Anat., Vol. X. 1873,
p. 196), mentions that the so-called cells in Osseous fishes which
Oellacher states to have migrated into the yolk, and which are clearly the
same as those mentioned by Owsjannikow, are really not cells, but
large nuclei. If this statement is correct the phenomena in
Osseous fishes are precisely the same as those I have described in the
Dog-fish.
[14]
This has been already made out by Kowalevsky, Würmern u.
Arthropoden,
loc. cit.
[15]
This groove is the only structure which it seems possible to compare with
the so-called primitive groove
of Birds. It is, however, doubtful
whether they are really homologous.
[16] For the specimens of this age I am indebted to Professor Huxley.
[17] If Romiti's observations (Archives für Mikr. Anatom. Vol. IX. p. 200) are correct, then the ordinary view of the Wolffian duct arising in Birds as a solid rod at the outer corner of the protovertebræ will have to be abandoned.
[18]
While correcting the proofs of this paper I have come across a memoir of
W. Müller (Ueber die Persistenz der Urniere bei Myxine
Glutinosa,
Jenaische Zeitschrift, Vol. VII. 1873), in
which he mentions that in Myxine the upper end of the Wolffian duct
communicates by numerous openings with the pleuro-peritoneal cavity; this
gives to the suggestion in the text a foundation of fact.
With Plate 5.
If the genealogical relationships of animals are to be mainly or largely determined on embryological evidence, it becomes a matter of great importance to know how far evidence of this kind is trustworthy.
The dependence to be placed on it has been generally assumed to be nearly complete. Yet there appears to be no à priori reason why natural selection should not act during the embryonic as well as the adult period of life; and there is no question that during their embryonic existence animals are more susceptible to external forces than after they have become full grown: indeed, an immense mass of evidence could be brought to shew that these forces do act upon embryos, and produce in them great alterations tending to obscure the genealogical inferences to be gathered from their developmental histories. Even the time-honoured layers form to this no exception. In Elasmobranchii, for instance, we find the notochord derived from the hypoblast and the spinal ganglia derived from the involuted epiblast of the neural canal, whilst in the higher vertebrates both of these organs are formed in the mesoblast. Such instances are leading embryologists to recognise the fact that the so-called layers are not quite constant and must not be absolutely depended upon in the determination of homologies. But though it is necessary to recognise the fact that great changes do occur in animals during their embryonic life, it is not necessary to conclude that all embryological evidence is thereby vitiated; but rather it becomes incumbent on us to attempt to determine which embryological features are ancestral and which secondary. For this purpose it is requisite [Pg 113] to ascertain what are the general characters of secondary features and how they are produced. Many vertebrates have in the first stages of their development a number of secondary characters which are due to the presence of food material in the ovum; the present essay is mainly an attempt to indicate how those secondary characters arose and to trace their gradual development. At the same time certain important ancestral characters of the early phases of the development of vertebrates, especially with reference to the formation of the hypoblast and mesoblast, are pointed out and their meaning discussed.
There are three orders of vertebrates of which no mention has been made, viz., the Mammals, the Osseous fishes, and the Reptiles. The first of these have been passed over because the accounts of their development are not sufficiently satisfactory, though as far as can be gathered from Bischoff's account of the dog and rabbit there would be no difficulty in shewing their relations with other vertebrates.
We also require further investigations on Osseous fishes, but it seems probable that they develop in nearly the same manner as the Elasmobranchii.
With reference to Reptiles we have no satisfactory investigations.
* * * * *
Amphioxus is the vertebrate whose mode of development in its earliest stages is simplest, and the modes of development of other vertebrates are to be looked upon as modifications of this due to the presence of food material in their ova. It is not necessary to conclude from this that Amphioxus was the ancestor of our present vertebrates, but merely that the earliest stages of development of this vertebrate ancestor were similar to those of Amphioxus.
The ovum of Amphioxus contains very little food material and its segmentation is quite uniform. The result of segmentation is a vesicle whose wall is formed of a single layer of cells. These are all of the same character, and the cavity of the vesicle called the segmentation cavity is of considerable size. A section of the embryo, as we may now call the ovum, is represented in Plate 5, fig. A I.
[Pg 114] The first change which occurs is the pushing in of one half of the wall of the vesicle towards the opposite half. At the same time by the narrowing of its mouth the hollow hemisphere so formed becomes again a vesicle[20].
Owing to its mode of formation the wall of this secondary vesicle is composed of two layers which are only separated by a narrow space, the remnant of the segmentation cavity.
Two of the stages in the formation of the secondary vesicle by this process of involution are shewn in Plate X, fig. A II , and A III. In the second of these the general growth has been very considerable, rendering the whole animal much larger than before. The cavity of this vesicle, A III , is that of the commencing alimentary canal whose final form is due to changes of shape undergone by this primitive cavity. The inner wall of the vesicle becomes converted into the wall of the alimentary canal or hypoblast, and also into part or the whole of the mesoblast.
During the involution the cells which are being involuted undergo a change of form, and before the completion of the process have acquired a completely different character to the cells forming the external wall of the secondary vesicle or epiblast. This change of character in the cells is already well marked in fig. A II. It is of great importance, since we shall find that some of the departures from this simple mode of development, which characterise other vertebrates, are in part due to the distinction between the hypoblast and epiblast cells appearing during segmentation, and not subsequently as in Amphioxus during the involution of the hypoblast.
Kowalevsky (Entwicklungsgeschichte des Amphioxus) originally believed that the narrow mouth of the vesicle (according to Mr Lankester's terminology blastopore) became the anus of the adult. He has since, and certainly correctly, given up this view. The opening of the involution becomes closed up and the adult anus is no doubt formed as in all other vertebrates by a pushing in from the exterior, though it probably corresponds in position very closely with the point of closing up of the original involution.
[Pg 115] The mode of formation of the mesoblast is not certainly known in Amphioxus; we shall find, however, that for all other vertebrates it arises from the cells which are homologous with the involuted cells of this animal.
Since food material is a term which will be very often employed, it will be well to explain exactly the sense in which it will be used. It will be used only with reference to those passive highly refractive particles which are found embedded in most ova.
In some eggs, of which the hen's egg may be taken as a familiar example, the yolk-spherules or food material form the larger portion of the ovum, and a distinction is frequently made between the germinal disc and the yolk.
This distinction is, however, apt to lead to a misconception of the true nature of the egg. There are strong grounds for believing that the so-called yolk, equally with the germinal disc, is composed of an active protoplasmic basis endowed with the power of growth, in which passive yolk-spherules are embedded; but that the part ordinarily called the yolk contains such a preponderating amount of yolk-spherules that the active basis escapes detection, and does not exhibit the same power of growth as the germinal disc.
With the exception of mammals, whose development requires to be more completely investigated, Amphioxus is as far as we know the only vertebrate whose ovum does not contain a large amount of food material.
In none of these (vertebrate) yolk-containing ova is the food material distributed uniformly. It is always concentrated much more at one pole than at the other, and the pole at which it is most concentrated may be conveniently called the lower pole of the egg.
In eggs in which the distribution of food material is not uniform segmentation does not take place with equal rapidity through all parts of the egg, but its rapidity is, roughly speaking, inversely proportional to the quantity of food material.
When the quantity of food material in a part of the egg becomes very great, segmentation does not occur at all; and even in those cases where the quantity of food yolk is not too great to prevent segmentation the resulting segmentation [Pg 116] spheres are much larger than where the yolk-granules are more sparsely scattered.
The Frog is the vertebrate whose development comes nearest to that of Amphioxus, as far as the points we are at present considering are concerned. But it will perhaps facilitate the understanding of their relations shortly to explain the diagrammatic sections which I have given of an animal supposed to be intermediate in its development between the Frog and Amphioxus. Plate 5, fig. B I, represents a longitudinal section of this hypothetical egg at the close of segmentation. The lower pole, coloured yellow, represents the part containing more yolk material, and the upper pole, coloured blue, that with less yolk. Owing to the presence of this yolk the lower pole even at the close of segmentation is composed of cells of a different character to those of the upper pole. In this respect this egg can already be distinguished from that of Amphioxus, in which no such difference between the two poles is apparent at the corresponding period (Plate 5, fig. A I).
The segmentation cavity in this ovum is not quite so large proportionately as in Amphioxus, and the encroachment upon it is due to the larger bulk of the lower pole of the egg. In fig. B II the involution of the lower pole has already commenced; this involution is (1) not quite symmetrical, and (2) on the ventral side (the left side) the epiblast cells forming the upper part of the egg are growing round the cells of the lower pole of the egg or lower layer cells. Both of these peculiarities are founded upon what happens in the Frog and the Selachian, but it is to be noticed that the change from the lower layer cells being involuted towards the epiblast cells, to the epiblast cells growing round the lower layer cells, is a necessary consequence of the increased bulk of the latter.
In this involution not only are the cells of the lower pole pushed on, but also some of those of the upper or yellow portion; so that in this as in all other cases the true distinction between the epiblast and hypoblast does not appear till the involution to form the latter is completed. In the next stage, B III, the involution has become nearly completed and the opening to the exterior or blastopore quite constricted.
The segmentation cavity has been entirely obliterated, as [Pg 117] would have been found to be the case with Amphioxus had the stage a little older than that on Plate 5, A III, been represented. The cavity marked (al), as was the case with Amphioxus, is that of the alimentary canal.
The similarities between the mode of formation of the hypoblast and alimentary canal in this animal and in Amphioxus are so striking and the differences between the two cases so slight that no further elucidation is required. One or two points need to be spoken of in order to illustrate what occurs in the Frog. When the involution to form the alimentary canal occurs, certain of the lower layer cells (marked hy) become distinguished from the remainder of the lower layer cells as a separate layer and form the hypoblast which lines the alimentary canal. It is to be noticed that the cells which form the ventral epithelium of the alimentary canal are not so soon to be distinguished from the other lower layer cells as those which form its dorsal epithelium. This is probably a consequence of the more active growth, indicated by the asymmetry of the involution, on the dorsal side, and is a fact with important bearings in the ova with more food material. The cells marked m and coloured red also become distinguished as a separate layer from the remainder of the hypoblast and form the mesoblast. The remainder of the lower layer cells form a mass equivalent to the yolk-sac of many vertebrates, and are not converted directly into the tissues of the animal.
Another point to be noticed is the different relation of epiblast cells to the hypoblast cells at the upper and lower side of the mouth of the involution. Above it, on its dorsal side, the epiblast and hypoblast are continuous with one another. On its ventral side they are primitively not so continuous. This is due to the epiblast, as was before mentioned, growing round the lower layer cells on the ventral side, vide B II, and merely remaining continuous with them on the dorsal. The importance of these two points will appear when we come to speak of other vertebrates.
The next animal whose development it is necessary to speak of is the Frog, and its differences from the mode of development are quite easy to follow and interpret. Segmentation is again not uniform, and results in the formation of an upper layer of [Pg 118] smaller cells and a lower one of larger; in the centre is a segmentation cavity. The stage at the close of segmentation is represented in C I. From the diagram it is apparent that the lower layer cells occupy a larger bulk than they did in the previous animal (Plate 5, B I), and tend to encroach still more upon the segmentation cavity, otherwise the differences between the two are unimportant. There are, however, two points to be noted. In the first place, although the cells of the upper pole are distinguished in the diagrams from the lower by their colour, it is not possible at this stage to say what will become epiblast and what hypoblast. In the second place the cells of the upper pole or epiblast consist of two layers—an outer called the epidermic layer and an inner called the nervous. In the previous cases the epiblast consisted of a single layer of cells. The presence of these two layers is due to a distinction which, arising in most other vertebrates late, in the Frog arises early. In most other vertebrates in the later stages of development the epiblast consists of an outer layer of passive and an inner of active cells. In the Frog and other Batrachians these two layers become distinguished at the commencement of development.
In the next stage (C II) we find that the involution to form the alimentary canal has commenced (al), but that it is of a very different character to the involution in the previous case. It consists in the growing inwards of a number of cells from the point x (C I) towards the segmentation cavity. The cells which grow in this way are partly the blue cells and partly the smaller yellow ones. At first this involuted layer of cells is only separated by a slit from the remainder of the lower layer cells; but by the stage represented in C II this has widened into an elongated cavity (al). In its formation this involution pushes backwards the segmentation cavity, which finally disappears in the stage C III. The point x remains practically stationary, but by the general growth of the epiblast, mesoblast and hypoblast, becomes further removed from the segmentation cavity in C II than in C I. On the opposite side of the embryo to that at which the involution occurs the epiblast cells as before, grow round the lower layer cells. The commencement of this is already apparent in C I, and in C II the process is nearly completed, [Pg 119] though there is still a small mass of yolk filling up the blastopore. The features of this involution are in the main exaggerations of what was supposed to occur in the previous animal. The asymmetry of the involution is so great that it is completely one-sided and results, in the first instance, in a mere slit; and the whole process of enclosing the yolk by epiblast is effected by the epiblast cells on the side of the egg opposite to the involution.
The true mesoblast and hypoblast are formed precisely as in the previous case. The involuted cells become separated into two layers, one forming the dorsal epithelium of the alimentary canal, and a layer between this and the epiblast forming the mesoblast. There is also a layer of mesoblast accompanying the epiblast which encloses the yolk, which is derived from the smaller yellow cells at y (C I). The edge of this mesoblast, m´, forms a thickened ridge, a feature which persists in other vertebrates.
It is a point of some importance for understanding the relation between the mode of formation of the alimentary canal in the Frog and other vertebrates to notice that on the ventral surface the cells which are to form the epithelium of the alimentary canal become distinguished as such very much later than do those to form its dorsal epithelium, and are derived not from the involuted cells but from the primitive large yolk-cells. It is indeed probable that only a very small portion of epithelium of the ventral wall of the mid-gut is in the end derived from these larger yolk-cells. The remainder of the yolk-cells (C III, and C II, yk) form the yolk mass and do not become directly formed into the tissues of the animal.
In the last stage I have represented for the frog, C III , there are several features to be noticed.
The direct connection at their hind-ends between the cavities of the neural and alimentary canals is the most important of these. This is a result of the previous continuity of the epiblast and hypoblast at the point x, and is a feature almost certainly found in Amphioxus, but which I will speak of more fully in my account of the Selachian's development. The opening of the blastopore called the anus of Rusconi is now quite narrowed, it does not become the anus of the adult. It may be noticed that at the front end of the embryo the primitive dorsal [Pg 120] epithelium of the alimentary canal is growing in such a way as to form the epithelium both of the dorsal and ventral surfaces of the fore-gut.
In spite of various features rendering the development of the Frog more difficult of comprehension than that of most other vertebrates, it is easy to see that the step between it and Amphioxus is not a very great one, and will very likely be bridged over at some future time, when our knowledge of the development of other forms becomes greater.
From the Frog to the Selachian is a considerable step, but I have again hypothetically sketched a type intermediate between them whose development agrees in some important points with that of Pelobates fuscus as described by Bambeke. The points of agreement, though not obvious at first sight, I shall point out in the course of my description.
The first stage (D I), at the close of segmentation, deserves careful attention. The segmentation cavity by the increase of the food yolk is very much diminished in size, and, what is still more important, has as it were sunk down so as to be completely within the lower layer cells. The roof of the segmentation cavity is thus formed of epiblast and lower layer cells, a feature which Bambeke finds in Pelobates fuscus and which is certainly found in the Selachians. In the Frog we found that the segmentation cavity began to be encroached on by the lower layer cells, and from this it is only a small step to find these cells creeping still further up and forming the roof of the cavity. In the lower layer cells themselves we find an important new feature, viz. that during segmentation they become divided in two distinct parts—one of these where the segments owing to the presence of much food yolk are very large, and the other where the segments are much smaller.
The separation between these two is rather sharp. Even this separation was foreshadowed in the Frog's egg, in which a number of lower layer cells were much smaller and more active at the two sides of the segmentation cavity than elsewhere. The segmentation cavity at first lies completely within the region of the small spheres. The larger cells serve almost entirely as food yolk. The epiblast, as is normal with vertebrates, consists of a single layer of columnar cells.
[Pg 121] In the next stage (D II) the formation of the alimentary canal (al) has commenced, but it is to be observed that there is in this case no true involution.
As an accompaniment to the encroachment upon the segmentation cavity, which was a feature of the last stage, the cells to form the walls of the alimentary canal have come to occupy their final position during segmentation and without the intermediation of an involution, and traces only of the involution, are to be found in (1) a split in the lower layer cells which passes along the line separating the small and the large lower layer cells; and (2) in the epiblast becoming continuous with the hypoblast on the dorsal side of the mouth of this split. It is even possible that at this point a few cells (though certainly only a very small number) of those marked blue in D I become involuted. This point in this, as in all other cases, is the tail end of the embryo. The other features of this stage are as follows:—(1) The segmentation cavity has become smaller and less conspicuous than it was. (2) The epiblast cells have begun to grow round the yolk even in a more conspicuous manner than they did in the Frog, and are accompanied by a layer of mesoblast cells which again becomes thickened at its edge. The mesoblast cells in the region of the body are formed in the same way as before, viz. by the separation of a layer to form the epithelium of the alimentary canal, the other cells remaining as mesoblast; and as in the Frog, or in a more conspicuous manner, we find that the dorsal surface only of the alimentary cavity has a wall formed of a distinct layer of cells, but on the ventral side the cavity is at first closed in by the large spheres of the yolk only. The formation of the alimentary canal by a split and not by an involution is exactly what Bambeke finds in Pelobates.
The next stage, D III, is about an equivalent age to C III in the Frog. It exhibits the same connection between the neural and the alimentary canals as was found there.
The alimentary canal is beginning to become closed in below, and this occurs near the two ends earlier than in the middle. The cells to form the ventral wall are derived from the large yolk-cells. The non-formation of the ventral wall of the alimentary canal so soon in the middle as at the ends is an [Pg 122] early trace of the umbilical canal found in Birds and Selachians, by which the alimentary tract is placed in communication with the yolk-sac. The segmentation cavity has by this stage completely vanished, and the epiblast with its accompanying mesoblast has spread completely round the yolk material so as to form the ventral wall of the body.
Though in some points this manner of development may seem to differ from that of the Frog, there is really a fundamental agreement between the two, and between this mode of development and that of the Selachians we shall find the agreement to be very close.
After segmentation we find that the egg of a Selachian consists of two parts—one of these called the germinal disc or blastoderm, and the other the yolk. The former of these corresponds with the epiblast and the part of the lower pole composed of smaller segments in the last-described egg, and the latter to the larger segments of the lower pole. This latter division, owing to the quantity of yolk which it contains, has not undergone segmentation, but its homology with the larger segments of the previous eggs is proved (1) by its containing a number of nuclei (E I, n), which become the nuclei of true cells and enter the blastoderm, and (2) by the presence in it of a number of lines forming a network similar to that of many cells. The segmentation cavity, as before, lies completely within the lower layer cells.
The next stage, E II, is almost precisely similar to the second stage of the last egg. As there, the primitive involution is merely represented by a split separating the yolk and the germinal disc, and on the dorsal side alone is there a true cellular wall for this split, and at the dorsal mouth of the split the alimentary epithelium becomes continuous with the epiblast.
The segmentation cavity has become diminished, and round the yolk the epiblast, accompanied by a layer of mesoblast, is commencing to grow. In this growth all parts of the blastoderm take a share except that part where the epiblast and hypoblast are continuous. This manner of growth is precisely what occurs in the Frog, though there it is not so easily made out; and not all the investigators who have studied the Frog have understood the exact meaning of the appearances they have [Pg 123] seen and drawn. This similarity of relation of the epiblast to the yolk in the two cases is a further confirmation of the identity of the Selachian's yolk with the large yolk-spheres of the previous eggs.
The next stage, E III, is in many ways identical with the corresponding stage in the last-described egg, and in the same way as in that case the neural and alimentary canals are placed in communication with each other.
The mode in which this occurs will be easily gathered from a comparison of E II and E III. It is the same for the Selachians and Batrachians. The neural canal (nc) is by the stage figured E III , completely formed in the way so well known in the Bird, and between the roof of the canal and the external epiblast a layer of mesoblast has already grown in. The floor of the neural canal is the same layer marked ep in E II , and therefore remains continuous with the hypoblast at x; and when by a simultaneous process the roof of the neural canal and the ventral wall of the alimentary become formed by the folding over of one continuous layer (the epiblast and hypoblast continuous at the point x), the two canals, viz. the neural and alimentary, are necessarily placed in communication at their hind-ends, as is seen in the diagram.
There are several important points of difference between E III and D III. In the first place, owing to the larger size of the yolk mass in E III , the epiblast, accompanied by mesoblast, has not proceeded nearly so far round it as in the previous case. It is also worth notice that at the right as well as at the left end of the germinal disc the epiblast is commencing to grow round the yolk. The yolk has, however, become surrounded to a much smaller extent on the right hand than on the left. Since, in the earlier stage, the epiblast became continuous with the hypoblast at x, it is not from sections obvious how this occurs. I have therefore appended a diagram to explain it (E´). The blastoderm rests like a disc on the yolk and grows over it on all sides, except at the point where the epiblast and hypoblast are continuous (x). This point becomes as it were left in a bay. Next the two sides of the bay coalesce, the bay becomes obliterated, and the effect produced is exactly as if the blastoderm had grown round the yolk at the point x (corresponding with the [Pg 124] tail of the embryo) as well as everywhere else. It thus comes about that the final point where the various parts of the blastoderm meet and completely enclose the yolk mass does not correspond with the anus of Rusconi of the Frog, but is at some little distance from the hind-end of the embryo. In other words, the position of the blastopore in the Selachian is not the same as in the Frog.
Another point deserving attention is the formation of the ventral wall of the alimentary canal. This takes place in two ways—partly by a folding-in at the sides and end, and partly from cells formed around the nuclei (n) in the yolk. From these a large portion of the ventral wall of the mid-gut is formed.
The folding-in of the sheet of hypoblast to assist in the closing-in of the ventral wall of the alimentary canal is a consequence of the flattened form of the original alimentary slit which is far too wide to form the cavity of the final canal. In the Bird whose development must next be considered this folding-in is a still more prominent feature in the formation of the alimentary canal. As in the last case, the alimentary canal is widely open in the middle to the yolk at the time when its two ends are closed below and shut off from it; still later this opening becomes very narrow and forms the duct of the so-called umbilical cord which places the yolk-sac in communication with the alimentary canal. As the young animal becomes larger the yolk-sac ceases to communicate directly with the alimentary canal, and is carried about by it for some time as an appendage and only at a later period shrivels up.
The mesoblast is formed in a somewhat different way in the Sharks than
in other vertebrates. It becomes split off from the hypoblast, not in the
form of a single sheet as in other vertebrates, but as two lateral sheets,
one on each side of the middle line and separated from one another by a
considerable interval; whilst the notochord is derived not as in other
vertebrates from the mesoblast, but from the hypoblast (vide F. M. Balfour, Development of Selachians[21],
Journal of Microscopical Science,
Oct., 1874).
[Pg 125] Between the Selachians and the Aves there is a considerable gulf, which it is more difficult satisfactorily to bridge over than in the previous cases; owing to this I have not attempted to give any intermediate stage between them.
The first stage of the Bird (F I) is very similar in many respects to the corresponding stage in the Selachian. The segmentation cavity is, however, a less well-defined formation, and it may even be doubted whether a true segmentation cavity, homologous with the segmentation cavity in the previously described eggs, is present. On the floor of the cavity which is formed by the yolk are a few larger cells known as formative cells which, according to Götte's observations, are derived from the yolk, in a somewhat similar manner to the cells which were formed around the nuclei in the Selachian egg, and which helped to form the ventral wall of the alimentary canal. Another point to be noticed is that the segmentation cavity occupies a central position, and not one to the side as in the Selachian.
The yolk is proportionately quite as large as in the Selachian's egg, but, as in that case, there can be little or no doubt of its being homologous with the largest of the segmentation spheres of the previous eggs. It does not undergo segmentation. The epiblast is composed of columnar cells, and extends a short way beyond the edge of the lower layer cells.
In the next stage the more important departures from the previous type of development become visible.
The epiblast spreads uniformly over the yolk-sac and not on the one side only as in the former eggs.
This is due to the embryo (indicated in F II by a thickening of the cells) lying in the centre and not at the edge of the blastoderm. A necessary consequence of this is, that the epiblast does not, as in the previous cases, become continuous with the hypoblast at the tail end of the embryo. This continuity, being of no functional importance, could easily be dispensed with, and the central position of the embryo may perhaps be explained by supposing the process, by which in the Selachian egg the blastopore ceases to correspond in position with the opening of the alimentary slit or anus of Rusconi (vide E´), to occur quite early during segmentation instead of at a late period of development. [Pg 126] For the possibility of such a change in the date of formation, the early appearance of the nervous and epidermic layers in the Frog affords a parallel.
The epiblast in its growth round the yolk is only partially accompanied by mesoblast, which, however, is thickened at its extreme edge as in the Frog. Owing to the epiblast not becoming continuous with the hypoblast at the tail end of the embryo, the alimentary slit is not open to the exterior. The hypoblast is formed by some of the lower layer cells becoming distinguished as a separate layer; the remainder of the lower layer cells become the mesoblast.
The formation of the mesoblast and hypoblast out of the lower layer cells has been accepted for the Bird by most observers, but has been disputed by several, and recently by Kölliker. These have supposed that the mesoblast is derived from the epiblast. I feel convinced that these observers are in the wrong, and that the mesoblast is genuinely derived from the lower layer cells.
The greater portion of the alimentary cavity consists of the original segmentation cavity (vide diagrams). This feature of the segmentation cavity of Birds sharply distinguishes it from any segmentation cavity of other eggs, and renders it very doubtful whether the similarly named cavities of the Bird and of other vertebrates are homologous. On the floor of the cavity are still to be seen some of the formative cells, but observers have not hitherto found that they take any share in forming the ventral wall of the alimentary canal.
The features of the next stage are the necessary consequences of those of the last.
The ventral wall of the alimentary canal is entirely formed by a folding-in of the sheet of hypoblast.
The more rapid folding-in at the head still indicates the previous more vigorous growth there, otherwise there is very little difference between the forms of the fold at the head and tail. The alimentary canal does not of course, at this or any period, communicate with the neural tube, since the epiblast and hypoblast are never continuous. The other features, such as the growth of the epiblast round the yolk-sac, are merely continuations of what took place in the last stage.
[Pg 127] In the development of a yolk-sac as a distinct appendage, and its absorption within the body, at a later period, the bird fundamentally resembles the Dog-fish.
Although there are some difficulties in deriving the type of development exhibited by the Bird directly from that of the Selachian, it is not very difficult to do so directly from Amphioxus. Were the alimentary involution to remain symmetrical as in Amphioxus, and the yolk-containing part of the egg to assume the proportions it does in the Bird, we should obtain a mode of development which would not be very dissimilar to that of the Bird. The epiblast would necessarily overgrow the yolk uniformly on all sides and not in the unsymmetrical fashion of the Selachian egg. A confirmation of this view might perhaps be sought for in the complete difference between the types of circulation of the yolk-sac in Birds and Selachians; but this is not so important as might at first sight appear, since it is not from the Selachian egg but from some Batrachian that it would be necessary to derive the Reptiles' and Birds' eggs.
If this view of the Bird's egg be correct, we are compelled to suppose that the line of ancestors of Birds and Reptiles did not include amongst them the Selachians and the Batrachians, or at any rate Selachians and Batrachians which develop on the type we now find.
The careful investigation of the development of some Reptiles might very probably throw light upon this important point. In the meantime it is better to assume that the type of development of Birds is to be derived from that of the Frog and Selachians.
Summary.—If the views expressed in this paper are correct, all the modes of development found in the higher vertebrates are to be looked upon as modifications of that of Amphioxus. It is, however, rather an interesting question whether it is possible to suppose that the original type was not that of Amphioxus, but of some other animal, say, for instance, that of the Frog, and that this varied in two directions,—on the one hand towards Amphioxus, in the reverse direction to the course of variation presupposed in the text; and on the other hand in the direction towards the Selachians as before.
The answer to this question must in my opinion be in the [Pg 128] negative. It is quite easy to conceive the food material of the Frog's egg completely vanishing, but although this would entail simplifications of development and possibly even make segmentation uniform, there would, as far as I can see, be no cause why the essential features of difference between the Frog's mode of development and that of Amphioxus should change. The asymmetrical and slit-like form of involution on the one side and the growth of the epiblast over the mesoblast on the other side, both characteristics of the present Frog's egg, would still be features in the development of the simplified egg.
In the Mammal's egg we probably have an example of a Reptile's egg simplified by the disappearance of the food material; and when we know more of Mammalian embryology it will be very interesting to trace out the exact manner in which this simplification has affected the development. It is also probable that the eggs of Osseous fish are fundamentally simplified Selachian eggs; in which case we already know that the diminution of food material has affected but very slightly the fundamental features of development.
One common feature which appears prominently in reviewing the embryology of vertebrates as a whole is the derivation of the mesoblast from the hypoblast; in other words, we find that it is from the layer corresponding to that which becomes involuted in Amphioxus so as to line the alimentary cavity that the mesoblast is split off.
That neither the hypoblast or mesoblast can in any sense be said to be derived from the epiblast is perfectly clear. When the egg of Amphioxus is in the blastosphere stage we cannot speak of either an epiblast or hypoblast. It is not till the involution or what is equivalent has occurred, converting the single-walled vesicle into a double-walled one, that we can speak of these two layers. It might seem scarcely necessary to insist upon this point, so clear is it without explanation, were it not that certain embryologists have made a confusion about it.
The derivation of the mesoblast from the hypoblast is the more interesting, since it is not confined to the vertebrates, but has a very wide extension amongst the invertebrates. In the cases (whose importance has been recently insisted upon by Professor Huxley), of the Asteroids, the Echinoids, Sagitta, and [Pg 129] others, in which the body-cavity arises as an outgrowth of the alimentary canal and the somatopleure and splanchnopleure are formed from that outgrowth, it is clear without further remark that the mesoblast is derived from the hypoblast. For the Echinoderms in which the water-vascular system and muscular system arise as a solid outgrowth of the wall of the alimentary canal there can also be no question as to the derivation of the mesoblast from the hypoblast.
Amongst other worms, in addition to Sagitta, the investigations of Kowalevsky seem to shew that in Lumbricus the mesoblast is derived from the hypoblast.
Amongst Crustaceans, Bobretsky's[22] observations on Oniscus (Zeitschrift für wiss. Zoologie, 1874) lead to the same conclusion.
In Insects Kowalevsky's observations lead to the conclusion that mesoblast and hypoblast arise from a common mass of cells; Ulianin's observations bring out the same result for the abnormal Poduridæ, and Metschnikoff's observations shew that this also holds for Myriapods.
In Molluscs the point is not so clear.
In Tunicates, even if we are not to include them amongst vertebrates[23], the derivation of mesoblast from hypoblast is without doubt.
Without going further into details it is quite clear that the derivation of the mesoblast from the hypoblast is very general amongst invertebrates.
It will hardly be disputed that primitively the muscular system of the body-wall could not have been derived from the layer of cells which lines the alimentary canal. We see indeed in Hydra and the Hydrozoa that in its primitive differentiation, as could have been anticipated beforehand, the muscular system of the body is derived from the epiblast cells. What, then, is the explanation of the widespread derivation of the mesoblast, including the muscular system of the body, from the hypoblast?
[Pg 130] The explanation of it may, I think, possibly be found, and at all events the suggestion seems to me sufficiently plausible to be worth making, in the fact that in many cases, and probably this applies to the ancestors of the vertebrates, the body-cavity was primitively a part of the alimentary.
Mr Lankester, who has already entered into this line of speculation, even suggests (Q. J. of Micr. Science, April, 1875) that this applies to all higher animals. It might then be supposed that the muscular system of part of the alimentary canal took the place of the primitive muscular system of the body; so that the whole muscular system of higher animals would be primitively part of the muscular system of the digestive tract.
I put this forward merely as a suggestion, in the truth of which I feel no confidence, but which may perhaps induce embryologists to turn their attention to the point. If we accept it for the moment, the supplanting of the body muscular system by that of the digestive tract may hypothetically be supposed to have occurred in the following way.
When the diverticulum or rather paired diverticula were given off from the alimentary canal they would naturally become attached to the body-wall, and any contractions of their intrinsic muscles would tend to cause movements in the body-wall. So far there is no difficulty, but there is a physiological difficulty in explaining how it can have happened that this secondary muscular system can have supplanted the original muscular system of the body.
The following suggestions may lessen this difficulty, though perhaps they hardly remove it completely. If we suppose that the animal in which these diverticula appeared had a hard test and was not locomotive, the intrinsic muscular system of the body would naturally completely atrophy. But since the muscular system of the diverticula from the stomach would be required to keep up the movement of the nutritive fluid, it would not atrophy, and were the test subsequently to become soft and the animal locomotive, would naturally form the muscular system of the body. Or even were the animal locomotive in which the diverticula appeared, it is conceivable that the two systems might at first coexist together; that either (1) subsequently [Pg 131] owing to the greater convenience of early development, the two systems might acquire a development from the same mass of cells and those the cells of the inner or hypoblast layer, so that the derivation of the body muscles from the hypoblast would only be apparent and not real, or (2) owing to their being better nourished as they would necessarily be, and to their possibly easier adaptability to some new form of movement of the animal, the muscle-cells of the alimentary canal might become developed exclusively whilst the original muscular system atrophied.
I only hold this view provisionally till some better explanation is given of the cases of Sagitta and the Echinoderms, as well as of the nearly universal derivation of the mesoblast from the hypoblast. The cases of this kind may be due to some merely embryonic changes and have no meaning in reference to the adult condition, but I think that we have no right to assume this till some explanation of the embryonic can be suggested.
For vertebrates, I have shewn that in Selachians the body-cavity at first extends quite to the top of what becomes the muscle plate, so that the line or space separating the two layers of the muscle plate (vide Balfour, 'Development of Elasmobranch Fishes[24],' Quart. Journ. of Micro. Science for Oct., 1874. Plate XV, fig. 11a, 11b, 12a, mp.) is a portion of the original body-cavity. If this is a primitive condition, which is by no means certain, we have a condition which we might expect, in which both the inner and the outer wall of the primitive body-cavity assists in forming the muscular system of the body.
It is very possible that the formation of the mesoblast as two masses, one on each side of the middle line as occurs in Selachians, and which as I pointed out in the paper quoted above also takes place in some worms, is a remnant of the primitive formation of the body-cavity as paired outgrowth of the alimentary canal. This would also explain the fact that in Selachians the body-cavity consists at first of two separate portions, one on each side of the alimentary canal, which only subsequently [Pg 132] become united below and converted into a single cavity (vide loc. cit.[25], Plate XIV, fig. 8b, pp).
In the Echinoderms we find instances where the body-cavity and water-vascular system arise as an outgrowth from the alimentary canal, which subsequently becomes constricted off from the latter (Asteroids and Echinoids), together with other instances (Ophiura, Synapta) where the water-vascular system and body-cavity are only secondarily formed in a solid mass of mesoblast originally split off from the walls of the alimentary canal.
These instances shew us how easily a change of this kind may take place, and remove the difficulty of understanding why in vertebrates the body-cavity never communicates with the alimentary.
The last point which I wish to call attention to is the blastopore or anus of Rusconi.
This is the primitive opening by which the alimentary canal communicates with the exterior, or, in other words, the opening of the alimentary involution. It is a distinctly marked structure in Amphioxus and the Batrachians, and is also found in a less well-marked form in the Selachians; in Birds no trace of it is any longer to be seen. In all those vertebrates in which it is present, it closes up and does not become the anus of the adult. The final anus nevertheless corresponds very closely in position with the anus of Rusconi. Mr Lankester has shewn (Quart. Journ. of Micro. Science for April, 1875) that in invertebrates as well as vertebrates the blastopore almost invariably closes up. It nevertheless corresponds as a rule very nearly in position either with the mouth or with the anus.
If this opening is viewed, as is generally done, as really being the mouth in some cases and the anus in others, it becomes very difficult to believe that the blastopore can in all cases represent the same structure. In a single branch of the animal kingdom it sometimes forms the mouth and sometimes the anus: thus for instance in Lumbricus it is the mouth (according to Kowalevsky), in Palæmon (Bobretzky) the anus. Is it credible that the mouth and anus have become changed, the one for the other?
If, on the other hand, we accept the view that the blastopore [Pg 133] never becomes either the one or the other of these openings, it is, I think, possible to account for its corresponding in position with the mouth in some cases or the anus in others.
That it would soon come to correspond either with the mouth or anus (probably with the earliest formed of these in the embryo), wherever it was primitively situated, follows from the great simplification which would be effected by its doing so. This simplification consists in the greater facility with which the fresh opening of either mouth or anus could be made where the epiblast and hypoblast were in continuity than elsewhere. Even a change of correspondence from the position of the mouth to that of the anus or vice versa could occur. The mode in which this might happen is exemplified by the case of the Selachians. I pointed out in the course of this paper how the final point of envelopment of the yolk became altered in Selachians so as to cease to correspond with the anus of Rusconi; in other words, how the position of the blastopore became changed. In such a case, if the yolk material again became diminished, the blastopore would correspond in position with neither mouth nor anus, and the causes which made it correspond in position with the anus before, would again operate, and make it correspond in position perhaps with the mouth. Thus the blastopore might absolutely cease to correspond in position with the anus and come to correspond in position with the mouth.
It is hardly possible to help believing that the blastopore primitively represented a mouth. It may perhaps have lost this function owing to an increase of food yolk in the ovum preventing its being possible for the blastopore to develop directly into a mouth, and necessitating the formation of a fresh mouth. If such were the case, there would be no reason why the blastopore should ever again serve functionally as a mouth in the descendants of the animal which developed this fresh mouth.
[Pg 134] EXPLANATION OF PLATE 5.
COMPLETE LIST OF REFERENCES.
al. Cavity of alimentary canal. bl. Blastoderm. ch. Notochord. ep. Epiblast. em. Embryo. f. Formative cells. hy. Hypoblast. ll. Lower layer cells. m. Mesoblast. n. Nuclei of yolk of Selachian egg. nc. Neural canal. sg. Segmentation cavity. x. Point where epiblast and hypoblast are continuous at the mouth of the alimentary involution. This point is always situated at the tail end of the embryo. yk. Yolk.
Epiblast is coloured blue, mesoblast red, and hypoblast yellow. The lower layer cells before their separation into hypoblast and mesoblast are also coloured green.
A I, A II, A III. Diagrammatic sections of Amphioxus in its early stages (founded upon Kowalevsky's observations).
B I, B II, B III. Diagrammatic longitudinal sections of an hypothetical animal, intermediate between Amphioxus and Batrachians, in its early stages.
C I, C II, C III. Diagrammatic longitudinal sections of Bombinator igneus in its early stages (founded upon Götte's observations). in C III the neural canal is completed, which was not the case in B III. The epiblast in C III has been diagrammatically represented as a single layer.
D I, D II, D III. Diagrammatic longitudinal sections of an animal, intermediate between Batrachians and Selachians, in its early stages.
E I, E II, E III. Diagrammatic longitudinal sections of a Selachian in its early stages.
E´. Surface view of the yolk of a Selachian's egg to shew the manner in which it is enclosed by the Blastoderm. The yolk is represented yellow and the Blastoderm blue.
F I, F II, F III. Diagrammatic longitudinal sections of a Bird in its early stages.
[19] From the Quarterly Journal of Microscopical Science, Vol. XV. 1875.
[20] I have been able to make at Naples observations which confirm the account of the invagination of Amphioxus as given by Kowalevsky, though my observations are not nearly so complete as those of the Russian naturalist.
[21] Paper No. V, p. 82 et seq. in this edition.
[22]
He says, p. 182: Bevor aber die Hälfte der Eioberfläche
von den Embryonalzellen bedeckt ist, kommt die erste gemeinsame Anlage des
mittleren und unteren Keimblattes zum Vorschein.
[23] Anton Dohrn, Der Ursprung des Wirbelthieres. Leipzig, 1875.
[24] Paper No. V, p. 60 et seq. of this edition, pl. 4, figs. 11a, 11b, 12a, mp.
[25] Pl. 3 of this edition, fig. 8b, pp.
Recent discoveries[27] as to the mode of development and anatomy of the urinogenital system of Selachians, Amphibians, and Cyclostome fishes, have greatly increased our knowledge of this system of organs, and have rendered more possible a comparison of the types on which it is formed in the various orders of vertebrates.
[Pg 136] The following paper is an attempt to give a consecutive history of the origin of this system of organs in vertebrates and of the changes which it has undergone in the different orders.
For this purpose I have not made use of my own observations alone, but have had recourse to all the Memoirs with which I am acquainted, and to which I have access. I have commenced my account with the Selachians, both because my own investigations have been directed almost entirely to them, and because their urinogenital organs are, to my mind, the most convenient for comparison both with the more complicated and with the simpler types.
On many points the views put forward in this paper will be found to differ from those which I expressed in my paper (loc. cit.) which give an account of my original[28] discovery of the segmental organs of Selachians, but the differences, with the exception of one important error as to the origin of the Wolffian duct, are rather fresh developments of my previous views from the consideration of fresh facts, than radical changes in them.
* * * * *
In Selachian embryos an intermediate cell-mass, or middle plate of mesoblast is formed, as in birds, from a partial fusion of the somatic and splanchnic layers of the mesoblast at the outer border of the protovertebræ. From this cell-mass the whole of the urinogenital system is developed.
At about the time when three visceral clefts have appeared, there arises from the intermediate cell-mass, opposite the fifth protovertebra, a solid knob, from which a column of cells grows backwards to opposite the position of the future anus (Fig. 1. pd.).
Fig. 1. Two sections of a Pristiurus Embryo with three visceral clefts.
The sections are to shew the development of the segmental duct (pd) or primitive duct of the kidneys. In A (the anterior of the two sections) this appears as a solid knob projecting towards the epiblast. In B is seen a section of the column which has grown backwards from the knob in A.
spn. rudiment of a spinal nerve; mc. medullary canal; ch. notochord; X. string of cells below the notochord; mp. muscle-plate; mp´. specially developed portion of muscle-plate; ao. dorsal aorta; pd. segmental duct; so. somatopleura; sp. splanchnopleura; pp. pleuro-peritoneal or body-cavity; ep. epiblast; al. alimentary canal.
This knob projects outwards toward the epiblast, and the column lies at first between the mesoblast and epiblast. The knob and column do not long remain solid. The knob becoming hollow acquires a wide opening into the pleuro-peritoneal or body-cavity, and the column a lumen; so that by the time that five visceral clefts have appeared, the two together form a [Pg 137] duct closed behind, but communicating in front by a wide opening with the pleuro-peritoneal cavity.
Before these changes are accomplished, a series of solid[29] outgrowths of elements of the 'intermediate cell-mass' appear at the uppermost corner of the body-cavity. These soon become hollow and appear as involutions from the body-cavity, curling round the inner and dorsal side of the previously formed duct.
One involution of this kind makes its appearance for each protovertebra, and the first belongs to the protovertebra immediately behind the anterior end of the duct whose development has just been described. In Pristiurus there are in all 29 of these at this period. The last two or three arise from that portion of the body-cavity, which at this stage still exists behind the anus. The first-formed duct and the subsequent involutions are the rudiments of the whole of the urinary system. [Pg 138] The duct is the primitive duct of the kidney[30]; I shall call it in future the segmental duct; and the involutions are the commencements of the segmental tubes which constitute the body of the kidney. I shall call them in future segmental tubes.
Soon after their formation the segmental tubes become convoluted, and their blind ends become connected with the segmental duct of the kidney. At the same time, or rather before this, the blind posterior termination of each of the segmental ducts of the kidneys unites with and opens into one of the horns of the cloaca. At this period the condition of affairs is represented in Fig. 2.
Fig. 2. Diagram of the primitive condition of the Kidney in a Selachian Embryo.
pd. segmental duct. It opens at o into the body-cavity and at its other extremity into the cloaca; x. line along which the division appears which separates the segmental duct into the Wolffian duct above and the Müllerian duct below; st. segmental tubes. They open at one end into the body-cavity, and at the other into the segmental duct.
There is at pd, the segmental duct of the kidneys, opening in front (o) into the body-cavity, and behind into the cloaca, and there are a series of convoluted segmental tubes (st), each opening at one end into the body-cavity, and at the other into the duct (pd).
The next important change which occurs is the longitudinal division of the segmental duct of the kidneys into Müller's duct, or the oviduct, and the duct of the Wolffian bodies or Leydig's duct. The splitting[31] is effected by the growth of a wall of cells [Pg 139] which divides the duct into two parts (fig. 3, wd. and md.). It takes place in such a way that the front end of the segmental duct, anterior to the entrance of the first segmental tube, together with the ventral half of the rest of the duct, is split off from its dorsal half as an independent duct (vide fig. 2, x).
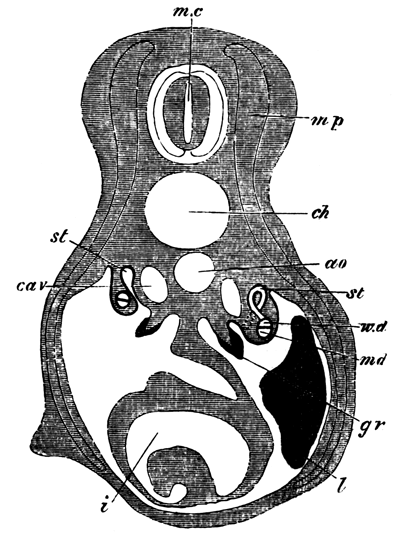
Fig. 3. Transverse section of a Selachian Embryo illustrating the formation of the Wolffian and Müllerian ducts by the longitudinal splitting of the segmental duct.
mc. medullary canal; mp. muscle-plate; ch. notochord; ao. aorta; cav. cardinal vein; st. segmental tube. On the one side the section passes through the opening of a segmental tube into the body-cavity. On the other this opening is represented by dotted lines, and the opening of the segmental tube into the Wolffian duct has been cut through; wd. Wolffian duct; md. Müllerian duct. The Müllerian duct and the Wolffian duct together constitute the primitive segmental duct; gr. The germinal ridge with the thickened germinal epithelium; l. liver; i. intestine with spiral valve.
The dorsal portion also forms an independent duct, and into it the segmental tubes continue to open. Such at least is the [Pg 140] method of splitting for the female—for the male the splitting is according to Professor Semper, of a more partial character, and consists for the most part in the front end of the duct only being separated off from the rest. The result of these changes is the formation in both sexes of a fresh duct which carries off the excretions of the segmental involutions, and which I shall call the Wolffian duct—while in the female there is formed another complete and independent duct, which I shall call the Müllerian duct, or oviduct, and in the male portions only of such a duct.
The next change which takes place is the formation of another duct from the hinder portion of the Wolffian duct, which receives the secretion of the posterior segmental tubes. This secondary duct unites with the primary or Wolffian duct near its termination, and the primary ducts of the two sides unite together to open to the exterior by a common papilla.
Slight modifications of the posterior terminations of these ducts are found in different genera of Selachians (vide Semper, Centralblatt für Med. Wiss. 1874, No. 59), but they are of no fundamental importance.
These constitute the main changes undergone by the segmental duct of the kidneys and the ducts derived from it; but the segmental tubes also undergo important changes. In the majority of Selachians their openings into the body-cavity, or, at any rate, the openings of a large number of them, persist through life; but the investigations of Dr Meyer[32] render it very probable that the small portion of each segmental tube adjoining the opening becomes separated from the rest and becomes converted into a sort of lymph organ, so that the openings of the segmental tubes in the adult merely lead into lymph organs and not into the gland of the kidneys.
These constitute the whole changes undergone in the female, but in the male the open ends of a varying number (according to the species) of the segmental tubes become connected with the testis and, uniting with the testicular follicles, serve to carry away the seminal fluid[33]. The spermatozoa have therefore to [Pg 141] pass through a glandular portion of the kidneys before they enter the Wolffian duct, by which they are finally carried away to the exterior.
In the adult female, then, there are the following parts of the urinogenital system (fig. 4):
(1) The oviduct, or Müller's duct (fig. 4, md.), split off from the segmental duct of the kidneys. Each oviduct opens at its upper end into the body-cavity, and behind the two oviducts have independent communications with the cloaca. The oviducts serve simply to carry to the exterior the ova, and have no communication with the glandular portion of the kidneys.
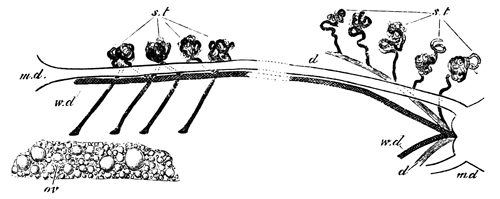
Fig. 4. Diagram of the arrangement of the Urinogenital Organs in an adult Female Selachian.
md. Müllerian duct; wd. Wolffian duct; st. segmental tubes; d. duct of the posterior segmental tubes; ov. ovary.
(2) The Wolffian ducts (fig. 4, wd.) or the remainder of the segmental ducts of the kidneys. Each Wolffian duct ends blindly in front, and the two unite behind to open by a common papilla into the cloaca.
This duct receives the secretion of the whole anterior end of the kidneys[34], that is to say, of all the anterior segmental tubes.
(3) The secondary duct (fig. 4, d.) belonging to the lower portion of the kidneys opening into the former duct near its termination.
(4) The segmental tubes (fig. 4, st) from whose convolutions and outgrowths the kidney is formed. They may be divided [Pg 142] into two parts, according to the duct by which their secretion is carried off.
In the male the following parts are present:
(1) The Müllerian duct (fig. 5, md.), consisting of a small remnant, attached to the liver, which represents the foremost end of the oviduct of the female.
(2) The Wolffian duct (fig. 5, wd), which precisely corresponds to the Wolffian duct of the female, except that, in addition to functioning as the duct of the anterior part of the kidneys, it also serves to carry away the semen. In the female it is straight, but has in the adult male a very tortuous course (vide fig. 5).
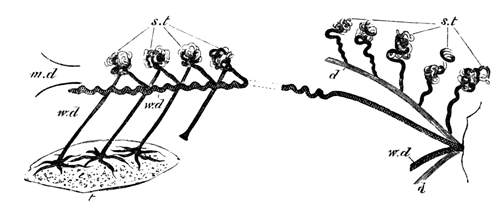
Fig. 5. Diagram of the arrangement of the Urinogenital Organs in an adult male selachian.
md. rudiment of Müllerian duct; wd. Wolffian duct, which also serves as vas deferens; st. segmental tubes. The ends of three of those which in the female open into the body-cavity, have in the male united with the testicular follicles, and serve to carry away the products of the testis; d. duct of the posterior segmental tubes; t. testis.
(3) the duct (fig. 5, d.) of the posterior portion of the kidneys, which has the same relations as in the female.
(4) The segmental tubes (fig. 5, st.). These have the same relations as in the female, except that the most anterior two, three or more, unite with the testicular follicles, and carry away the semen into the Wolffian duct.
* * * * *
The mode of arrangement and the development of these parts suggest a number of considerations.
In the first place it is important to notice that the segmental tubes develop primitively as completely independent [Pg 143] organs[35], one of which appears in each segment. If embryology is in any way a repetition of ancestral history, it necessarily follows that these tubes were primitively independent of each other. Ancestral history, as recorded in development, is often, it is true, abridged; but it is clear that though abridgement might prevent a series of primitively separate organs from appearing as such, yet it would hardly be possible for a primitively compound organ, which always retained this condition, to appear during development as a series of separate ones. These considerations appear to me to prove that the segmented ancestors of vertebrates possessed a series of independent and segmental excretory organs.
Both Professor Semper and myself, on discovering these organs, were led to compare them and state our belief in their identity with the so-called segmental organs of Annelids.
This view has since been fairly generally accepted. The segmental organs of annelids agree with those of vertebrates in opening at one end into the body-cavity, but differ in the fact that each also communicates with the exterior by an independent opening, and that they are never connected with each other.
On the hypothesis of the identity of the vertebrate segmental tubes with the annelid segmental organs, it becomes essential to explain how the external openings of the former may have become lost.
This brings us at once to the origin of the segmental duct of the kidneys, by which the secretion of all the segmental tubes was carried to the exterior, and it appears to me that a right understanding of the vertebrate urinogenital system depends greatly upon a correct view of the origin of this duct. I would venture to repeat the suggestion which I made in my original paper (loc. cit.) that this duct is to be looked upon as the most anterior of the segmental tubes which persist in vertebrates. [Pg 144] In favour of this view are the following anatomical and embryological facts. (1) It develops in nearly the same manner as the other segmental tubes, viz. in Selachians as a solid outgrowth from the intermediate cell-mass, which subsequently becomes hollowed so as to open into the body-cavity: and in Amphibians and Osseous and Cyclostome fishes as a direct involution from the body-cavity. (2) In Amphibians, Cyclostomes and Osseous fishes its upper end develops a glandular portion, by becoming convoluted in a manner similar to the other segmental tubes. This glandular portion is often called either the head-kidney or the primitive kidney. It is only an embryonic structure, but is important as demonstrating the true nature of the primitive duct of the kidneys.
We may suppose that some of the segmental tubes first united, possibly in pairs, and that then by a continuation of this process the whole of them coalesced into a common gland. One external opening sufficed to carry off the entire secretion of the gland, and the other openings therefore atrophied.
This history is represented in the development of the dog-fish in an abbreviated form, by the elongation of the first segmental tube (segmental duct of the kidney) and its junction with each of the posterior segmental tubes. Professor Semper looks upon the primitive duct of the kidneys as a duct which arose independently, and was not derived from metamorphosis of the segmental organs. Against this view I would on the one hand urge the consideration, that it is far easier to conceive of the transformation by change of function (comp. Dohrn, Functionswechsel, Leipzig, 1875) of a segmental organ into a segmental duct, than to understand the physiological cause which should lead, in the presence of so many already formed ducts, to the appearance of a totally new one. By its very nature a duct is a structure which can hardly arise de novo. We must even suppose that the segmental organs of Annelids were themselves transformations of still simpler structures. On the other hand I would point to the development in this very duct amongst Amphibians and Osseous fishes of a glandular portion similar to that of a segmental tube, as an à posteriori proof of its being a metamorphosed segmental tube. The development in insects of a longitudinal tracheal duct by the coalescence of a [Pg 145] series of transverse tracheal tubes affords a parallel to the formation of a duct from the coalescence of a series of segmental tubes.
Though it must be admitted that the loss of the external openings of the segmental organs requires further working out, yet the difficulties involved in their disappearance are not so great as to render it improbable that the vertebrate segmental organs are descended from typical annelidan ones.
The primitive vertebrate condition, then, is probably that of an early stage of Selachian development while there is as yet a segmental duct,—the original foremost segmental tube opening in front into the body-cavity and behind into the cloaca; with which duct all the segmental tubes communicate. Vide Fig. 2.
The next condition is to be looked upon as an indirect result of the segmental duct serving as well for the products of the generative organs as the secretions of the segmental tubes.
As a consequence of this, the segmental duct became split into a ventral portion, which served alone for the ova, and a dorsal portion which received the secretion of the segmental tubes. The lower portion, which we have called the oviduct, in some cases may also have received the semen as well as the ova. This is very possibly the case with Ceratodus (vide Günther, Trans. of Royal Society, 1871), and the majority of Ganoids (Hyrtl, Denkschriften Wien, Vol. VIII.). In the majority of other cases the oviduct exists in the male in a completely rudimentary form; and the semen is carried away by the same duct as the urine.
In Selachians the transportation of the semen from the testis to the Wolffian duct is effected by the junction of the open ends of two or three or more segmental tubes with the testicular follicles, and the modes in which this junction is effected in the higher vertebrates seem to be derivatives from this. If the views here expressed are correct it is by a complete change of function that the oviduct has come to perform its present office. And in the bird and higher vertebrates no trace, or only the very slightest (vide p. 165) of the primitive urinary function is retained during embryonic or adult life.
The last feature in the anatomy of the Selachians which [Pg 146] requires notice is the division of the kidney into two portions, an anterior and posterior. The anatomical similarity between this arrangement and that of higher vertebrates (birds, &c.) is very striking. The anterior one precisely corresponds, anatomically, to the Wolffian body, and the posterior one to the true permanent kidney of higher vertebrates: and when we find that in the Selachians the duct for the anterior serves also for the semen as does the Wolffian duct of higher vertebrates, this similarity seems almost to amount to identity. A discussion of the differences in development in the two cases will come conveniently with the account of the bird; but there appear to me the strongest grounds for looking upon the kidneys of Selachians as equivalent to both the Wolffian bodies and the true kidneys of the higher vertebrates.
The condition of the urinogenital organs in Selachians is by no means the most primitive found amongst vertebrates.
The organs of both Cyclostomous and Osseous fishes, as well as those of Ganoids, are all more primitive; and in the majority of points the Amphibians exhibit a decidedly less differentiated condition of these organs than do the Selachians.
In Cyclostomous fishes the condition of the urinary system is very simple. In Myxine (vide Joh. Müller Myxinoid fishes, and Wilhelm Müller, Jenaische Zeitschrift, 1875, Das Urogenitalsystem des Amphioxus u. d. Cyclostomen) there is a pair of ducts which communicate posteriorly by a common opening with the abdominal pore. From these ducts spring a series of transverse tubules, each terminating in a Malpighian corpuscle. These together constitute the mass of the kidneys. About opposite the gall-bladder the duct of the kidney (the segmental duct) narrows very much, and after a short course ends in a largish glandular mass (the head-kidney), which communicates with the pericardial cavity by a number of openings.
In Petromyzon the anatomy of the kidneys is fundamentally the same as in Myxine. They consist of the two segmental ducts, and a number of fine branches passing off from these, which become convoluted but do not form Malpighian tufts. The head-kidney is absent in the adult.
W. Müller (loc. cit.) has given a short but interesting account of the development of the urinary system of Petromyzon. He [Pg 147] finds that the segmental ducts develop first of all as simple involutions from the body-cavity. The anterior end of each then develops a glandular portion which comes to communicate by a number of openings with the body-cavity. Subsequently to the development of this glandular portion the remainder of the kidneys appears in the posterior portion of the body-cavity; and before the close of embryonic life the anterior glandular portion atrophies.
The comparison of this system with that of a Selachian is very simple. The first developed duct is the segmental duct of a Selachian, and the glandular portion developed at its anterior extremity, which is permanent in Myxine but embryonic in Petromyzon, is, as W. Müller has rightly recognized, equivalent to the head-kidney of Amphibians, which remains undeveloped in Selachians. It is, according to my previously stated view, the glandular portion of the first segmental organ or the segmental duct. The series of orifices by which this communicates with the body-cavity are due to the division of the primary opening of the segmental duct. This is shewn both by the facts of their development in Petromyzon given by Müller, as well as by the occurrence of a similar division of the primary orifice in Amphibians, which is mentioned later in this paper. In a note in my original paper (loc. cit.) I stated that these openings were equivalent to the segmental involutions of Selachians. This is erroneous, and was due to my not having understood the description given in a preliminary paper of Müller (Jenaische Zeitschrift, 1873). The large development of this glandular mass in the Cyclostome and Osseous fishes and in embryo Amphibians, implies that it must at one time have been important. Its earlier development than the remainder of the kidneys is probably a result of the specialized function of the first segmental organ.
The remainder of the kidney in Cyclostomes is equivalent to the kidney of Selachians. Its development from segmental involutions has not been recognized. If these segmental involutions are really absent it may perhaps imply that the simplicity of the Cyclostome kidneys, like that of so many other of their organs, is a result of degeneration rather than a primitive condition.
[Pg
148] In Osseous fishes the segmental duct of the kidneys develops,
as the observations of Rosenberg[36] (Teleostierniere,
Inaug. Disser.
Dorpat, 1867) and Oellacher (Zeitschrift für
Wiss. Zool. 1873) clearly prove, by an involution from the
body-cavity. This involution grows backwards in the form of a duct and
opens into the cloaca. The upper end of this duct (the most anterior
segmental tube) becomes convoluted, and forms a glandular body, which has
no representative in the urinary apparatus of Selachians, but whose
importance, as indicating the origin of the segmental duct of the kidneys,
I have already insisted upon.
The rest of the kidney becomes developed at a later period, probably in the same way as in Selachians; but this, as far as I know, has not been made out.
The segmental duct of the kidneys forms the duct for this new gland, as in embryo Selachians (Fig. 2), but, unlike what happens in Selachians, undergoes no further changes, with the exception of a varying amount of retrogressive metamorphosis of its anterior end. The kidneys of Osseous fish usually extend from just behind the head to opposite the anus, or even further back than this. They consist for the most part of a broader anterior portion, an abdominal portion reaching from this to the anus, and, as in those cases in which the kidneys extend further back than the anus, of a caudal portion.
The two ducts (segmental ducts of the kidneys) lie, as a rule, in the lower part of the kidneys on their outer borders, and open almost invariably into a urinary bladder. In some cases they unite before opening into the bladder, but generally have independent openings.
This bladder, which is simply a dilatation of the united lower ends of the primitive kidney-ducts, and has no further importance, is almost invariably present, but in many cases lies unsymmetrically either to the right or the left. It opens to the exterior by a very minute opening in the genito-urinary papilla, immediately behind the genital pore. There are, however, a few cases in which the generative and urinary organs have a [Pg 149] common opening. For further details vide Hyrtl, Denk. der k. Akad. Wien, Vol. II.
It is possible that the generative ducts of Osseous fishes are derived from a splitting from the primitive duct of the kidney, but this is discussed later in the paper.
In Osseous fishes we probably have an embryonic condition of the Selachian kidneys retained permanently through life.
* * * * *
In the majority of Ganoids the division of the segmental duct of the kidney into two would seem to occur, and the ventral duct of the two (Müllerian duct), which opens at its upper end into the body-cavity, is said to serve as an excretory duct for both male and female organs.
The following are the more important facts which are known about the generative and urinary ducts of Ganoids.
In Spatularia (vide Hyrtl, Geschlechts u. Harnwerkzeuge bei den Ganoiden, Denkschriften der k. Akad. Wien, Vol. VIII.) the following parts are found in the female.
(1) The ovaries stretching along the whole length of the abdominal cavity.
(2) The kidneys, which are separate and also extend along the greater part of the abdominal cavity.
(3) The ureters lying on the outer borders of the kidneys. Each ureter dilates at its lower end into an elongated wide tube, which continues to receive the ducts from the kidneys. The two ureters unite before terminating and open behind the anus.
(4) The two oviducts (Müllerian ducts). These open widely into the abdominal cavity, at about two-thirds of the distance from the anterior extremity of the body-cavity. Each opens by a narrow pore into the dilated ureter of its side.
In the male the same parts are found as in the female, but Hyrtl found that the Müllerian duct of the left side at its entrance into the ureter became split into two horns, one of which ended blindly. On the right side the opening of the Müllerian duct was normal.
In the Sturgeon (vide J. Müller, Bau u. Grenzen d. Ganoiden, Berlin Akad. 1844; Leydig, Fischen u. Reptilien, and Hyrtl, Ganoiden) the same parts are found as in Spatularia.
[Pg 150] The kidneys extend along the whole length of the body-cavity; and the ureter, which does not reach the whole length of the kidneys, is a thin-walled wide duct lying on the outer side. On laying it open the numerous apertures of the tubules for the kidney are exposed. The Müllerian duct, which opens in both sexes into the abdominal cavity, ends, according to Leydig, in the cases of some males, blindly behind without opening into the ureter, and Müller makes the same statement for both sexes. It was open on both sides in a female specimen I examined[37], and Hyrtl found it invariably so in both sexes in all the specimens he examined.
Both Rathke and Stannius (I have been unable to refer to the original papers) believed that the semen was carried off by transverse ducts directly into the ureter, and most other observers have left undecided the mechanism of the transportation of the semen to the exterior. If we suppose that the ducts Rathke saw really exist they might perhaps be supposed to enter not directly into the ureter, but into the kidney, and be in fact homologous with the vasa efferentia of the Selachians. The frequent blind posterior termination of the Müllerian duct is in favour of the view that these ducts of Rathke are really present.
In Polypterus (vide Hyrtl, Ganoiden) there is, as in other Ganoids, a pair of Müllerian ducts. They unite at their lower ends. The ureters are also much narrower than in previously described Ganoids and, after coalescing, open into the united oviducts. The urinogenital canal, formed by coalescence of the Müllerian ducts and ureters, has an opening to the exterior immediately behind the anus.
In Amia (vide Hyrtl) there is a pair of Müllerian ducts which, as well as the ureters, open into a dilated vesicle. This vesicle appears as a continuation of the Müllerian ducts, but receives a number of the efferent ductules of the kidneys. There is a single genito-urinary pore behind the anus.
In Ceratodus (Günther, Phil. Trans. 1871) the kidneys are small and confined to the posterior extremity of the abdomen. The generative organs extend however along the greater part of [Pg 151] the length of the abdominal cavity. In both male and female there is a long Müllerian duct, and the ducts of the two sides unite and open by a common pore into a urinogenital cloaca which communicates with the exterior by the same opening as the alimentary canal. In both sexes the Müllerian duct has a wide opening near the anterior extremity of the body-cavity. The ureters coalesce and open together into the urinogenital cloaca dorsal to the Müllerian ducts. It is not absolutely certain that the semen is transported to the exterior by the Müllerian duct of the male, which is perhaps merely a rudiment as in Amphibia. Dr Günther failed however to find any other means by which it could be carried away.
The genital ducts of Lepidosteus differ in important particulars from those of the other Ganoids (vide Müller, loc. cit. and Hyrtl, loc. cit.).
In both sexes the genital ducts are continuous with the investments of the genital organs.
In the female the dilated posterior extremities of the ureters completely invest for some distance the generative ducts, whose extremities are divided into several processes, and end in a different way on the two sides. A similar division and asymmetry of the ducts is mentioned by Hyrtl as occurring in the male of Spatularia, and it seems not impossible that on the hypothesis of the genital ducts being segmental tubes these divisions may be remnants of primitive glandular convolutions. The ureters in both sexes dilate as in other Ganoids at their posterior extremities, and unite with one another. The unpaired urinogenital opening is situated behind the anus. In the male the dilated portion of the ureters is divided into a series of partitions which are not present in the female.
Till the embryology of the secretory system of Ganoids has been worked out, the homologies of their generative ducts are necessarily a matter of conjecture. It is even possible that what I have called the Müllerian duct in the male is functionless, as with Amphibians, but that, owing to the true ducts of the testis having been overlooked, it has been supposed to function as the vas deferens. Günther's (loc. cit.) injection experiments on Ceratodus militate against this view, but I do not think they can be considered as conclusive as long as the [Pg 152] mechanism for the transportation of the semen to the exterior has not been completely made out. Analogy would certainly lead us to expect the ureter to serve in Ganoids as the vas deferens.
The position of the generative ducts might in some cases lead to the supposition that they are not Müllerian ducts, or, in other words, the most anterior pair of segmental organs but a pair of the posterior segmental tubes.
What are the true homologies of the generative ducts of Lepidosteus, which are continuous with the generative glands, is somewhat doubtful. It is very probable that they may represent the similarly functioning ducts of other Ganoids, but that they have undergone further changes as to their anterior extremities.
It is, on the other hand, possible that their generative ducts are the same structures as those ducts of Osseous fishes, which are continuous with the generative organs. These latter ducts are perhaps related to the abdominal pores, and had best be considered in connection with these; but a completely satisfactory answer to the questions which arise in reference to them can only be given by a study of their development.
In the Cyclostomes the generative products pass out by an abdominal pore, which communicates with the peritoneal cavity by two short tubes[38], and which also receives the ducts of the kidneys.
Gegenbaur suggests that these are to be looked upon as Müllerian ducts, and as therefore developed from the segmental ducts of the kidneys. Another possible view is that they are the primitive external openings of a pair of segmental organs. In Selachians there are usually stated to be a pair of abdominal pores. In Scyllium I have only been able to find, on each side, a large deep pocket opening to the exterior, but closed below towards the peritoneal cavity, so that in it there seem to be no abdominal pores[39]. In the Greenland Shark (Læmargus Borealis) [Pg 153] Professor Turner (Journal of Anat. and Phys. Vol. VIII.) failed to find either oviduct or vas deferens, but found a pair of large open abdominal pores, which he believes serve to carry away the generative products of both sexes. Whether the so-called abdominal pores of Selachians usually end blindly as in Scyllium, or, as is commonly stated, open into the body-cavity, there can be no question that they are homologous with true abdominal powers.
The blind pockets of Scyllium appear very much like the remains of primitive involutions from the exterior, which might easily be supposed to have formed the external opening of a pair of segmental organs, and this is probably the true meaning of abdominal pores. The presence of abdominal pores in all Ganoids in addition to true genital ducts and of these pockets or abdominal pores in Selachians, which are almost certainly homologous with the abdominal pores of Ganoids and Cyclostomes, and also occur in addition to true Müllerian ducts, speak strongly against the view that the abdominal pores have any relation to Müllerian ducts. Probably therefore the abdominal pores of the Cyclostomous fishes (which seem to be of the same character as other abdominal pores) are not to be looked on as rudimentary Müllerian ducts.
We next come to the question which I reserved while speaking of the kidneys of Osseous fishes, as to the meaning of their genital ducts.
In the female Salmon and the male and female Eel, the generative products are carried to the exterior by abdominal pores, and there are no true generative ducts. In the case of most other Osseous fish there are true generative ducts which are continuous with the investment of the generative organs[40] and [Pg 154] have generally, though not always, an opening or openings independent of the ureter close behind the rectum, but no abdominal pores are present. It seems, therefore, that in Osseous fish the generative ducts are complementary to abdominal pores, which might lead to the view that the generative ducts were formed by a coalescence of the investment of the generative glands with the short duct of abdominal pore.
Against this view there are, however, the following facts:
(1) In the cases of the salmon and the eel it is perfectly true that the abdominal pore exactly corresponds with the opening of the genital duct in other Osseous fishes, but the absence of genital ducts in these cases must rather be viewed, as Vogt and Pappenheim (loc. cit.) have already insisted, as a case of degeneration than of a primitive condition. The presence of genital ducts in the near allies of the Salmonidæ, and even in the male salmon, are conclusive proofs of this. If we admit that the presence of an abdominal pore in Salmonidæ is merely a result of degeneration, it obviously cannot be used as an argument for the complementary nature of abdominal pores and generative ducts.
(2) Hyrtl (Denkschriften der k. Akad. Wien, Vol. 1) states that in Mormyrus oxyrynchus there is a pair of abdominal pores in addition to true generative ducts. If his statements are correct, we have a strong argument against the generative ducts of Osseous fishes being related to abdominal pores. For though this is the solitary instance of the presence of both a genital opening and abdominal pores known to me in Osseous fishes, yet we have no right to assume that the abdominal pores of Mormyrus are not equivalent to those of Ganoids and Selachians. It must be admitted, with Gegenbaur, that embryology alone can elucidate the meaning of the genital ducts of Osseous fishes.
In Lepidosteus, as was before mentioned, the generative ducts, though continuous with the investment of the generative bodies, unite with the ureters, and in this differ from the generative ducts of Osseous fishes. The relation, indeed, of the [Pg 155] generative ducts of Lepidosteus to the urinary ducts is very similar to that existing in other Ganoid fishes; and this, coupled with the fact that Lepidosteus possesses a pair of abdominal pores on each side of the anus[41], makes it most probable that its generative ducts are true Müllerian ducts.
* * * * *
In the Amphibians the urinary system is again more primitive than in the Selachians.
The segmental duct of the kidneys is formed[42] by an elongated fold arising from the outer wall of the body-cavity, in the same position as in Selachians. This fold becomes constricted into a canal, closed except at its anterior end, which remains open to the body-cavity. This anterior end dilates, and grows out into two horns, and at the same time its opening into the body-cavity becomes partly constricted, and so divided into three separate orifices, one for each horn and a central one between the two. The horns become convoluted, blood channels appearing between their convolutions, and a special coil of vessels is formed arising from the aorta and projecting into the body-cavity near the openings of the convolutions. These formations together constitute the glandular portion[43] of the original anterior segmental tube or segmental duct of the kidneys. I have already pointed out the similarity which this organ exhibits to the head-kidneys of Cyclostome fishes in its mode of formation, especially with reference to the division of the primitive opening. The lower end of the segmental duct unites with a horn of the cloaca.
After the formation of the gland just described the remainder of the kidney is formed.
[Pg 156] This arises in the same way as in Selachians. A series of involutions from the body-cavity are developed; these soon form convoluted tubes, which become branched and interlaced with one another, and also unite with the primitive duct of the kidneys. Owing to the branching and interlacing of the primitive segmental tubes, the kidney is not divided into distinct segments in the same way as with the Selachians. The mode of development of these segmental tubes was discovered by Götte. Their openings are ciliated, and, as Spengel (loc. cit.) and Meyer (loc. cit.) have independently discovered, persist in most adult Amphibians. As both these investigators have pointed out, the segmental openings are in the adult kidneys of most Amphibians far more numerous than the vertebral segments to which they appertain. This is due to secondary changes, and is not to be looked upon as the primitive state of things. At this stage the Amphibian kidneys are nearly in the same condition as the Selachian, in the stage represented in Fig. 2. In both there is the segmental duct of the kidneys, which is open in front, communicates with the cloaca behind, and receives the whole secretion from the kidneys. The parallelism between the two is closely adhered to in the subsequent modifications of the Amphibian kidney, but the changes are not completed so far in Amphibians as in Selachians. The segmental duct of the Amphibian kidney becomes, as in Selachians, split into a Müllerian duct or oviduct, and a Wolffian duct or duct for the kidney.
The following points about this are noteworthy:
(1) The separation of the two ducts is never completed, so that they are united together behind, and for a short distance, blend and form a common duct; the ducts of the two sides so formed also unite before opening to the exterior.
(2) The separation of the two ducts does not occur in the form of a simple splitting, as in Selachians. But the efferent ductules from the kidney gradually alter their points of entrance into the primitive duct. Their points of entrance become carried backwards further and further, and since this process affects the anterior ducts proportionally more than the posterior, the efferent ducts finally all meet and form a common duct which unites with the Müllerian duct near its posterior extremity. [Pg 157] This process is not always carried out with equal completeness. In the tailless Amphibians, however, the process is generally[44] completed, and the ureters (Wolffian ducts) are of considerable length. Bufo cinereus, in the male of which the Müllerian ducts are very conspicuous, serves as an excellent example of this.
In the Salamander (Salamandra maculosa), Figs. 6 and 7, the process is carried out with greater completeness in the female than in the male, and this is the general rule in Amphibians. In the male Proteus, the embryonic condition would seem to be retained almost in its completeness so that the ducts of the kidney open directly and separately into the still persisting primitive duct of the kidney. The upper end of the duct nevertheless extends some distance beyond the end of the kidney and opens into the abdominal cavity. In the female Proteus, on the other hand, the separation into a Müllerian duct and a ureter is quite complete. The Newt (Triton) also serves as an excellent example of the formation of distinct Müllerian and Wolffian ducts being much more complete in the female than the male. In the female Newt all the tubules from the kidney open into a duct of some length which unites with the Müllerian duct near its termination, but in the male the anterior segmental tubes, including those which, as will be afterwards seen, serve as vasa efferentia of the testis, enter the Müllerian duct directly, while the posterior unite as in the female into a common duct before joining the Müllerian duct. For further details as to the variations exhibited in the Amphibians, the reader is referred to Leydig, Anat. Untersuchung, Fischen u. Reptilien. Ditto, Lehrbuch der Histologie, Menschen u. Thiere. Von Wittich, Siebold u. Kölliker, Zeitschrift, Vol. IV. p. 125.
The different conditions of completeness of the Wolffian ducts observable amongst the Amphibians are instructive in reference to the manner of development of the Wolffian duct in Selachians. The mode of division in the Selachians of the segmental duct of the kidney into a Müllerian and Wolffian [Pg 158] duct is probably to be looked upon as an embryonic abbreviation of the process by which these two ducts are formed in Amphibians. The fact that this separation into Müllerian and Wolffian ducts proceeds further in the females of most Amphibians than in the males, strikingly shews that it is the oviductal function of the Müllerian duct which is the indirect cause of its separation from the Wolffian duct. The Müllerian duct formed in the way described persists almost invariably in both sexes, and in the male sometimes functions as a sperm reservoir; e.g. Bufo cinereus. In the embryo it carries at its upper end the glandular mass described above (Kopfniere), but this generally atrophies, though remnants of it persist in the males of some species (e.g. Salamandra). Its anterior end opens, in most cases by a single opening, into the perivisceral cavity in both sexes, and is usually ciliated. As the female reaches maturity, the oviduct dilates very much; but it remains thin and inconspicuous in the male.
The only other developmental change of importance is the connection of the testes with the kidneys. This probably occurs in the same manner as in Selachians, viz. from the junction of the open ends of the segmental tubes with the follicles of the testes. In any case the vessels which carry off the semen constitute part of the kidney, and the efferent duct of the testis is also that of the kidney. The vasa efferentia from the testis either pass through one or two nearly isolated anterior portions of the kidney (Proteus, Triton) or else no such special portion of the kidney becomes separated from the rest, and the vasa efferentia enter the general body of the kidney.
* * * * *
In the male Amphibian, then, the urinogenital system consists of the following parts (Fig. 6):
(1) Rudimentary Müllerian ducts, opening anteriorly into the body-cavity, which sometimes carry aborted Kopfnieren.
(2) The partially or completely formed Wolffian ducts (ureters) which also serve as the ducts for the testes.
(3) The kidneys, parts of which also serve as the vasa efferentia, and whose secretion, together with the testicular products, is carried off by the Wolffian ducts.
[Pg 159] (4) The united lower parts of Wolffian and Müllerian ducts which are really the lower unsplit part of the segmental ducts of the kidneys.
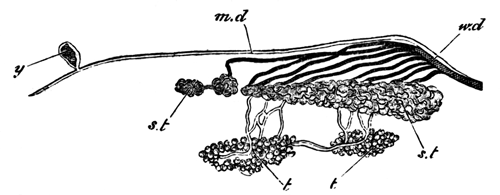
Fig. 6. Diagram of the Urinogenital Organs of a Male Salamander.
(Copied from Leydig's Histologie des Menschen u. der Thiere.)
md. Müller's duct (rudimentary); y. remnant of the secretory portion of the segmental duct Kopfniere; Wd. Wolffian duct; a less complete structure in the male than in the female; st. segmental tubes or kidney. The openings of these into the body-cavity are not inserted in the figure; t. testis. Its efferent ducts form part of the kidney.
In the female, there are (Fig. 7)
(1) The Müllerian ducts which function as the oviducts.
(2) The Wolffian ducts.
(3) The kidneys.
(4) The united Müllerian and Wolffian ducts as in the male.
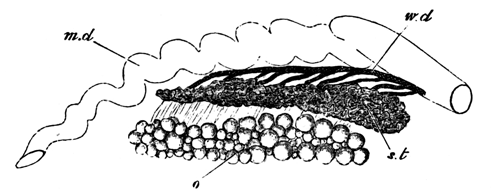
Fig. 7. Diagram of the Urinogenital Organs of a Female Salamander.
(Copied from Leydig's Histologie des Menschen u. der Thiere.)
Md. Müller's duct or oviduct; Wd. Wolffian duct or the duct of the kidneys; st. segmental tubes or kidney. The openings of these into the body-cavity are not inserted in the figure; o. ovary.
The urinogenital organs of the adult Amphibians agree in almost all essential particulars with those of Selachians. The [Pg 160] ova are carried off in both by a specialized oviduct. The Wolffian duct, or ureter, is found both in Selachians and Amphibians, and the relations of the testis to it are the same in both, the vasa efferentia of the testes having in both the same anatomical peculiarities.
The following points are the main ones in which Selachians and Amphibians differ as to the anatomy of the urinogenital organs; and in all but one of these, the organs of the Amphibian exhibit a less differentiated condition than do those of the Selachian.
(1) A glandular portion (Kopfniere) belonging to the first segmental organ (segmental duct of the kidneys) is found in all embryo Amphibians, but usually disappears, or only leaves a remnant in the adult. It has not yet been found in any Selachian.
(2) The division of the primitive duct of the kidney into the Müllerian duct and the Wolffian duct is not completed so far in Amphibians as Selachians, and in the former the two ducts are confluent at their lower ends.
(3) The permanent kidney exhibits in Amphibians no distinction into two glands (foreshadowing the Wolffian bodies and true kidneys of higher vertebrates), as it does in the Selachians.
(4) The Müllerian duct persists in its entirety in male Amphibians, but only its upper end remains in male Selachians.
(5) The openings of the segmental tubes into the body-cavity correspond in number with the vertebral segments in most Selachians, but are far more numerous than these in Amphibians. This is the chief point in which the Amphibian kidney is more differentiated than the Selachian.
* * * * *
The modifications in development which the urinogenital system has suffered in higher vertebrates (Sauropsida and Mammalia) are very considerable; nevertheless it appears to me to be possible with fair certainty to trace out the relationship of its various parts in them to those found in the Ichthyopsida. The development of urinogenital organs has been far more fully worked out for the bird than for any other member of the amniotic vertebrates; but, as far as we know, [Pg 161] there are no essential variations except in the later periods of development throughout the division. These later variations, concerning for the most part the external apertures of the various ducts, are so well known and have been so fully described as to require no notice here. The development of these parts in the bird will therefore serve as the most convenient basis for comparison.
In the bird the development of these parts begins by the appearance of a column of cells on the upper surface of the intermediate cell-mass (Fig. 8, W.d). As in Selachians, the intermediate cell-mass is a group of cells between the outer edge of the protovertebræ and the upper end of the body-cavity. The column of cells thus formed is the commencement of the duct of the Wolffian body. Its development is strikingly similar to that of the segmental duct of the kidney in Selachians. I shall attempt when I have given an account of the development of the Müllerian duct to speak of the relations between the Selachian duct and that of the bird.
Romiti (Archiv f. Micr. Anat. Vol. X.) has recently stated that the Wolffian duct develops as an involution from the body-cavity. The fact that the specimens drawn by Romiti to support this view are too old to determine such a point, and the inspection of a number of specimens made by my friend Mr Adam Sedgwick of Trinity College, who, at my request, has been examining the urinogenital organs of the fowl, have led me to the conclusion that Romiti is in error in differing from his predecessors as to the development of the Wolffian duct. The solid string of cells to form the Wolffian duct lies at first close to the epiblast, but, by the alteration in shape which the protovertebræ undergo and the general growth of cells around it, becomes gradually carried downwards till it lies close to the germinal epithelium which lines the body-cavity. While undergoing this change of position it also acquires a lumen, but ends blindly both in front and behind. Towards the end of the fourth day the Wolffian duct opens into a horn of the cloaca. The cells adjoining its inner border commence, as it passes down on the third day, to undergo histological changes, which, by the fourth day, result in the formation of a series of ducts and Malpighian tufts which form the mass of the Wolffian body[45]. [Pg 162]
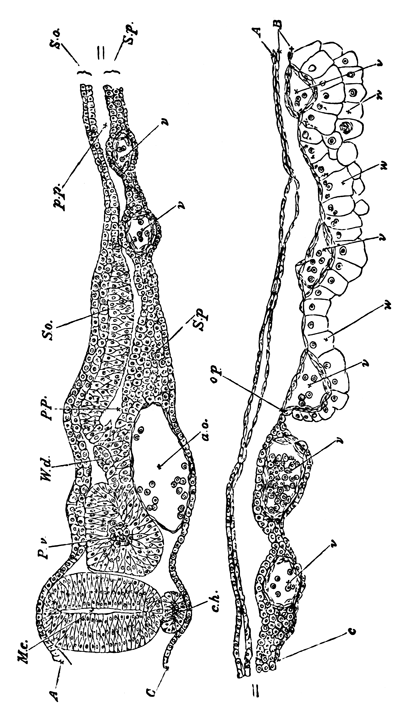
Fig. 8. Transverse section through the Dorsal region of an Embryo Fowl of 45 h. To shew the mode of Formation of the Wolffian Duct.
A. epiblast; B. mesoblast; C. hypoblast; M.c. medullary canal; Pv. Protovertebræ; W.d. Wolffian duct; so. Somatopleure; Sp. Splanchnopleure; pp. pleuro-peritoneal cavity; ch. notochord; ao. dorsal aorta; v. blood-vessels.
[Pg 163]The Müllerian duct arises in the form of an involution, whether at first solid or hollow, of the germinal epithelium, and, as I am satisfied, quite independently of the Wolffian duct. It is important to notice that its posterior end soon unites with the Wolffian duct, from which however it not long after becomes separated and opens independently into the cloaca. The upper end remains permanently open to the body-cavity, and is situated nearly opposite the extreme front end of the Wolffian body.
Between the 80th and 100th hour of incubation the ducts of the permanent kidneys begin to make their appearance. Near its posterior extremity each Wolffian duct becomes expanded, and from the dorsal side of this portion a diverticulum is constricted off, the blind end of which points forwards. This is the duct of the permanent kidneys, and around its end the kidneys are found. It is usually stated that the tubules of the permanent kidneys arise as outgrowths from the duct, but this requires to be worked over again.
The condition of the urinogenital system in birds immediately after the formation of the permanent kidneys is strikingly similar to its permanent condition in adult Selachians. There is the Müllerian duct in both opening in front into the body-cavity and behind into the cloaca. In both the kidneys consist of two parts—an anterior and posterior—which have been called respectively Wolffian bodies and permanent kidneys in birds and Leydig's glands and the kidneys in Selachians.
The duct of the permanent kidney, which at first opens into that of the Wolffian body, subsequently becomes further split off from the Wolffian duct, and opens independently into the cloaca.
[Pg 164] The subsequent changes of these parts are different in the two sexes.
In the female the Müllerian ducts[46] persist and become the oviducts. Their anterior ends remain open to the body-cavity. The changes in their lower ends in the various orders of Sauropsida and Mammalia are too well known to require repetition here. The Wolffian body and duct atrophy: there are left however in many cases slight remnants of the anterior extremity of the body forming the parovarium of the bird, and also frequently remnants of the posterior portion of the gland as well as of the duct. The permanent kidney and its duct remain unaltered.
In the male the Müllerian duct becomes almost completely obliterated. The Wolffian duct persists and forms the vas deferens, and the anterior so-called sexual portion of the Wolffian body also persists in an altered form. Its tubules unite with the seminiferous tubules, and also form the epididymis. Unimportant remnants of the posterior part of the Wolffian body also persist, but are without function. In both sexes the so-called permanent kidneys form the sole portion of the primitive uriniferous system which persists in the adult.
In considering the relations between the modes of development of the urinogenital organs of the bird and of the Selachians, the first important point to notice is, that whereas in the Selachians the segmental duct of the kidneys is first developed and subsequently becomes split into the Müllerian and Wolffian ducts; in the bird these two ducts develop independently. This difference in development would be accurately described by saying that in birds the segmental duct of the kidneys develops as in Selachians, but that the Müllerian duct develops independently of it.
Since in Selachians the Wolffian duct is equivalent to the segmental duct of the kidneys with the Müllerian removed from it, when in birds the Müllerian duct develops independently of the segmental kidney duct, the latter becomes the same as the Wolffian duct.
[Pg 165] The second mode of stating the difference in development in the two cases represents the embryological facts of the bird far better than the other method.
It explains why the Wolffian duct appears earlier than the Müllerian and not at the same time, as one might expect according to the other way of stating the case. If the Wolffian duct is equivalent to the segmental duct of Selachians, it must necessarily be the first duct to develop; and not improbably the development of the Müllerian duct would in birds be expected to occur at the time corresponding to that at which the primitive duct in Selachians became split into two ducts.
It probably also explains the similarity in the mode of development of the Wolffian duct in birds and the primitive duct of the kidneys in Selachians.
This way of stating the case is also in accordance with theoretical conclusions. As the egg-bearing function of the Müllerian duct became more and more confirmed we might expect that the adult condition would impress itself more and more upon the embryonic development, till finally the Müllerian duct ceased to be at any period connected with the kidneys, and the history of its origin ceased to be traceable in its development. This seems to have actually occurred in the higher vertebrates, so that the only persisting connection between the Müllerian duct and the urinary system is the brief but important junction of the two at their lower ends on the sixth or seventh day. This junction justly surprised Waldeyer (Eierstock u. Ei, p. 129), but receives a complete and satisfactory explanation on the hypothesis given above.
The original development of the segmental tubes is in the bird solely retained in the tubules of the Wolffian body arising independently of the Wolffian duct, and I have hitherto failed to find that there is a distinct division of the Wolffian bodies into segments corresponding with the vertebral segments.
I have compared the permanent kidneys to the lower portion of the kidneys of Selachians. The identity of the anatomical condition of the adult Selachian and embryonic bird which has been already pointed out speaks strongly in favour of this view; and when we further consider that the duct of [Pg 166] the permanent kidneys is developed in nearly the same way as the supposed homologous duct in Selachians, the suggested identity gains further support. The only difficulty is the fact that in Selachians the tubules of the part of the kidneys under comparison develop as segmental involutions in point of time anteriorly to their duct, while in birds they develop in a manner not hitherto certainly made out but apparently in point of time posteriorly to their duct. But when the immense modifications in development which the whole of the gland of the excretory organ has undergone in the bird are considered, I do not think that the fact I have mentioned can be brought forward as a serious difficulty.[TN5]
The further points of comparison between the Selachian and the bird are very simple. The Müllerian duct in its later stages behaves in the higher vertebrates precisely as in the lower. It becomes in fact the oviduct in the female and atrophies in the male. The behaviour of the Wolffian duct is also exactly that of the duct which I have called the Wolffian duct in Ichthyopsida, and in the tubules of the Wolffian body uniting with the tubuli seminiferi we have represented the junction of the segmental tubes with the testis in Selachians and Amphibians. It is probably this junction of two independent organs which led Waldeyer to the erroneous view that the tubuli seminiferi were developed from the tubules of the Wolffian body.
With the bird I conclude the history of the origin of the urinogenital system of vertebrates. I have attempted, and I hope succeeded, in tracing out by the aid of comparative anatomy and embryology the steps by which a series of independent and simple segmental organs like those of Annelids have become converted into the complicated series of glands and ducts which constitute the urinogenital system of the higher vertebrates. There are no doubt some points which require further elucidation amongst the Ganoid and Osseous fishes. The most important points which appear to me still to need further research, both embryological and anatomical, are the abdominal pores of fishes, the generative ducts of Ganoids, especially Lepidosteus, and the generative ducts of Osseous fishes.
[Pg 167] The only further point which requires discussion is the embryonic layer from which these organs are derived.
I have shewn beyond a doubt (loc. cit.) that in Selachians these organs are formed from the mesoblast. The unanimous testimony of all the recent investigators of Amphibians leads to the same conclusion. In birds, on the other hand, various investigators have attempted to prove that these organs are derived from the epiblast. The proof they give is the following: the epiblast and mesoblast appear fused in the region of the axis cord. From this some investigators have been led to the conclusion that the whole of the mesoblast is derived from the upper of the two primitive embryonic layers. To these it may be replied that, even granting their view to be correct, it is no proof of the derivation of the urinogenital organs from the epiblast, since it is not till the complete formation of the three layers that any one of them can be said to exist. Others look upon the fusion of the two layers as a proof of the passage of cells from the epiblast into the mesoblast. An assumption in itself, which however is followed by the further assumption that it is from these epiblast cells that the urinogenital system is derived! Whatever may have been the primitive origin of the system, its mesoblastic origin in vertebrates cannot in my opinion be denied.
Kowalewsky (Embryo. Stud. an Vermen u. Arthropoda, Mem. Akad. St Petersbourg, 1871) finds that the segmental tubes of Annelids develop from the mesoblast. We must therefore look upon the mesoblastic origin of the excretory system as having an antiquity greater even than that of vertebrates.
[26] From the Journal of Anatomy and Physiology, Vol. X. 1875.
[27] The more important of these are:—
Semper—Ueber die Stammverwandtschaft der Wirbelthiere u. Anneliden. Centralblatt f. Med. Wiss. 1874, No. 35.
Semper—Segmentalorgane bei ausgewachsenen Haien. Centralblatt f. Med. Wiss. 1874, No. 52.
Semper—Das Urogenitalsystem der höheren Wirbelthiere. Centralblatt f. Med. Wiss. 1874, No. 59.
Semper—Stammesverwandtschaft d. Wirbelthiere u. Wirbellosen. Arbeiten aus Zool. Zootom. Inst. Würzburg. II Band.
Semper—Bildung u. Wachstum der Keimdrüsen bei den Plagiostomen. Centralblatt f. Med. Wiss. 1875, No. 12.
Semper—Entw. d. Wolf. u. Müll. Gang. Centralblatt f. Med. Wiss. 1875, No. 29.
Alex. Schultz—Phylogenie d. Wirbelthiere. Centralblatt f. Med. Wiss. 1874, No. 51.
Spengel—Wimpertrichtern i. d. Amphibienniere. Centralblatt f. Med. Wiss. 1875, No. 23.
Meyer—Anat. des Urogenitalsystems der Selachier u. Amphibien. Sitzb. Naturfor. Gesellschaft. Leipzig, 30 April, 1875.
F. M. Balfour—Preliminary Account of development of Elasmobranch fishes. Quart. Journ. of Micro. Science, Oct. 1874. (This edition, Paper V. p. 60 et seq.)
W. Müller—Persistenz der Urniere bei Myxine glutinosa. Jenaische Zeitschrift, 1873.
W. Müller—Urogenitalsystem d. Amphioxus u. d. Cyclostomen. Jenaische Zeitschrift, 1875.
Alex. Götte—Entwicklungsgeschichte der Unke (Bombinator igneus).
[28] These organs were discovered independently by Professor Semper and myself. Professor Semper's preliminary account appeared prior to my own which was published (with illustrations) in the Quarterly Journal of Mic. Science. Owing to my being in South America, I did not know of Professor Semper's investigations till several months after the publication of my paper.
[29] These outgrowths are at first solid in both Pristiurus, Scyllium and Torpedo, but in Torpedo attain a considerable length before a lumen appears in them.
[30] This duct is often called either Müller's duct, the oviduct, or the duct of the primitive kidneys 'Urnierengang.' None of these terms are very suitable. A justification of the name I have given it will appear from the facts given in the later parts of this paper. In my previous paper I have always called it oviduct, a name which is very inappropriate.
[31] This splitting was first of all discovered and an account of it published by Semper (Centralblatt f. Med. Wiss. 1875, No. 29). I had independently made it out for the female a few weeks before the publication of Semper's account—but have not yet made observations about the point for the male.
My own previous account of the origin of the Wolffian duct (Quart. Journ. of Micros. Science, Oct. 1874, and this edition, Paper V.), is completely false, and was due to my not having had access to a complete series of my sections when I wrote the paper.
[32] Sitzun.[TN4] der Naturfor. Gesellschaft, Leipzig, 30 April, 1875.
[33] We owe to Professor Semper the discovery of the arrangement of the seminal ducts. Centralblatt f. Med. Wiss. 1875, No. 12.
[34] This upper portion of the kidneys is called Leydig's gland by Semper. It would be better to call it the Wolffian body, for I shall attempt to shew that it is homologous with the gland so named in Sauropsida and Mammalia.
[35] Further study of my sections has shewn me that the initial independence of these organs is even more complete than might be gathered from the description in my paper (loc. cit.). I now find, as I before conjectured, that they at first correspond exactly with the muscle-plates, there being one for each muscle-plate. This can be seen in the fresh embryos, but longitudinal sections shew it in an absolutely demonstrable manner.
[36] I am unfortunately only acquainted with Dr Rosenberg's paper from an abstract.
[37] For this specimen I am indebted to Dr Günther.
[38] According to Müller (Myxinoiden, 1845) there is in Myxine an abdominal pore with two short canals leading into it, and Vogt and Pappenheim (An. Sci. Nat. Part IV. Vol. XI.) state that in Petromyzon there are two such pores, each connected with a short canal.
[39] My own rough examination of preserved specimens was hardly sufficient to enable me to determine for certain the presence or absence of these pores. Mr Bridge, of Trinity College, has, however, since then commenced a series of investigations on this point, and informs me that these pores are certainly absent in Scyllium as well as in other genera.
[40] The description of the attachment of the vas deferens to the testis in the Carp given by Vogt and Pappenheim (Ann. Scien. Nat. 1859) does not agree with what I found in the Perch (Perca fluvialis). The walls of the duct are in the Perch continuous with the investment of the testis, and the gland of the testis occupies, as it were, the greater part of the duct; there is, however, a distinct cavity corresponding to what Vogt and P. call the duct, near the border of attachment of the testis into which the seminal tubules open. I could find at the posterior end of the testis no central cavity which could be distinguished from the cavity of this duct.
[41] This is mentioned by Müller (Ganoid fishes, Berlin Akad. 1844), Hyrtl (loc. cit.), and Günther (loc. cit.), and through the courtesy of Dr Günther I have had an opportunity of confirming the fact of the presence of the abdominal pores on two specimens of Lepidosteus in the British Museum.
[42] My account of the development of these parts in Amphibians is derived for the most part from Götte, Die Entwicklungsgeschichte der Unke.
[43] It is called Kopfniere (head-kidney), or Urniere (primitive kidney), by German authors. Leydig correctly looks upon it as together with the permanent kidney constituting the Urniere of Amphibians. The term Urniere is one which has arisen in my opinion from a misconception; but certainly the Kopfniere has no greater right to the appellation than the remainder of the kidney.
[44] In Bombinator igneus, Von Wittich stated that the embryonic condition was retained. Leydig, Anatom. d. Amphib. u. Reptilien, shewed that this is not the case, but that in the male the Müllerian duct is very small, though distinct.
[45] This account of the origin of the Wolffian body differs from that given by Waldeyer, and by Dr Foster and myself (Elements of Embryology, Foster and Balfour), but I have been led to alter my view from an inspection of Mr Sedgwick's preparations, and I hope to shew that theoretical considerations lead to the expectation that the Wolffian body would develop independently of the duct.
[46] The right oviduct atrophies in birds, and the left alone persists in the adult.
With Plates 22 and 23.
In the course of an inquiry into the development of Elasmobranch Fishes, my attention has recently been specially directed to the first appearance and early stages of the spinal nerves, and I have been led to results which differ so materially from those of former investigators, that I venture at once to lay them before the Society. I have employed in my investigations embryos of Scyllium canicula, Scyllium stellare, Pristiurus, and Torpedo. The embryos of the latter animal, especially those hardened in osmic acid, have proved by far the most favourable for my purpose, though, as will be seen from the sequel, I have been able to confirm the majority of my conclusions on embryos of all the above-mentioned genera.
A great part of my work was done at the Zoological Station founded by Dr Dohrn at Naples; and I have to thank both Dr Dohrn and Dr Eisig for the uniformly obliging manner in which they have met my requirements for investigation. I have more recently been able to fill up a number of lacunæ in my observations by the study of embryos bred in the Brighton Aquarium; for these I am indebted to the liberality of Mr Lee and the directors of that institution.
The first appearance of the Spinal Nerves in Pristiurus.
In a Pristiurus-embryo, at the time when two visceral clefts become visible from the exterior (though there are as yet [Pg 169] no openings from without into the throat), a transverse section through the dorsal region exhibits the following features (Pl. 22, fig. A):—
The external epiblast is formed of a single row of flattened elongated cells. Vertically above the neural canal the cells of this layer are more columnar, and form the rudiment of the primitively continuous dorsal fin.
The neural canal (nc) is elliptical in section, and its walls are composed of oval cells two or three deep. The wall at the two sides is slightly thicker than at the ventral and dorsal ends, and the cells at the two ends are also smaller than elsewhere. A typical cell from the side walls of the canal is about 1/1900 inch in its longest diameter. The outlines of the cells are for the most part distinctly marked in the specimens hardened in either chromic or picric acid, but more difficult to see in those prepared with osmic acid; their protoplasm is clear, and in the interior of each is an oval nucleus very large in proportion to the size of its cell. The long diameter of a typical nucleus is about 1/3000 inch, or about two-thirds of that of the cell.
The nuclei are granular, and very often contain several especially large and deeply stained granules; in other cases only one such is present, which may then be called a nucleolus.
In sections there may be seen round the exterior of the neural tube a distinct hyaline membrane: this becomes stained of a brown colour with osmic acid, and purple or red with hæmatoxylin or carmine respectively. Whether it is to be looked upon as a distinct membrane differentiated from the outermost portion of the protoplasm of the cells, or as a layer of albumen coagulated by the reagents applied, I am unable to decide for certain. It makes its appearance at a very early period, long before that now being considered; and similar membranes are present around other organs as well as the neural tube. The membrane is at this stage perfectly continuous round the whole exterior of the neural tube as well on the dorsal surface as on the ventral.
The section figured, whose features I am describing, belongs to the middle of the dorsal region. Anteriorly to this point the spinal cord becomes more elliptical in section, and the spinal canal more lanceolate; posteriorly, on the other hand, the spinal [Pg 170] canal and tube become more nearly circular in section. Immediately beneath the neural tube is situated the notochord (ch). It exhibits at this stage a central area rich in protoplasm, and a peripheral layer very poor in protoplasm; externally it is invested by a distinct cuticular membrane.
Beneath the notochord is a peculiar rod of cells, constricted from the top of the alimentary canal[48]. On each side and below this are the two aortæ, just commencing to be formed, and ventral to these is the alimentary canal.
On each side of the body two muscle-plates are situated; their upper ends reach about one-third of the way up the sides of the neural tube. The two layers which together constitute the muscle-plates are at this stage perfectly continuous with the somatic and splanchnic layers of the mesoblast, and the space between the two layers is continuous with the body-cavity. In addition to the muscle-plates and their ventral continuations, there are no other mesoblast-cells to be seen. The absence of all mesoblastic cells dorsal to the superior extremities of the muscles is deserving of special notice.
Very shortly after this period and, as a rule, before a third visceral cleft has become visible, the first traces of the spinal nerves make their appearance.
First Stage.—The spinal nerves do not appear at the same time along the whole length of the spinal canal, but are formed first of all in the neck and subsequently at successive points posterior to this.
Their mode of formation will be most easily understood by referring to Pl. 22, figs. B I, B II, B III, which are representations of three sections taken from the same embryo. B I is from the region of the heart; B II belongs to a part of the body posterior to this, and B III to a still posterior region.
In most points the sections scarcely differ from Pl. 22, fig. A, which, indeed, might very well be a posterior section of the embryo to which these three sections belong.
The chief point, in addition to the formation of the spinal nerves, which shews the greater age of the embryo from which the sections were taken is the complete formation of the aortæ.
[Pg 171] The upper ends of the muscle-plates have grown no further round the neural canal than in fig. A, and no scattered mesoblastic connective-tissue cells are visible.
In fig. A the dorsal surface of the neural canal was as completely rounded off as the ventral surface; but in fig. B III this has ceased to be the case. The cells at the dorsal surface of the neural canal have become rounder and smaller and begun to proliferate, and the uniform outline of the neural canal has here become broken (fig. B III, pr). The peculiar membrane completely surrounding the canal in fig. A now terminates just below the point where the proliferation of cells is taking place.
The prominence of cells which springs in this way from the top of the neural canal is the commencing rudiment of a pair of spinal nerves. In fig. B II, a section anterior to fig. B III, this formation has advanced much further (fig. B II, pr). From the extreme top of the neural canal there have now grown out two club-shaped masses of cells, one on each side; they are perfectly continuous with the cells which form the extreme top of the neural canal, and necessarily also are in contact with each other dorsally. Each grows outwards in contact with the walls of the neural canal; but, except at the point where they take their origin, they are not continuous with its walls, and are perfectly well separated by a sharp line from them.
In fig. B I, though the club-shaped processes still retain their attachment to the summit of the neural canal, they have become much longer and more conspicuous.
Specimens hardened in both chromic acid (Pl. 22, fig. C) and picric acid give similar appearances as to the formation of these bodies.
In those hardened in osmic acid, though the mutual relations of the masses of cells are very clear, yet it is difficult to distinguish the outlines of the individual cells.
In the chromic acid specimens (fig. C) the cells of these rudiments appear rounded, and each of them contains a large nucleus.
I have been unable to prepare longitudinal sections of this stage, either horizontal or vertical, to shew satisfactorily the extreme summit of the spinal cord; but I would call attention [Pg 172] to the fact that the cells forming the proximal portion of the outgrowth are seen in every transverse section at this stage, and therefore exist the whole way along, whereas the distal portion is seen only in every third or fourth section, according to the thickness of the sections. It may be concluded from this that there appears a continuous outgrowth from the spinal canal, from which discontinuous processes grow out.
In specimens of a very much later period (Pl. 23, fig. I) the proximal portions of the outgrowth are unquestionably continuous with each other, though their actual junctions with the spinal cord are very limited in extent. The fact of this continuity at a later period is strongly in favour of the view that the posterior branches of the spinal nerves arise from the first as a continuous outgrowth of the spinal cord, from which a series of distal processes take their origin. I have, however, failed to demonstrate this point absolutely. The processes, which we may call the nerve-rudiments, are, as appears from the later stages, equal in number to the muscle-plates.
It may be pointed out, as must have been gathered from the description above, that the nerve-rudiments have at this stage but one point of attachment to the spinal cord, and that this one corresponds with the dorsal or posterior root of the adult nerve.
The rudiments are, in fact, those of the posterior root only.
The next or second stage in the formation of these structures to which I would call attention occurs at about the time when three to five visceral clefts are present. The disappearance from the notochord in the anterior extremity of the body of a special central area rich in protoplasm serves as an excellent guide to the commencement of this epoch.
Its investigation is beset with far greater difficulties than the previous one. This is owing partly to the fact that a number of connective-tissue cells, which are only with great difficulty to be distinguished from the cells which compose the spinal nerves, make their appearance around the latter, and partly to the fact that the attachment of the spinal nerves to the neural canal becomes much smaller, and therefore more difficult to study.
Fortunately, however, in Torpedo these peculiar features [Pg 173] are not present to nearly the same extent as in Pristiurus and Scyllium.
The connective-tissue cells, though they appear earlier in Torpedo than in the two other genera, are much less densely packed, and the large attachment of the nerves to the neural canal is retained for a longer period.
Under these circumstances I consider it better, before proceeding with this stage, to give a description of the occurrences in Torpedo, and after that to return to the history of the nerves in the genera Pristiurus and Scyllium.
The development of the Spinal Nerves in Torpedo.
The youngest Torpedo-embryo in which I have found traces of the spinal nerves belongs to the earliest part of what I called the second stage.
The segmental duct[49] is just appearing, but the cells of the notochord have not become completely vacuolated. The rudiments of the spinal nerves extend half of the way towards the ventral side of the spinal cord; they grow out in a most distinct manner from the dorsal surface of the spinal cord (Pl. 22, fig. D a, pr); but the nerve-rudiments of the two sides are no longer continuous with each other at the dorsal median line, as in the earlier Pristiurus-embryos. The cells forming the proximal portion of the rudiment have the same elongated form as the cells of the spinal cord, but the remaining cells are more circular.
From the summit of the muscle-plates (mp) an outgrowth of connective tissue has made its appearance (c), which eventually fills up the space between the dorsal surface of the cord and the external epiblast. There is not the slightest difficulty in distinguishing the connective-tissue cells from the nerve-rudiment. I believe that in this embryo the origin of the nerves from the neural canal was a continuous one, though naturally the peripheral ends of the nerve-rudiments were separate from each other.
The most interesting feature of the stage is the commencing formation of the anterior roots. Each of these arises (Pl. 22, [Pg 174] fig. D a, ar) as a small but distinct outgrowth from the epiblast of the spinal cord, near the ventral corner of which it appears as a conical projection. Even from the very first it has an indistinct form of termination and a fibrous appearance, while the protoplasm of which it is composed becomes very attenuated towards its termination.
The points of origin of the anterior roots from the spinal cord are separated from each other by considerable intervals. In this fact, and also in the nerves of the two sides never being united with each other in the ventral median line, the anterior roots exhibit a marked contrast to the posterior.
There exists, then, in Torpedo-embryos by the end of this stage distinct rudiments of both the anterior and posterior roots of the spinal nerves. These rudiments are at first quite independent of and disconnected with each other, and both take their rise as outgrowths of the epiblast of the neural canal.
The next Torpedo-embryo (Pl. 22, fig. D b), though taken from the same female, is somewhat older than the one last described. The cells of the notochord are considerably vacuolated; but the segmental duct is still without a lumen. The posterior nerve-rudiments are elongated, pear-shaped bodies of considerable size, and, growing in a ventral direction, have reached a point nearly opposite the base of the neural canal. They still remain attached to the top of the neural canal, though the connexion has in each case become a pedicle so narrow that it can only be observed with great difficulty.
It is fairly certain that by this stage each posterior nerve-rudiment has its own separate and independent junction with the spinal cord; their dorsal extremities are nevertheless probably connected with each other by a continuous commissure.
The cells composing the rudiments are still round, and have, in fact, undergone no important modifications since the last stage.
The important feature of the section figured (fig. D b), and one which it shares with the other sections of the same embryo, is the appearance of connective-tissue cells around the nerve-rudiment. These cells arise from two sources; one of these is supplied by the vertebral rudiments, which at the end of [Pg 175] the last stage (Pl. 22, fig. C, vr) become split off from the inner layer of the muscle-plates. The vertebral rudiments have in fact commenced to grow up on each side of the neural canal, in order to form the mass of cells out of which the neural arches are subsequently developed.
The dorsal extremities of the muscle-plates form the second source of these connective-tissue cells. These latter cells lie dorsal and external to the nerve-rudiments.
The presence of this connective tissue, in addition to the nerve-rudiments, removes the possibility of erroneous interpretations in the previous stages of the Pristiurus-embryo.
It might be urged that the two masses which I have called nerve-rudiments are nothing else than mesoblastic connective tissue commencing to develop around the neural canal, and that the appearance of attachment to the neural canal which they present is due to bad preparation or imperfect observation. The sections of both this and the last Torpedo-embryo which I have been describing clearly prove that this is not the case. We have, in fact, in the same sections the developing connective tissue as well as the nerve-rudiments, and at a time when the latter still retains its primitive attachment to the neural canal. The anterior root (fig. D b, ar) is still a distinct conical prominence, but somewhat larger than in the previously described embryo; it is composed of several cells, and the cells of the spinal cord in its neighbourhood converge towards its point of origin.
In a Torpedo-embryo (Pl. 22, fig. D c) somewhat older than the one last described, though again derived from the oviduct of the same female, both the anterior and the posterior rudiments have made considerable steps in development.
In sections taken from the hinder part of the body I found that the posterior rudiments nearly agreed in size with those in fig. D b.
It is, however, still less easy than there to trace the junction of the posterior rudiments with the spinal cord, and the upper ends of the rudiments of the two sides do not nearly meet.
In a considerable series of sections I failed to find any case [Pg 176] in which I could be absolutely certain that a junction between the nerve and the spinal cord was effected; and it is possible that in course of the change of position which this junction undergoes there may be for a short period a break of continuity between the nerve and the cord. This, however, I do not think probable. But if it takes place at all, it takes place before the nerve becomes functionally active, and so cannot be looked upon as possessing any physiological significance.
The rudiment of the posterior nerve in the hinder portion of the body is still approximately homogeneous, and no distinction of parts can be found in it.
In the same region of the body the anterior rudiment retains nearly the same condition as in the previous stage, though it has somewhat increased in size.
In the sections taken from the anterior part of the same embryo the posterior rudiment has both grown in size and also commenced to undergo histological changes by which it has become divided into a root, a ganglion, and a nerve.
The root (fig. D c, pr) consists of small round cells which lie close to the spinal cord, and ends dorsally in a rounded extremity.
The ganglion (g) consists of larger and more elongated cells, and forms an oval mass enclosed on the outside by the downward continuation of the root, having its inner side nearly in contact with the spinal cord.
From its ventral end is continued the nerve, which is of considerable length, and has a course approximately parallel to that of the muscle-plate. It forms a continuation of the root rather than of the ganglion.
Further details in reference to the histology of the nerve-rudiment at this stage are given later in this paper, in the description of Pristiurus-embryos, of which I have a more complete series of sections than of the Torpedo-embryos.
When compared with the nerve-rudiment in the posterior part of the same embryo, the nerve-rudiment last described is, in the first place, considerably larger, and has secondly undergone changes, so that it is possible to recognize in it parts which can be histologically distinguished as nerve and ganglion.
The developmental changes which have taken place in the [Pg 177] anterior root are not less important than those in the posterior. The anterior root now forms a very conspicuous cellular prominence growing out from the ventral corner of the spinal cord (fig. D c, ar). It has a straight course from the spinal cord to the muscle-plate, and there shews a tendency to turn downwards at an open angle: this, however, is not represented in the specimen figured. The cells of which it is composed each contain a large oval nucleus, and are not unlike the cells which form the posterior rudiment. The anterior and posterior nerves are still quite unconnected with each other; and in those sections in which the anterior root is present the posterior root of the same side is either completely absent or only a small part is to be seen. The cells of the spinal cord exhibit a slight tendency to converge towards the origin of the anterior nerve-root.
In the spinal cord itself the epithelium of the central canal is commencing to become distinguished from the grey matter, but no trace of the white matter is visible.
I have succeeded in making longitudinal vertical sections of this stage, which prove that the ends of the posterior roots adjoining the junction with the cord are all connected with each other (Pl. 22, fig. D d).
If the figure representing a transverse section of the embryo (fig. D c) be examined, or better still the figure of a section of the slightly older Scyllium-embryo (Pl. 23, fig. HI or II), the posterior root will be seen to end dorsally in a rounded extremity, and the junction with the spinal cord to be effected, not by the extremity of the nerve, but by a part of it at some little distance from this.
It is from these upper ends of the rudiments beyond the junction with the spinal cord that I believe the commissures to spring which connect together the posterior roots.
My sections shewing this for the stage under consideration are not quite as satisfactory as is desirable; nevertheless they are sufficiently good to remove all doubt as to the presence of these commissures.
A figure of one of these sections is represented (Pl. 22, fig. D d). In this figure pr points to the posterior roots and x to the commissures uniting them.
[Pg 178] In a stage somewhat subsequent to this I have succeeded in making longitudinal sections, which exhibit these junctions with a clearness which leaves nothing to be desired.
It is there effected (Pl. 23, fig. L) in each case by a protoplasmic commissure with imbedded nuclei[50]. Near its dorsal extremity each posterior root dilates, and from the dilated portion is given off on each side the commissure uniting it with the adjoining roots.
Considering the clearness of this formation in this embryo, as well as in the embryo belonging to the stage under description, there cannot be much doubt that at the first formation of the posterior rudiments a continuous outgrowth arises from the spinal cord, and that only at a later period do the junctions of the roots with the cord become separated and distinct for each nerve.
I now return to the more complete series of Pristiurus-embryos, the development of whose spinal nerves I have been able to observe.
Second Stage of the Spinal Nerves in Pristiurus.
In the youngest of these (Pl. 22, fig. E) the notochord has undergone but very slight changes, but the segmental duct has made its appearance, and is as much developed as in the Torpedo-embryo from which fig. D b was taken.
(The embryo from which fig. E a was derived had three visceral clefts.)
There have not as yet appeared any connective-tissue cells dorsal to the top of the muscle-plates, so that the posterior nerve-rudiments are still quite free and distinct.
The cells composing them are smaller than the cells of the neural canal; they are round and nucleated; and, indeed, in their histological constitution the nerve-rudiments exhibit no important deviations from the previous stage, and they have hardly increased in size. In their mode of attachment to the neural tube an important change has, however, already commenced to be visible.
In the previous stage the two nerve-rudiments met above the [Pg 179] summit of the spinal cord and were broadly attached to it there; now their points of attachment have glided a short distance down the sides of the spinal cord[51].
The two nerve-rudiments have therefore ceased to meet above the summit of the canal; and in addition to this they appear in section to narrow very much before becoming united with its walls, so that their junctions with these appear in a transverse section to be effected by at most one or two cells, and are, comparatively speaking, very difficult to observe.
In an embryo but slightly older than that represented in Fig. E a the first rudiment of the anterior root becomes visible. This appears, precisely as in Torpedo, in the form of a small projection from the ventral corner of the spinal cord (fig. E b, ar).
The second step in this stage (Pl. 22, fig. F) is comparable, as far as the connective-tissue is concerned, with the section of Torpedo (Pl. 22, fig. D d). The notochord (the histological details of whose structure are not inserted in this figure) is rather more developed, and the segmental duct, as was the case with the corresponding Torpedo-embryo, has become hollow at its anterior extremity.
The embryo from which the section was taken possessed five visceral clefts, but no trace of external gills.
In the section represented, though from a posterior part of the body, the dorsal nerve-rudiments have become considerably larger than in the last embryo; they now extend beyond the base of the neural canal. They are surrounded to a great extent by mesoblastic tissue, which, as in the case of the Torpedo, takes its origin from two sources, (1) from the commencing vertebral bodies, (2) from the summits of the muscle-plates.
It is in many cases very difficult, especially with chromic-acid specimens, to determine with certainty the limits of the rudiments of the posterior root.
[Pg 180] In the best specimens a distinct bordering line can be seen, and it is, as a rule, possible to state the characters by which the cells of the nerve-rudiments and vertebral bodies differ. The more important of these are the following:—(1) The cells of the nerve-rudiment are distinctly smaller than those of the vertebral rudiment; (2) the cells of the nerve-rudiment are elongated, and have their long axis arranged parallel to the long axis of the nerve-rudiment, while the cells surrounding them are much more nearly circular.
The cells of the nerve-rudiment measure about 1/1600 × 1/4500 to 1/1600 × 1/3200 inch, those of the vertebral rudiment 1/1600 × 1/1900 inch. The greater difficulty experienced in distinguishing the nerve-rudiment from the connective-tissue in Pristiurus than in Torpedo arises from the fact that the connective-tissue is much looser and less condensed in the latter than in the former.
The connective-tissue cells which have grown out from the muscle-plates form a continuous arch over the dorsal surface of the neural tube (vide Pl. 22, fig. F): and in some specimens it is difficult to see whether the arch is formed by the rudiment of the posterior root or by connective-tissue. It is, however, quite easy with the best specimens to satisfy one's self that it is from the connective-tissue, and not the nerve-rudiment, that the dorsal investment of the neural canal is derived.
As in the previous case, the upper ends of each pair of posterior nerve-rudiments are quite separate from one another, and appear in sections to be united by a very narrow root to the walls of the neural canal at the position indicated in fig. F[52].
The cells forming the nerve-rudiments have undergone slight modifications; they are for the most part more distinctly elongated than in the earlier stage, and appear slightly smaller in comparison with the cells of the neural canal.
They possess as yet no distinctive characters of nerve-cells. They stain more deeply with osmic acid than the cells around them, but with hæmatoxylin there is but a very slight difference in intensity between their colouring and that of the neighbouring connective-tissue cells.
The anterior roots have grown considerably in length, but [Pg 181] their observation is involved in the same difficulties with chromic-acid specimens as that of the posterior rudiments.
There is a further difficulty in observing the anterior roots, which arises from the commencing formation of white matter in the cord. This is present in all the anterior sections of the embryo from which fig. F is taken. When the white matter is formed the cells constituting the junction of the anterior nerve-root with the spinal cord undergo the same changes as the cells which are being converted into the white matter of the cord, and become converted into nerve-fibres; these do not stain with hæmatoxylin, and thus an apparent space is left between the nerve-root and the spinal cord. This space by careful examination may be seen to be filled up with fibres. In osmic acid sections, although even in these the white matter is stained less deeply than the other tissues, it is a matter of comparative ease to observe the junction between the anterior nerve root and the spinal cord.
I have been successful in preparing satisfactory longitudinal sections of embryos somewhat older than that shewn in fig. F, and they bring to light several important points in reference to the development of the spinal nerves. Three of these sections are represented in Pl. 22, figs. G1, G2, and G3.
The sections are approximately horizontal and longitudinal. G1 is the most dorsal of the three; it is not quite horizontal though nearly longitudinal. The section passes exactly through the point of attachment of the posterior roots to the walls of the neural canal.
The posterior rudiments appear as slight prominences of rounded cells projecting from the wall of the neural canal. From transverse sections the attachment of the nerves to the wall of the neural canal is proved to be very narrow, and from these sections it appears to be of some length in the direction of the long axis of the embryo. A combination of the sections taken in the two directions leads to the conclusion that the nerves at this stage thin out like a wedge before joining the spinal cord.
The independent junctions of the posterior rudiments with the spinal cord at this stage are very clearly shewn, though the rudiments are probably united with each other just dorsal to their junction with the spinal cord.
[Pg 182] The nerves correspond in number with the muscle-plates, and each arises from the spinal cord, nearly opposite the middle line of the corresponding muscle-plates (figs. G1 and G2).
Each nerve-rudiment is surrounded by connective-tissue cells, and is separated from its neighbours by a considerable interval.
At its origin each nerve-rudiment lies opposite the median portion of a muscle-plate (figs. G1 and G2); but, owing to the muscle-plate acquiring an oblique direction, at the level of the dorsal surface of the notochord it appears in horizontal sections more nearly opposite the interval between two muscle-plates (figs. G2 and G3).
In horizontal sections I find masses of cells which make their appearance on a level with the ventral surface of the spinal cord. I believe I have in some sections successfully traced these into the spinal cord, and I have little doubt that they are the anterior roots of the spinal nerves; they are opposite the median line of the muscle-plates, and do not appear to join the posterior roots (vide fig. G3, ar).
At the end of this period or second stage the main characters of the spinal nerves in Pristiurus are the following:—
(1) The posterior nerve-rudiments form somewhat wedge-shaped masses of tissue attached dorsally to the spinal cord.
(2) The cells of which they are composed are typical undifferentiated embryonic cells, which can hardly be distinguished from the connective-tissue cells around them.
(3) The nerves of each pair no longer meet above the summit of the spinal canal, but are independently attached to its sides.
(4) Their dorsal extremities are probably united by commissures.
(5) The anterior roots have appeared; they form small conical projections from the ventral corner of the spinal cord, but have no connexion with the posterior rudiments.
The Third Stage of the Spinal Nerves in Pristiurus.
With the third stage the first distinct histological differentiations of the nerve-rudiments commence. Owing to the [Pg 183] changes both in the nerves themselves and in the connective-tissue around them, which becomes less compact and its cells stellate, the difficulty of distinguishing the nerves from the surrounding cells vanishes; and the difficulties of investigation in the later stages are confined to the modes of attachment of the nerves to the neural canal, and the histological changes which take place in the rudiments themselves.
The stage may be considered to commence at the period when the external gills first make their appearance as small buds from the walls of the visceral clefts. Already, in the earliest rudiments of the posterior root of this period now figured, a number of distinct parts are visible (Pl. 23, fig. H I).
Surrounding nearly the whole structure there is present a delicate investment similar to that which I mentioned as surrounding the neural canal and other organs; it is quite structureless, but becomes coloured with all staining reagents. I must again leave open the question whether it is to be looked upon as a layer of coagulated protoplasm or as a more definite structure. This investment completely surrounds the proximal portion of the posterior root, but vanishes near its distal extremity.
The nerve-rudiment itself may be divided into three distinct portions:—(1) the proximal portion, in which is situated the pedicle of attachment to the wall of the neural canal; (2) an enlarged portion, which may conveniently, from its future fate, be called the ganglion; (3) a distal portion beyond this. The proximal portion presents a fairly uniform diameter, and ends dorsally in a rounded expansion; it is attached remarkably enough, not by its extremity, but by its side, to the spinal cord. The dorsal extremities of the posterior nerves are therefore free; as was before mentioned, they probably serve as the starting-point of the longitudinal commissures between the posterior roots.
The spinal cord at this stage is still made up of fairly uniform cells, which do not differ in any important particulars from the cells which composed it during the last stage. The outer portion of the most peripheral layer of cells has already begun to be converted into the white matter.
The delicate investment spoken of before still surrounds the [Pg 184] whole spinal cord, except at the points of junction of the cord with the nerve-rudiments. Externally to this investment, and separated from it for the most part by a considerable interval, a mesoblastic sheath (Pl. 23, fig. H I, i) for the spinal cord is beginning to be formed.
The attachment of the nerve-rudiments to the spinal cord, on account of its smallness, is [TN6] still very difficult to observe. In many specimens where the nerve is visible a small prominence may be seen rising up from the spinal cord at a point corresponding to x (Pl. 23, fig. H I). It is, however, rare to see this prominence and the nerve continuous with each other: as a rule they are separated by a slight space, and frequently one of the cells of the mesoblastic investment of the spinal cord is interposed between the two. In some especially favourable specimens, similar to the one figured, there can be seen a distinct cellular prominence (fig. H I, x) from the spinal cord, which becomes continuous with a small prominence on the lateral border of the nerve-rudiment near its free extremity. The absence of a junction between the two in a majority of sections is only what might be expected, considering how minute the junction is.
Owing to the presence of the commissure connecting the posterior roots, some part of a nerve is present in every section.
The proximal extremity of the nerve-rudiment itself is composed of cells, which, by their smaller size and a more circular form, are easily distinguished from cells forming the ganglionic portion of the nerve.
The ganglionic portion of the nerve, by its externally swollen configuration, is at once recognizable in all the sections in which the nerve is complete. The delicate investment before mentioned is continuous around it. The cells forming it are larger and more elongated than the cells forming the upper portion of the nerve-rudiment: each of them possesses a large and distinct nucleus.
The remainder of the nerve rudiment forms the commencement of the true nerve. It can in this stage be traced only for a very small distance, and gradually fades away, in such a manner that its absolute termination is very difficult to observe.
The connective-tissue cells which surround the nerve-rudiment [Pg 185] are far looser than in the last stage, and are commencing to throw out processes and become branched.
The anterior root-nerve has grown very considerable since the last stage. It projects from the same region of the cord as before, but on approaching the muscle-plate takes a sudden bend downwards (fig. H II, ar).
I have failed to prove that the anterior and posterior roots are at this stage united.
Fourth Stage.
In an embryo but slightly more advanced than the one last described, important steps have been made in the development of the nerve-rudiment. The spinal cord itself now possesses a covering of white matter; this is thickest at the ventral portion of the cord, and extends to the region of the posterior root of the spinal nerve.
The junction of the posterior root with the spinal cord is easier to observe than in the last stage.
It is still effected by means of unaltered cells, though the cells which form the projection from the cord to the nerve are commencing to undergo changes similar to those of the cells which are being converted into white matter.
In the rudiment of the posterior root itself there are still three distinct parts, though their arrangement has undergone some alteration and their distinctness has become more marked (Pl. 23, fig. I I).
The root of the nerve (fig. I I, pr) consists, as before, of nearly circular cells, each containing a nucleus, very large in proportion to the size of the cell. The cells have a diameter of about 1/3000 of an inch. This mass forms not only the junction between the ganglion and the spinal canal, but is also continued into a layer investing the outer side of the ganglion and continuous with the nerve beyond the ganglion.
The cells which compose the ganglion (fig. I I, sp.g) are easily distinguished from those of the root. Each cell is elongated with an oval nucleus, large in proportion to the cell; and its protoplasm appears to be continued into an angular, not to say fibrous process, sometimes at one and more rarely at [Pg 186] both ends. The processes of the cells are at this stage very difficult to observe: figs. I a, I b, I c represent three cells provided with them and placed in the positions they occupied in the ganglion.
The relatively very small amount of protoplasm in comparison to the nucleus is fairly represented in these figures, though not in the drawing of the ganglion as a whole. In the centre of each nucleus is a nucleolus.
Fig. I b, in which the process points towards the root of the nerve, I regard as a commencing nerve-fibre: its more elongated shape seems to imply this. In the next stage special bundles of nerve-fibres become very conspicuous in the ganglion. The long diameter of an average ganglion-cell is about 1/1600 of an inch. The whole ganglion forms an oval mass, well separated both from the nerve-root and the nerve, and is not markedly continuous with either. On its outer side lies the downward process of the nerve-root before mentioned.
The nerve itself is still, as in the last case, composed of cells which are larger and more elongated than either the cells of the root or the ganglion.
The condition of the anterior root at this stage is hardly altered from what it was; it is composed of very small cells, which with hæmatoxylin stain more deeply than any other cell of the section. A figure of it is given in I II.
Horizontal longitudinal sections of this stage are both easy to make and very instructive. On Pl. 23, fig. K I is represented a horizontal section through a plane near the dorsal surface of the spinal cord: each posterior root is seen in this section to lie nearly opposite the anterior extremity of a muscle-plate.
In a more ventral plane (fig. K II) this relation is altered, and the posterior roots lie opposite the hinder parts of the muscle-plates.
The nerves themselves are invested by the hyaline membrane spoken of above; and surrounding this again there is present a delicate mesoblastic investment of spindle-shaped cells.
Longitudinal sections also throw light upon the constitution of the anterior nerve roots (vide fig. K II, ar). In the two segments on the left-hand side in this figure the anterior roots [Pg 187] are cut through as they are proceeding, in a more or less horizontal course, from the spinal cord to the muscle-plates.
Where the section (which is not quite horizontal) passes through the plane of the notochord, as on the right-hand side, the anterior roots are cut transversely. Each root, in fact, changes its direction, and takes a downward course.
The anterior roots are situated nearly opposite the middle of the muscle-plates: their section is much smaller than that of the posterior roots, and with hæmatoxylin they stain more deeply than any of the other cells in the preparation.
The anterior roots, so far as I have been able to observe, do not at this stage unite with the posterior; but on this point I do not speak with any confidence.
The period now arrived at forms a convenient break in the development of the spinal nerves; and I hope to treat the remainder of the subject, especially the changes in the ganglion, the development of the ganglion-cells, and of the nerve-fibres, in a subsequent paper.
I will only add that, not long after the stage last described, the posterior root unites with the anterior root at a considerable distance below the cord: this is shewn in Pl. 23, fig. L. Still later the portion of the root between the ganglion and the spinal cord becomes converted into nerve-fibres, and the ganglion becomes still further removed from the cord, while at the same time it appears distinctly divided into two parts.
As regards the development of the cranial nerves, I have made a few observations, which, though confessedly incomplete, I would desire to mention here, because, imperfect as they are, they seem to shew that in Elasmobranch Fishes the cranial nerves resemble the spinal nerves in arising as outgrowths from the central nervous system.
I have given a figure of the development of a posterior root of a cranial nerve in fig. M I. The section is taken from the same embryo as figs. B I, B II, and B III.
It passes through the anterior portion of a thickening of the external epiblast, which eventually becomes involuted as the auditory vesicle.
The posterior root of a nerve (VII) is seen growing out from the summit of the hind brain in precisely the same manner that [Pg 188] the posterior roots of the spinal nerves grow out from the spinal cord: it is the rudiment of the seventh or facial nerve. The section behind this (fig. M II), still in the region of the ear, has no trace of a nerve, and thus serves to shew the early discontinuity of the posterior nerve-rudiments which arise from the brain.
I have as yet failed to detect any cranial anterior roots like those of the spinal nerves[53]. The similarity in development between the cranial and spinal nerves is especially interesting, as forming an important addition to the evidence which at present exists that the cranial nerves are only to be looked on as spinal nerves, especially modified in connexion with the changes which the anterior extremity of the body has undergone in existing vertebrates.
* * * * *
My results may be summarized as follows:—
Along the extreme dorsal summit of the spinal cord there arises on each side a continuous outgrowth.
From each outgrowth processes corresponding in number to the muscle-plates grow downwards. These are the posterior nerve-rudiments.
The outgrowths, at first attached to the spinal cord throughout their whole length, soon cease to be so, and remain in connexion with it in certain spots only, which form the junctions of the posterior roots with the spinal cord.
The original outgrowth on each side remains as a bridge, uniting together the dorsal extremities of all the posterior rudiments. The points of junction of the posterior roots with the spinal cord are at first situated at the extreme dorsal summit of the latter, but eventually travel down, and are finally placed on the sides of the cord.
After these events the posterior nerve-rudiments grow rapidly in size, and become differentiated into a root (by which they are attached to the spinal canal), a ganglion, and a nerve.
The anterior roots, like the posterior, are outgrowths from the spinal cord; but the outgrowths to form them are from the [Pg 189] first discontinuous, and the points from which they originally spring remain as those by which they are permanently attached to the spinal cord, and do not, as in the case of the posterior roots, undergo a change of position. The anterior roots arise, not vertically below, but opposite the intervals between the posterior roots.
The anterior roots are at first quite separate from the posterior roots; but soon after the differentiation of the posterior rudiment into a root, ganglion, and nerve, a junction is effected between each posterior nerve and the corresponding anterior root. The junction is from the first at some little distance from the ganglion.
* * * * *
Investigators have hitherto described the spinal nerves as formed from part of the mesoblast of the protovertebræ. His alone, so far as I know, takes a different view.
His's[54] observations lead him to the conclusion that the posterior roots are developed as ingrowths from the external epiblast into the space between the protovertebræ and the neural canal. These subsequently become constricted off, unite with the neural canal and form spinal nerves.
These statements, which have not been since confirmed, diverge nearly to the same extent from my own results as does the ordinary account of the development of these parts.
Hensen (Virchow's Archiv, Vol. XXXI. 1864) also looks upon the spinal nerves as developed from the epiblast, but not as a direct result of his own observations[55].
Without attempting, for the present at least, to explain this divergence, I venture to think that the facts which I have just described have distinct bearings upon one or two important problems.
One point of general anatomy upon which they throw considerable light is the primitive origin of nerves.
So long as it was admitted that the spinal and cerebral nerves [Pg 190] developed in the embryo independently of the central nervous system, their mode of origin always presented to my mind considerable difficulties.
It never appeared clear how it was possible for a state of things to have arisen in which the central nervous system, as well as the peripheral terminations of nerves, whether motor or sensory, were formed independently of each other, while between them a third structure was developed which, growing in both directions (towards the centre and towards the periphery), ultimately brought the two into connexion.
That such a condition could be a primitive[TN7] one seemed scarcely possible.
Still more remarkable did it appear, on the supposition that the primitive mode of formation of these parts was represented in the developmental history of vertebrates, that we should find similar structural elements in the central and in the peripheral nervous systems.
The central nervous system arises from the epiblast, and yet contains precisely similar nerve-cells and nerve-fibres to the peripheral nervous system, which, if derived, as is usually stated, from the mesoblast, was necessarily supposed to have a completely different origin from the central nervous system.
Both of these difficulties are to a great extent removed by the facts of the development of these parts in Elasmobranchii.
If it be admitted that the spinal roots develop as outgrowths from the central nervous system in Elasmobranch Fishes, the question arises, how far can it be supposed to be possible that in other vertebrates the spinal roots and ganglia develop independently of the spinal cord, and only subsequently become united with it.
I have already insisted that this cannot be the primary condition; and though I am of opinion that the origin of the nerves in higher vertebrates ought to be worked over again, yet I do not think it impossible that, by a secondary adaptation, the nerve-roots might develop in the mesoblast[56].
[Pg 191] The presence of longitudinal commissures connecting the central ends of all the posterior roots is very peculiar. The commissures may possibly be looked on as outlying portions of the cord, rather than as parts of the nerves.
I have not up to this time followed their history beyond a somewhat early period in embryonic life, and am therefore unacquainted with their fate in the adult.
As far as I am aware, no trace of similar structures has been met with in other vertebrates.
The commissures have a very strong resemblance to those by which in Elasmobranch Fishes the glossopharyngeal nerve and the branches of the pneumogastric are united in an early embryonic stage[57].
I think it not impossible that the commissures in the two cases represent the same structures. If this is the case, it would seem that the junction of a number of nerves to form the pneumogastric is not a secondary state, but the remnant of a primary one, in which all the spinal nerves were united, as they embryonically are in Elasmobranchii.
One point brought out in my investigations appears to me to have bearings upon the origin of the central canal of the Vertebrate nervous system, and in consequence upon the origin of the Vertebrate group itself.
The point I allude to is the posterior nerve-rudiments making their first appearance at the extreme dorsal summit of the spinal cord.
The transverse section of the ventral nervous cord of an ordinary segmented worm consists of two symmetrical halves placed side by side.
If by a mechanical folding the two lateral halves of the nervous cord became bent towards each other, while into the groove formed between the two the external skin became pushed, we should have an approximation to the Vertebrate spinal cord. Such a folding might take place to give extra rigidity to the body in the absence of a vertebral column.
If this folding were then completed in such a way that the groove, lined by external skin and situated between the [Pg 192] two lateral columns of the nervous system, became converted into a canal, above and below which the two columns of the nervous system united, we should have in the transformed nervous cord an organ strongly resembling the spinal cord of Vertebrates.
This resemblance would even extend beyond mere external form. Let the ventral nervous cord of the common earthworm, Lumbricus agricola, be used for comparison[58], a transverse section of which is represented by Leydig[59] and Claparède. In this we find that on the ventral surface (the Annelidan ventral surface) of the nervous cord the ganglion-cells (grey matter) (k) are situated, and on the dorsal side the nerve-fibres or white matter (h). If the folding that I have supposed were to take place, the grey and white matters would have very nearly the relative situations which they have in the Vertebrate spinal cord.
The grey matter would be situated in the interior and surround the epithelium of the central canal, and the white matter would nearly surround the grey and form the anterior white commissure. The nerves would then arise, not from the sides of the nervous cord as in existing Vertebrates, but from its extreme ventral summit.
One of the most striking features which I have brought to light with reference to the development of the posterior roots, is the fact of their growing out from the extreme dorsal summit of the neural canal—a position analogous to the ventral summit of the Annelidan nervous cord. Thus the posterior roots of the nerves in Elasmobranchii arise in the exact manner which might have been anticipated were the spinal cord due to such a folding as I have suggested. The argument from the nerves becomes the stronger, from the great peculiarity in the position of the outgrowth, a feature which would be most perplexing without some such explanation as I have proposed. The central epithelium of the neural canal according to this view represents the external skin; and its ciliation is to be explained as a remnant of the ciliation of the external skin now found amongst many of the lower Annelids.
[Pg 193] I have, however, employed the comparison of the Vertebrate and Annelidan nervous cords, not so much to prove a genetic relation between the two as to shew the à priori possibility of the formation of a spinal canal and the à posteriori evidence we have of the Vertebrate spinal canal having been formed in the way indicated.
I have not made use of what is really the strongest argument for my view, viz. that the embryonic mode of formation of the spinal canal, by a folding in of the external epiblast, is the very method by which I have supposed the spinal canal to have been formed in the ancestors of Vertebrates.
My object has been to suggest a meaning for the peculiar primitive position of the posterior roots, rather than to attempt to explain in full the origin of the spinal canal.
EXPLANATION OF THE PLATES[60].
Plate 22.
Fig. A. Section through the dorsal region of an embryo of Scyllium stellare, with the rudiments of two visceral clefts. The section illustrates the general features at a period anterior to the appearance of the posterior nerve-roots.
nc. neural canal. mp. muscle-plate. ch. notochord. x. subnotochordal rod. ao. rudiment of dorsal aorta. so. somatopleure. sp. splanchnopleure. al. alimentary tract. All the parts of the section except the spinal cord are drawn somewhat diagrammatically.
Figs. B I, B II, B III. Three sections of a Pristiurus-embryo. B I is through the heart, B II through the anterior part of the dorsal region, and B III through a point slightly behind this. Drawn with a camera. (Zeiss CC ocul. 2.)
In B III there is visible a slight proliferation of cells from the dorsal summit of the neural canal.
In B II this proliferation definitely constitutes two club-shaped masses of cells (pr), both attached to the dorsal summit of the neural canal. The masses are the rudiments of the posterior nerve-roots.
In B I the rudiments of the posterior roots are of considerable length.
[Pg 194] pr. rudiment of posterior roots. nc. neural canal. mp. muscle-plate. ch. notochord. x. subnotochordal rod. ao. dorsal aorta. so. somatopleure. sp. splanchnopleure. al. alimentary canal. ht. heart.
Fig. C. Section from a Pristiurus-embryo, slightly older than B. Camera. (Zeiss CC ocul. 2.) The embryo from which this figure was taken was slightly distorted in the process of removal from the blastoderm.
vr. rudiment of vertebral body. Other reference letters as in previous figures.
Fig. D a. Section through the dorsal region of a Torpedo-embryo with three visceral clefts. (Zeiss CC ocul. 2.) The section shews the formation of the dorsal nerve-rudiments (pr) and of a ventral anterior nerve-rudiment (ar), which at this early stage is not distinctly cellular.
ar. rudiment of an anterior nerve-root. y. cells left behind on the separation of the external skin from the spinal cord. c. connective-tissue cells springing from the summit of the muscle-plates. Other reference letters as above.
Fig. D b. Section from dorsal region of a Torpedo-embryo somewhat older than D a. Camera. (Zeiss CC ocul. 2.) The posterior nerve-rudiment is considerably longer than in fig. Da, and its pedicle of attachment to the spinal cord is thinner. The anterior nerve-rudiment, of which only the edge is present in the section, is distinctly cellular.
m. mesoblast growing up from vertebral rudiment. sd. segmental duct.
Fig. D c. Section from a still older Torpedo-embryo. Camera. (Zeiss CC ocul. 2.) The connective-tissue cells are omitted. The rudiment of the ganglion (g) on the posterior root has appeared. The rudiment of the posterior nerve is much longer than before, and its junction with the spinal cord is difficult to detect. The anterior root is now an elongated cellular structure.
g. ganglion.
Fig. D d. Longitudinal and vertical section through a Torpedo-embryo of the same age as D c.
The section shews the commissures (x) uniting the posterior roots.
Fig. E a. Section of a Pristiurus-embryo belonging to the second stage. Camera. (Zeiss CC ocul. 2.) The section shews the constriction of the pedicle which attaches the posterior nerve-rudiments to the spinal cord.
pr. rudiment of posterior nerve-root. nc. neural canal. mp. muscle-plate. vr. vertebral rudiment. sd. segmental duct. ch. notochord. so. somatopleure. sp. splanchnopleure. ao. aorta. al. alimentary canal.
Fig. E b. Section of a Pristiurus-embryo slightly older than Ea. Camera. (Zeiss CC ocul. 2.) The section shews the formation of the anterior nerve-root (ar).
ar. rudiment of the anterior nerve-root.
Fig. F. Section of a Pristiurus-embryo with the rudiments of five visceral clefts. Camera. (Zeiss CC ocul. 2.)
The rudiment of the posterior root is seen surrounded by connective-tissue, from which it cannot easily be distinguished. The artist has not been very successful in rendering this figure.
[Pg 195] Figs. G1, G2, G3. Three longitudinal and horizontal sections of an embryo somewhat older than F. The embryo from which these sections were taken was hardened in osmic acid, but the sections have been represented without tinting. G1 is most dorsal of the three sections. Camera. (Zeiss CC ocul. 1.)
nc. neural canal. sp.c. spinal cord. pr. rudiment of posterior root. ar. rudiment of anterior root. mp. muscle-plate. c. connective-tissue cells. ch. notochord.
Plate 23.
Fig. H I. Section through the dorsal region of a Pristiurus-embryo in which the rudimentary external gills are present as very small knobs. Camera. (Zeiss CC ocul. 2.)
The section shews the commencing differentiation of the posterior nerve-rudiment into root (pr), ganglion (sp.g), and nerve (n), and also the attachment of the nerve-root to the spinal cord (x). The variations in the size and shape of the cells in the different parts of the nerve-rudiment are completely lost in the figure.
pr. posterior nerve-root. sp.g. ganglion of posterior root. n. nerve of posterior root. x. attachment of posterior root to spinal cord. w. white matter of spinal cord. i. mesoblastic investment to the spinal cord.
Fig. H II. Section through the same embryo as H I. (Zeiss CC ocul. 1.)
The section contains an anterior root, which takes its origin at a point opposite the interval between two posterior roots.
The white matter has not been very satisfactorily represented by the artist.
Figs. I I, I II. Two sections of a Pristiurus-embryo somewhat older than H. Camera. (Zeiss CC ocul. 1.)
The connective-tissue cells are omitted.
Figs. I a, I b, I c. Three isolated cells from the ganglion of one of the posterior roots of the same embryo.
Figs. K I, K II. Two horizontal longitudinal sections through an embryo in which the external gills have just appeared. K I is the most dorsal of the two sections. Camera. (Zeiss CC ocul. 1.)
The sections shew the relative positions of the anterior and posterior roots at different levels.
pr. posterior nerve-rudiment. ar. anterior nerve-rudiment. sp.c. spinal cord. n.c. neural canal. mp. muscle-plate. mp´. first-formed muscles.
Fig. L. Longitudinal and vertical section through the trunk of a Scyllium-embryo after the external gills have attained their full development. Camera. (Zeiss CC ocul. 1.)
The embryo was hardened in a mixture of chromic acid and osmic acid.
The section shews the commissures which dorsally unite the posterior roots, and also the junction of the anterior and posterior roots. The commissures are unfortunately not represented in the figure with great accuracy; their outlines are in nature perfectly regular, and not, as in the figure, notched at the junctions of the cells composing them. Their cells are apparently more or less completely fused, and certainly not nearly so clearly marked as in the figure. The commissures stain very deeply with the mixture of osmic and chromic acid, and form one of the most conspicuous [Pg 196] features in successful longitudinal sections of embryos so hardened. In sections hardened with chromic acid only they cannot be seen with the same facility.
sp.c. spinal cord. gr. grey matter. w. white matter. ar. anterior root. pr. posterior root. x. commissure uniting the posterior roots.
Figs. M I, M II. Two sections through the head of the same embryo as fig. B. M I, the foremost of the two, passes through the anterior part of the thickening of epiblast, which becomes involuted as the auditory vesicle. It contains the rudiment of the seventh nerve, VII. Camera. (Zeiss CC ocul. 2.)
VII. rudiment of seventh nerve. au. thickening of external epiblast, which becomes involuted as the auditory vesicle. n.c. neural canal. ch. notochord. pp. body-cavity in the head. so. somatopleure. sp. splanchnopleure. al. throat exhibiting an outgrowth to form the first visceral cleft.
[47] [From the Philosophical Transactions of the Royal Society of London, Vol. CLXVI. Pt. 1. Received October 5, Read December 16, 1875.]
[48]
Vide Balfour, Preliminary account of the
Development of Elasmobranch Fishes,
Quart. Journ. of Microsc. Science, Oct. 1874, p. 33. [This edition, p. 96.]
[49]
Vide Balfour, Origin and History of
Urinogenital Organs of Vertebrates,
Journal of Anatomy and
Physiology, Oct. 1875. [This edition,
No. VII.]
[50] This commissure is not satisfactorily represented in the figure. Vide Explanation of Plate 23.
[51] [May 18, 1876.—Observations I have recently made upon the development of the cranial nerves incline me to adopt an explanation of the change which takes place in the point of attachment of the spinal nerves to the cord differing from that enunciated in the text. I look upon this change as being apparent rather than real, and as due to a growth of the roof of the neural canal in the median dorsal line, which tends to separate the roots of the two sides more and more, and cause them to assume a more ventral position.]
[52] The artist has not been very successful in rendering this figure.
[53] [May 18, 1876.—Subsequent observations have led me to the conclusion that no anterior nerve-roots are to be found in the brain.]
[54] Erste Anlage des Wirbelthier-Leibes.
[55] [May 18, 1876.—Since the above was written Hensen has succeeded in shewing that in mammals the rudiments of the posterior roots arise in a manner closely resembling that described in the present paper; and I have myself, within the last few days, made observations which incline me to believe that the same holds good for the chick. My observations are as yet very incomplete.]
[56] [May 18, 1876.—Hensen's observations, as well as those recently made by myself on the chick, render it almost certain that the nerves in all Vertebrates spring from the spinal cord.]
[57]
Balfour, A Preliminary Account of the Development of Elasmobranch
Fishes,
Q. J. Micros. Sc. 1874, plate XV. fig. 14, v.g. [This edition, Pl.
4, fig. 14, vg].
[58] The nervous cords of other Annelids resemble that of Lumbricus in the relations of the ganglion-cells of the nerve-fibres.
[59] Tafeln zur vergleichenden Anatomie, Taf. iii. fig. 8.
[60] The figures on these Plates give a fair general idea of the appearance presented by the developing spinal nerves; but the finer details of the original drawings have in several cases become lost in the process of copying.
The figures which are tinted represent sections of embryos hardened in osmic acid; those without colour sections of embryos hardened in chromic acid.
During a short visit to Naples in January last, I was enabled, through the kindness of Dr Dohrn, to make some observations on the spinal nerves of Amphioxus. These were commenced solely with the view of confirming the statements of Stieda on the anatomy of the spinal nerves, which, if correct, appeared to me to be of interest in connection with the observations I had made that, in Elasmobranchii, the anterior and posterior roots arise alternately and not in the same vertical plane. I have been led to conclusions on many points entirely opposed to those of Stieda, but, before recording these, I shall proceed briefly to state his results, and to examine how far they have been corroborated by subsequent observers.
Stieda[62], from an examination of sections and isolated spinal cords, has been led to the conclusion that, in Amphioxus, the nerves of the opposite sides arise alternately, except in the most anterior part of the body, where they arise opposite each other. He also states that the nerves of the same side issue alternately from the dorsal and ventral corners of the spinal cord. He regards two of these roots (dorsal and ventral) on the same side as together equivalent to a single spinal nerve of higher vertebrates formed by the coalescence of a dorsal and ventral root.
Langerhans[63] apparently agrees with Stieda as to the facts about the alternation of dorsal and ventral roots, but differs [Pg 198] from him as to the conclusions to be drawn from those facts. He does not, for two reasons, believe that two nerves of Amphioxus can be equivalent to a single nerve in higher vertebrates: (1) Because he finds no connecting branch between two succeeding nerves, and no trace of an anastomosis. (2) Because he finds that each nerve in Amphioxus supplies a complete myotome, and he considers it inadmissible to regard the nerves, which in Amphioxus together supply two myotomes, as equivalent to those which in higher vertebrates supply a single myotome only.
Although the agreement as to facts between Langerhans and Stieda is apparently a complete one, yet a critical examination of the statements of these two authors proves that their results, on one important point at least, are absolutely contradictory. Stieda, Pl. III. fig. 19, represents a longitudinal and horizontal section through the spinal cord which exhibits the nerves arising alternately on the two sides, and represents each myotome supplied by one nerve. In his explanation of the figure he expressly states that the nerves of one plane only (i.e. only those with dorsal or only those with ventral roots) are represented; so that if all the nerves which issue from the spinal cord had been represented double the number figured must have been present. But since each myotome is supplied by one nerve in the figure, if all the nerves present were represented, each myotome would be supplied by two nerves.
Since Langerhans most emphatically states that only one nerve is present for each myotome, it necessarily follows that he or Stieda has made an important error; and it is not too much to say that this error is more than sufficient to counterbalance the value of Langerhans' evidence as a confirmation of Stieda's statements.
I commenced my investigations by completely isolating the nervous system of Amphioxus by maceration in nitric acid according to the method recommended by Langerhans[64]. On examining specimens so obtained it appeared that, for the greater length of the cord, the nerves arose alternately on the [Pg 199] two sides, as was first stated by Owsjannikow, and subsequently by Stieda and Langerhans; but to my surprise not a trace could be seen of a difference of level in the origin of the nerves of the same side.
The more carefully the specimens were examined from all points of view, the more certainly was the conclusion forced upon me, that nerves issuing from the ventral corner of the spinal cord, as described by Stieda, had no existence.
Not satisfied by this examination, I also tested the point by means of sections. I carefully made transverse sections of a successfully hardened Amphioxus, through the whole length of the body. There was no difficulty in seeing the dorsal roots in every third section or so, but not a trace of a ventral root was to be seen. There can, I think, be no doubt, that, had ventral roots been present, they must, in some cases at least, have been visible in my sections.
In dealing with questions of this kind it is no doubt difficult to prove a negative; but, since the two methods of investigation employed by me both lead to the same result, I am able to state with considerable confidence that my observations lend no support to the view that the alternate spinal nerves of Amphioxus have their roots attached to the ventral corner of the spinal cord.
How a mistake on this point arose it is not easy to say. All who have worked with Amphioxus must be aware how difficult it is to conserve the animal in a satisfactory state for making sections. The spinal cord, especially, is apt to be distorted in shape, and one of its ventral corners is frequently produced into a horn-like projection terminating in close contact with the sheath. In such cases the connective tissue fibres of the sheath frequently present the appearance of a nerve-like prolongation of the cord; and for such they might be mistaken if the sections were examined in a superficial manner. It is not, however, easy to believe that, with well conserved specimens, a mistake could be made on this point by so careful and able an investigator as Stieda, especially considering that the histological structure of the spinal nerves is very different from that of the fibrous prolongations of the sheath of the spinal cord.
[Pg 200] It only remains for me to suppose that the specimens which Stieda had at his disposal, were so shrunk as to render the origin of the nerves very difficult to determine.
The arrangement of the nerves of Amphioxus, according to my own observations, is as follows.
The anterior end of the central nervous system presents on its left and dorsal side a small pointed projection, into which is prolonged a diverticulum from the dilated anterior ventricle of the brain. This may perhaps be called the olfactory nerve, though clearly of a different character to the other nerves. It was first accurately described by Langerhans[65].
Vertically below the olfactory nerve there arise two nerves, which issue at the same level from the ventral side of the anterior extremity of the central nervous system. These form the first pair of nerves, and are the only pair which arise from the ventral portion of the cerebro-spinal cord. The two nerves, which form the second pair, arise also opposite each other but from the dorsal side of the cord. The first and second pair of nerves have both been accurately drawn and described by Langerhans: they, together with the olfactory nerve, can easily be seen in nervous systems which have been isolated by maceration.
In the case of the third pair of nerves, the nerve on the right-hand side is situated not quite opposite but slightly behind that on the left. The right nerve of the fourth pair is situated still more behind the left, and, in the case of the fifth pair, the nerve to the right is situated so far behind the left nerve that it occupies a position half-way between the left nerves of the fifth and sixth pairs. In all succeeding nerves the same arrangement holds good, so that they exactly alternate on two sides.
Such is the arrangement carefully determined by me from one specimen. It is possible that it may not be absolutely constant, but the following general statement almost certainly holds good.
All the nerves of Amphioxus, except the first pair, have their roots inserted in the dorsal part of the cord. In the case of [Pg 201] the first two pairs the nerves of the two sides arise opposite each other; in the next few pairs, the nerves on the right-hand side gradually shift backwards: the remaining nerves spring alternately from the two sides of the cord.
For each myotome there is a single nerve, which enters, as in the case of other fishes, the intermuscular septum. This point may easily be determined by means of longitudinal sections, or less easily from an examination of macerated specimens. I agree with Langerhans in denying the existence of ganglia on the roots of the nerves.
[61] From the Journal of Anatomy and Physiology, Vol. X. 1876.
[62] Mém. Acad. Pétersbourg, Vol. XIX.
[63] Archiv f. mikr. Anatomie, Vol. XII.
[64] Loc. cit.
[65] Loc. cit.
Published 1878.
The present Monograph is a reprint of a series of papers published in the Journal of Anatomy and Physiology during the years 1876, 1877 and 1878. The successive parts were struck off as they appeared, so that the earlier pages of the work were in print fully two years ago. I trust the reader will find in this fact a sufficient excuse for a certain want of coherence, which is I fear observable, as well as for the omission of references to several recent publications. The first and second chapters would not have appeared in their present form had I been acquainted, at the time of writing them, with the researches which have since been published, on the behaviour of the germinal vesicle and on the division of nuclei. I may also call attention to the valuable papers of Prof. His[66] on the formation of the layers in Elasmobranchii, and of Prof. Kowalevsky[67] on the development of Amphioxus, to both of which I would certainly have referred, had it been possible for me to do so.
Professor His deals mainly with the subjects treated of in Chapter III., and gives a description very similar to my own of the early stages of development. His interpretations of the observed changes are, however, very different from those at which I have arrived. Although this is not the place for a discussion of Prof. His's views, I may perhaps state that, in spite of the arguments he has brought forward in support of his position, I am still inclined to maintain the accuracy of my original account. The very striking paper on Amphioxus by Kowalevsky (the substance of which I understand to have been published in Russia at an earlier period) contains a confirmation of the views expressed in chapter VI. on the development [Pg 206] of the mesoblast, and must be regarded as affording a conclusive demonstration, that in the case of Vertebrata the mesoblast has primitively the form of a pair of diverticula from the walls of the archenteron.
* * * * *
The present Memoir, while differing essentially in scope and object from the two important treatises by Professors His[68] and Götte[69], which have recently appeared in Germany, has this much in common with them, that it deals monographically with the development of a single type: but here the resemblance ends. Both of these authors seek to establish, by a careful investigation of the development of a single species, the general plan of development of Vertebrates in general, if not of the whole animal kingdom. Both reject the theory of descent, as propounded by Mr Darwin, and offer completely fresh explanations of the phenomena of Embryology. Accepting, as I do, the principle of natural selection, I have had before me, in writing the Monograph, no such ambitious aim as the establishment of a completely new system of Morphology. My object will have been fully attained if I have succeeded in adding a few stones to the edifice, the foundations of which were laid by Mr Darwin in his work on the Origin of Species.
I may perhaps call attention to one or two special points in this work which seem to give promise of further results. The chapter on the Development of the Spinal and Cranial Nerves contains a modification of the previously accepted views on this subject, which may perhaps lead to a more satisfactory conception of the origin of nerves than has before been possible, and a more accurate account of the origin of the muscle-plates and vertebral column. The attempt to employ the embryological relations of the cephalic prolongations of the body-cavity, and of the cranial nerves, in the solution of the difficult problems of the Morphology of the head, may prove of use in the line of study so successfully cultivated by our great English Anatomist, Professor Huxley. Lastly, I venture to hope that my conclusions in reference to the relations of the sympathetic system and the suprarenal body, and to the development of the mesoblast, [Pg 207] the notochord, the limbs, the heart, the venous system, and the excretory organs, are not unworthy of the attention of Morphologists.
* * * * *
The masterly manner in which the systematic position of Elasmobranchii is discussed by Professor Gegenbaur, in the introduction to his Monograph on the Cranial Skeleton of the group, relieves me from the necessity of entering upon this complicated question. It is sufficient for my purpose that the Elasmobranch Fishes be regarded as forming one of the most primitive groups among Vertebrates, a view which finds ample confirmation in the importance of the results to which Prof. Gegenbaur and his pupils have been led in this branch of their investigations.
* * * * *
Though I trust that the necessary references to previous contributions in the same department of enquiry have not been omitted, the 'literature of the subject' will nevertheless be found to occupy a far smaller share of space than is usual in works of a similar character. This is an intentional protest on my part against, what appears to me, the unreasonable amount of space so frequently occupied in this way. The pages devoted to the 'previous literature' only weary the reader, who is not wise enough to skip them, and involve a great and useless expenditure of time on the part of any writer, who is capable of something better than the compilation of abstracts.
* * * * *
In conclusion, my best thanks are due to Drs Dohrn and Eisig for the uniformly kind manner in which they have forwarded my researches both at the Zoological Station in Naples, and after my return to England; and also to Mr Henry Lee and to the Manager and Directors of the Brighton Aquarium, who have always been ready to respond to my numerous demands on their liberality.
To my friend and former teacher Dr Michael Foster I tender my sincerest thanks for the never-failing advice and assistance which he has given throughout the whole course of the work.
[66] Zeitschrift f. Anat. u. Entwicklungsgeschichte, Bd. II.
[67] Archiv f. Micr. Anat. Bd. XIII.
[68] Erste Anlage des Wirbelthierleibes.
[69] Entwicklungsgeschichte der Unke.
| TABLE OF CONTENTS. |
|---|
| CHAPTER I. |
| THE RIPE OVARIAN OVUM, pp. 213-221. |
| Structure of ripe ovum. Atrophy of germinal vesicle. The extrusion of its membrane and absorption of its contents. Oellacher's observations on the germinal vesicle. Götte's observations. Kleinenberg's observations. General conclusions on the fate of the germinal vesicle. Germinal disc. |
| CHAPTER II. |
| THE SEGMENTATION, pp. 222-245. |
| Appearance of impregnated germinal disc. Stage with two furrows. Stage with twenty-one segments. Structure of the sides of the furrows. Later stages of segmentation. Spindle-shaped nuclei. Their presence outside the blastoderm. Knobbed nuclei. Division of nuclei. Conclusion of segmentation. Nuclei of the yolk. Asymmetry of the segmented blastoderm. Comparison of Elasmobranch segmentation with that of other meroblastic ova. Literature of Elasmobranch segmentation. |
| CHAPTER III. |
| FORMATION OF THE LAYERS, pp. 246-285. |
| Division of blastoderm into two layers. Formation of segmentation cavity. Disappearance of cells from floor of segmentation cavity. Nuclei of yolk and of blastoderm. Formation of embryonic rim. Appearance of a layer of cells on the floor of the segmentation cavity. Formation of mesoblast. Formation of medullary groove. Disappearance of segmentation cavity. Comparison of segmentation cavity of Elasmobranchii with that of other types. Alimentary cavity. Formation of mesoblast in two lateral plates. Protoplasmic network of yolk. Summary. Nature of meroblastic ova. Comparison of Elasmobranch development with that of other types. Its relation to the Gastrula. Haeckel's views on vertebrate Gastrula. Their untenable nature. Comparison of primitive streak with blastopore. Literature. |
| CHAPTER IV. |
| GENERAL FEATURES OF THE ELASMOBRANCH EMBRYO AT SUCCESSIVE STAGES, pp. 286-297. |
| Description of Stages A-Q. Enclosure of yolk by blastoderm. Relation of the anus of Rusconi to the blastopore. [Pg 210] |
| CHAPTER V. |
| STAGES B-G, pp. 298-314. |
| General features of the epiblast.—Original uniform constitution. Separation into lateral and central portions. The medullary groove.—Its conversion into the medullary canal. The mesoblast.—Its division into somatic and splanchnic layers. Formation of protovertebræ. The lateral plates. The caudal swellings. The formation of the body-cavity in the head. The alimentary canal.—Its primitive constitution. The anus of Rusconi. Floor formed by yolk. Formation of cellular floor from cells formed around nuclei of the yolk. Communication behind of neural and alimentary canals. Its discovery by Kowalevsky. Its occurrence in other instances. General features of the hypoblast. The notochord.—Its formation as a median thickening of the hypoblast. Possible interpretations to be put on this. Its occurrence in other instances. |
| CHAPTER VI. |
| DEVELOPMENT OF THE TRUNK DURING STAGES G TO K, pp. 315-360. |
| Order of treatment. External epiblast.—Characters of epiblast. Its late division into horny and epidermic layers. Comparison of with Amphibian epiblast. The unpaired fins. The paired fins.—Their formation as lateral ridges of epiblast. Hypothesis that the limbs are remnants of continuous lateral fins. Mesoblast.—Constitution of lateral plates of mesoblast. Their splanchnic and somatic layers. Body-cavity constituting space between them. Their division into lateral and vertebral plates. Continuation of body-cavity into vertebral plates. Protovertebræ. Division into muscle-plates and vertebral bodies. Development of muscle-plates. Disappearance of segmentation in tissue to form vertebral bodies. Body-cavity and parietal plates. Primitive independent halves of body-cavity. Their ventral fusion. Separation of anterior part of body-cavity as pericardial cavity. Communication of pericardial and peritoneal cavities. Somatopleure and splanchnopleure. Résumé. General considerations on development of mesoblast. Probability of lateral plates of mesoblast in Elasmobranchii representing alimentary diverticula. Meaning of secondary segmentation of vertebral column. The urinogenital system.—Development of segmental duct and segmental tubes as solid bodies. Formation of a lumen in them, and their opening into body-cavity. Comparison of segmental duct and segmental tubes. Primitive ova. Their position. Their structure. The notochord.—The formation of its sheath. The changes in its cells. |
| CHAPTER VII. |
| GENERAL DEVELOPMENT OF THE TRUNK FROM STAGE K TO THE CLOSE OF EMBRYONIC LIFE, pp. 361-377. |
| External epiblast.—Division into separate layers. Placoid scales. Formation of their enamel. Lateral line.—Previous investigations. Distinctness of lateral line and lateral nerve. Lateral nerve a branch of vagus. Lateral line a thickening of epiblast. Its greater width behind. Its conversion into a canal by its cells assuming a tubular arrangement. The formation of its segmental apertures. Mucous canals of the head. Their nerve-supply. Reasons for dissenting from Semper's and Götte's view of lateral nerve. Muscle-plates.—Their growth. Conversion of both layers into [Pg 211] muscles. Division into dorso-lateral and ventro-lateral sections. Derivation of limb-muscles from muscle-plates. Vertebral column and notochord.—Previous investigations. Formation of arches. Formation of cartilaginous sheath of notochord and membrana elastica externa. Differentiation of neural arches. Differentiation of hæmal arches. Segmentation of cartilaginous sheath of notochord. Vertebral and intervertebral regions. Notochord. |
| CHAPTER VIII. |
| DEVELOPMENT OF THE SPINAL NERVES AND OF THE SYMPATHETIC NERVOUS SYSTEM, pp. 378-396. |
| The spinal nerves.—Formation of posterior roots. Later formation of anterior roots. Development of commissure uniting posterior roots. Subsequent development of posterior roots. Their change in position. Development of ganglion. Further changes in anterior roots. Junction of anterior and posterior roots. Summary. General considerations.—Origin of nerves. Hypothesis explaining peripheral growth. Hensen's views. Later investigations. Götte. Calberla. Relations between Annelidan and Vertebrate nervous systems. Spinal canal. Dr Dohrn's views. Their difficulties. Hypothesis of dorsal coalescence of lateral nerve cords. Sympathetic nervous system.—Development of sympathetic ganglia on branches of spinal nerves. Formation of sympathetic commissure. |
| CHAPTER IX. |
| DEVELOPMENT OF THE ORGANS IN THE HEAD, pp. 397-445. |
| Development of the Brain, pp. 397-407. General history. Fore-brain.—Optic vesicles. Infundibulum. Pineal gland. Olfactory lobes. Lateral ventricles. Mid-brain. Hind-brain.—Cerebellum. Medulla.—Previous investigations. Huxley. Miklucho-Maclay. Wilder. Organs of sense, pp. 407-412. Olfactory organ.—Olfactory pit. Schneiderian folds. Eye. General development. Hyaloid membrane. Lens capsule. Processus falciformis. Auditory organs.—Auditory pit. Semicircular canals. Mouth involution and Pituitary body, pp. 412-414. Outgrowth of pituitary involution. Separation of pituitary sack. Junction with infundibulum. Development of cranial nerves, pp. 414-428. Early development of 5th, 7th, 8th, 9th and 10th cranial nerves. Distribution of the nerves in the adult. The fifth nerve.—Its division into ophthalmic and mandibular branches. Later formation of superior maxillary branch. Seventh and auditory nerves.—Separation of single rudiment into seventh and auditory. Forking of seventh nerve over hyomandibular cleft. Formation of anterior branch to form ramus ophthalmicus[TN8] superficialis of adult. General view of morphology of branches of seventh nerve. Glossopharyngeal and vagus nerves.—General distribution at stage L. Their connection by a commissure. Junction of the commissure with commissure connecting posterior roots of spinal nerves. Absence of anterior roots. Hypoglossal nerve. Mesoblast of Head, pp. 429-432. Body-cavity and myotomes of head.—Continuation of body-cavity into head. Its division into segments. Development of muscles from their walls. General mesoblast of head. Notochord in Head, P. 433. Hypoblast of the Head, pp. 433-434. The formation of the gill-slits. Layer from which gills are derived. Segmentation of the Head, pp. 434-440. Indication of segmentation afforded by (1) cranial nerves, (2) visceral clefts, (3) head-cavities. Comparison of results obtained. |
| CHAPTER X. [Pg 212] |
| THE ALIMENTARY CANAL, pp. 446-459. |
| The solid œsophagus.—Œsophagus originally hollow. Becomes solid during Stage K. The postanal section of the alimentary tract.—Continuity of neural and alimentary canals. Its discovery by Kowalevsky. The postanal section of gut. Its history in Scyllium. Its disappearance. The cloaca and anus.—The formation of the cloaca. Its junction with segmental ducts. Abdominal pockets. Anus. The thyroid body.—Its formation in region of mandibular arch. It becomes solid. Previous investigations. The pancreas.—Arises as diverticulum from dorsal side of duodenum. Its further growth. Formation of duct. The liver.—Arises as ventral diverticulum of duodenum. Hepatic cylinders. Comparison with other types. The subnotochordal rod.—Its separation from dorsal wall of alimentary canal. The section of it in the trunk. In the head. Its disappearance. Views as to its meaning. |
| CHAPTER XI. |
| THE VASCULAR SYSTEM AND VASCULAR GLANDS, pp. 460-478. |
| The heart.—Its development. Comparison with other types. Meaning of double formation of heart. The general circulation. The venous system. The primitive condition of. Comparison of, with Amphioxus and Annelids. The cardinal veins. Relations of caudal vein. The circulation of the yolk-sack.—Previous observations. Various stages. Difference of type in amniotic Vertebrates. The vascular glands.—Suprarenal and interrenal bodies. Previous investigations. The suprarenal bodies.—Their structure in the adult. Their development from the sympathetic ganglia. The interrenal body.—Its structure in the adult. Its independence of suprarenal bodies. Its development. |
| CHAPTER XII. |
| THE ORGANS OF EXCRETION, pp. 479-520. |
| Previous investigations. Excretory organs and genital ducts in adult. In male.—Kidney and Wolffian body. Wolffian duct. Ureters. Cloaca. Seminal bladders. Rudimentary oviduct. In female.—Wolffian duct. Ureters. Cloaca.—Segmental openings. Glandular tubuli of kidney. Malpighian bodies. Accessory Malpighian bodies. Relations of to segmental tubes. Vasa efferentia. Comparison of Scyllium with other Elasmobranchii. Development of segmental tubes. Their junction with segmental duct. Their division into four segments. Formation of Malpighian bodies. Connection between successive segments. Morphological interest of. Development of Müllerian and Wolffian ducts. In female—General account. Formation of oviduct as nearly solid cord. Hymen. In male—Rudimentary Müllerian duct.—Comparison of development of Müllerian duct in Birds and Elasmobranchii. Own researches. Urinal cloaca. Formation of Wolffian body and kidney proper.—General account. Details of formation of ureters. Vasa efferentia.—Views of Semper and Spengel. Difficulties of Semper's views. Unsatisfactory result of own researches. General homologies. Résumé. Postscript. |
The ripe ovum is nearly spherical, and, after the removal of its capsule, is found to be unprovided with any form of protecting membrane.
My investigations on the histology of the ripe ovarian ovum have been made with the ova of the Gray Skate (Raja batis) only, and owing to a deficiency of material are somewhat imperfect.
The bulk of the ovum is composed of yolk spherules, imbedded in a protoplasmic matrix. Dr Alexander Schultz[70], who has studied with great care the constitution of the yolk, finds, near the centre of the ovum, a kernel of small yolk spherules, which is succeeded by a zone of spherules which gradually increase in size as they approach the surface. But, near the surface, he finds a layer in which they again diminish in size and exhibit numerous transitional forms on the way to molecular yolk granules. These Dr Schultz regards as in a retrogressive condition.
Another interesting feature about the yolk is the presence in it of a protoplasmic network. Dr Schultz has completely confirmed, and on some points enlarged, my previous observations on this subject[71]. Dr Schultz's confirmation is the more important, since he appears to be unacquainted with my previous investigations. In my paper (loc. cit.), after giving a description of the network I make the following statement as to its distribution.
[Pg 214] A specimen of this kind is represented
in Plate 14, fig. 2, ny, where the meshes
of the network are seen to be finer immediately around the nuclei, and
coarser in the intervals. The specimen further shews, in the clearest
manner, that this network is not divided into areas, each representing a
cell and each containing a nucleus. I do not know to what extent this
network extends into the yolk. I have never yet seen the limits of it,
though it is very common to see the coarsest yolk-granules lying in its
meshes. Some of these are shewn in Plate 14, fig. 2, y.k.
[This edition, p. 65.]
Dr Schultz, by employing special methods of hardening and cutting sections of the whole egg, has been able to shew that this network extends, in the form of fine radial lines, from the centre to the circumference; and he rightly states, that it exhibits no cell-like structures. I have detected this network extending throughout the whole yolk in young eggs, but have failed to see it with the distinctness which Dr Schultz attributes to it in the ripe ovum. Since it is my intention to enter fully both into the structure and meaning of this network in my account of a later stage, I say no more about it here.
At one pole of the ripe ovum a slight examination demonstrates the presence of a small circular spot, sharply distinguished from the remainder of the yolk by its lighter colour. Around this spot is an area which is also of a lighter colour than the yolk, and the outer border of which gradually shades into the normal tint of the yolk. If a section be made through this part (vide Pl. 6, fig. 1) the circular spot will be found to be the germinal vesicle, and the area around it a disc of yolk containing smaller spherules than the surrounding parts. The germinal vesicle possessed the same structure in both the ripe eggs examined by me; and, in both, it was situated quite on the external surface of the yolk.
In one of my specimens it was flat above, but convex below; in the other and, on the whole, the better preserved of the two, it had the somewhat quadrangular but rather irregular section represented in Pl. 6, fig. 1. It consisted of a thickish membrane and its primitive contents. The membrane surrounded the upper part of the contents and exhibited numerous folds and creases (vide fig. 1). As it extended downwards it became thinner, and completely disappeared at some little distance from the lower end of the contents. These, therefore, rested below on the yolk. At its circumference the membrane of the disc was [Pg 215] produced into a kind of fold, forming a rim which rested on the surface of the yolk.
In neither of my specimens is the cavity in the upper part of the membrane filled by the contents; and the upper part of the membrane is so folded and creased that sections through almost any portion of it pass through the folds. The regularity of the surface of the yolk is not broken by the germinal vesicle, and the yolk around exhibits not the slightest signs of displacement. In the germinal vesicle figured the contents are somewhat irregular in shape; but in my other specimen they form a regular mass concave above and convex below. In both cases they rest on the yolk, and the floor of the yolk is exactly moulded to suit the surface of the contents of the germinal vesicle. The contents have a granular aspect, but differ in constitution from the surrounding yolk. Each germinal vesicle measured about one-fiftieth of an inch in diameter.
It does not appear to me possible to suppose that the peculiar appearances which I have drawn and described are to be looked upon as artificial products either of the chromic acid, in which the ova were hardened, or of the instrument with which sections of them were made. It is hardly conceivable that chromic acid could cause a rupture of the membrane and the ejection of the contents of the vesicle. At the same time the uniformity of the appearances in the different sections, the regularity of the whole outline of the egg, and the absence of any signs of disturbance in the yolk, render it impossible to believe that the structures described are due to faults of manipulation during or before the cutting of the sections.
We can only therefore conclude that they represent the real state of the germinal vesicle at this period. No doubt they alone do not supply a sufficient basis for any firm conclusions as to the fate of the germinal vesicle. Still, if they cannot sustain, they unquestionably support certain views. The natural interpretation of them is that the membrane of the germinal vesicle is in the act of commencing to atrophy, preparatory to being extruded from the egg, while the contents of the germinal vesicle are about to be absorbed.
In favour of the extrusion of the membrane rather than its absorption are the following features:
[Pg 216] (1) The thickness of its upper surface. (2) The extension of its edge over the yolk. (3) Its position external to the yolk.
In favour of the view that the contents will be left behind and absorbed when the membrane is pushed out, are the following features of my sections:
(1) The rupture of the membrane of the germinal vesicle on its lower surface. (2) The position of the contents almost completely below the membrane of the vesicle and surrounded by yolk.
In connection with this subject, Oellacher's valuable observations upon the behaviour of the germinal vesicle in Osseous Fishes and in Birds at once suggest themselves[72]. Oellacher sums up his results upon the behaviour of the germinal vesicle in Osseous Fishes in the following way (p. 12):
The germinal vesicle of the Trout's egg, at a
period when the egg is very nearly ripe, lies near the surface of the
germinal disc which is aggregated together in a hollow of the yolk....
After this a hole appears in the membrane of the germinal vesicle, which
opens into the space between the egg-membrane and the germinal disc. The
hole widens more and more, and the membrane frees itself little by little
from the contents of the germinal vesicle, which remain behind in the form
of a ball on the floor of the cavity formed in this way. The cavity becomes
flatter and flatter and the contents are pushed up further and further from
the germinal disc. When the hollow, in which lie the contents of the
original germinal vesicle, completely vanishes, the covering membrane
becomes inverted ... and the membrane is spread out on the convex surface
of the germinal disc as a circular, investing structure. It is clear that
by the removal of the membrane the contents of the germinal vesicle become
lost.
These very definite statements of Oellacher tell strongly against my interpretation of the appearance presented by the germinal vesicle of the ripe Skate's egg. Oellacher's account is so precise, and his drawings so fully bear out his interpretations, that it is very difficult to see where any error can have crept in.
On the other hand, with the exception of those which Oellacher has made, there cannot be said to be any satisfactory observations demonstrating the extrusion of the germinal vesicle from the ovum. Oellacher has observed this definitely for the Trout, but his observations upon the same point in the Bird would quite as well bear the interpretation that the membrane alone became pushed out, as that this occurred to the germinal vesicle, contents and all.
[Pg 217] While, then, there are on the one hand Oellacher's observations on a single animal, hitherto unconfirmed, there are on the other very definite observations tending to shew that the germinal vesicle has in many cases an altogether different fate. Götte[73], not to mention other observers before him, has in the case of Batrachian's eggs traced out with great precision the gradual atrophy of the germinal vesicle, and its final absorption into the matter of the ovum.
Götte distinguishes three stages in the degeneration of the germinal vesicle of Bombinator's egg. In the first stage the germinal vesicle has begun to travel up towards the surface of the egg. It retains nearly its primitive condition, but its contents have become more opaque and have partly withdrawn themselves from the thin membrane. The germinal spots are still circular, but in some cases have increased in size. The most important feature of this stage is the smaller size of the germinal vesicle than that of the cavity of the yolk in which it lies, a condition which appears to demonstrate the commencing atrophy of the vesicle.
In the next stage the cavity containing the germinal vesicle has vanished without leaving a trace. The germinal vesicle itself has assumed a lens-like form, and its borders are irregular and pressed in here and there by yolk. Of the membrane of the germinal vesicle, and of the germinal spots, only scanty remnants are to be seen, many of which lie in the immediately adjoining yolk.
In the last stage no further trace of a distinct germinal vesicle is present. In its place is a mass of very finely granular matter, which is without a distinct border and graduates into the surrounding yolk and is to be looked on as a remnant of the germinal vesicle.
This careful investigation of Götte proves beyond a doubt that in Batrachians neither the membrane, nor the contents of the germinal vesicle, are extruded from the egg.
In Mammalia, Van Beneden[74] finds that the germinal vesicle becomes invisible, though he does not consider that it absolutely ceases to exist. He has not traced the steps of the process with the same care as Götte, but it is difficult to believe that an [Pg 218] extrusion of the vesicle in the way described by Oellacher would have escaped his notice.
Passing from Vertebrates to Invertebrates, we find that almost every careful investigator has observed the disappearance, apparent or otherwise, of the germinal vesicle, but that very few have watched with care the steps of the process.
The so-called Richtungskörper has been supposed to be the extruded remnant of the germinal vesicle. This view has been especially adopted and supported by Oellacher (loc. cit.), and Flemming[75].
The latter author regards the constant presence of this body, and the facility with which it can be stained, as proofs of its connection with the germinal vesicle, which has, however, according to his observations, disappeared before the appearance of the Richtungskörper.
Kleinenberg[76], to whom we are indebted for the most precise observations we possess on the disappearance of the germinal vesicle, gives the following account of it, pp. 41 and 42.
"We left the germinal vesicle as a vesicle with a distinct doubly contoured membrane, and equally distributed granular contents, in which the germinal spot had appeared.... The germinal vesicle reaches 0.06 mm. in diameter, and at the same time its contents undergo a separation. The greater part withdraws itself from the membrane and collects as a dense mass around the germinal spot, while closely adjoining the membrane there remains only a very thin but unbroken lining of the plasmoid material. The intermediate space is filled with a clear fluid, but the layer which lines the membrane retains its connection with the mass around the germinal vesicle by means of numerous fine threads which traverse the space filled with fluid.... At about the time when the formation of the pseudocells in the egg is completed the germinal spot undergoes a retrogressive metamorphosis, it loses its circular outline and it now appears as if coagulated; then it breaks up into small fragments, and I am fairly confident that these become dissolved. The germinal vesicle ... becomes, on the egg assuming a spherical form, drawn into an eccentric position towards the pole of the egg directed outwards, where it lies close to the surface and only covered by a very thin layer of plasma. In this situation its degeneration now begins, and ends in its complete disappearance. The granular contents become more and more fluid; at the same time part of them pass out through the membrane. This, which so far was firmly stretched, next collapses to a somewhat egg-like sac, whose wall is thickened and in places folded.
[Pg 219]The inner mass which up to this time has
remained compact now breaks up into separate highly refractive bodies, of
spherical or angular form and of very different sizes; between them, here
and there, are scattered drops of a fluid fat.... I am very much inclined
to regard the solid bodies in question as fat or as that peculiar
modification of albuminoid bodies which we recognise as the certain
forerunner of the formation of fat in so many pathologically altered
tissues; and therefore to refer the disappearance of the germinal vesicle
to a fatty degeneration. On one occasion I believe that I observed an
opening in the membrane at this stage; if this is a normal condition it
would be possible to believe that its solid contents passed out and were
taken up in the surrounding plasma. What becomes of the membrane I am
unable to say; in any case the germinal vesicle has vanished to the very
last trace before impregnation occurs.
Kleinenberg clearly finds that the germinal vesicle disappears completely before the appearance of the Richtungskörper, in which he states a pseudocell or yolk-sphere is usually found.
The connection between the Richtungskörper and the germinal vesicle is not a result of strict observation, and there can be no question that the evidence in the case of invertebrates tends to prove that the germinal vesicle in no case disappears owing to its extrusion from the egg, but that if part of it is extruded from the egg as Richtungskörper this occurs when its constituents can no longer be distinguished from the remainder of the yolk. This is clearly the case in Hydra, where, as stated above, one of the pseudocells or yolk-spheres is usually found imbedded in the Richtungskörper.
My observations on the Skate tend to shew that, in its case, the membrane of the germinal vesicle is extruded from the egg, though they do not certainly prove this. That conclusion is however supported by the observations of Schenk[77]. He found in the impregnated, but not yet segmented, germinal disc a cavity which, as he suggests, might well have been occupied by the germinal vesicle. It is not unreasonable to suppose that the membrane, being composed of formed matter and able only to take a passive share in vital functions, could, without thereby influencing the constitution of the ovum, be ejected.
If we suppose, and this is not contradicted by observation, that the Richtungskörper is either only the metamorphosed membrane of the germinal vesicle with parts of the yolk, or part of the yolk alone, and assume that in Oellacher's observations [Pg 220] only the membrane and not the contents were extruded from the egg, it would be possible to frame a consistent account of the behaviour of the germinal vesicle throughout the animal kingdom, which may be stated in the following way.
The germinal vesicle usually before, but sometimes immediately after impregnation undergoes atrophy and its contents become indistinguishable from the remainder of the egg. In those cases in which its membrane is very thick and resistent, e.g. Osseous and Elasmobranch Fishes, Birds, etc., this may be incapable of complete resorption, and be extruded bodily from the egg. In the case of most ova, it is completely absorbed, though at a subsequent period it may be extruded from the egg as the Richtungskörper. In all cases the contents of the germinal vesicle remain in the ovum.
In some cases the germinal vesicle is stated to persist and to undergo division during the process of segmentation; but the observations on this point stand in need of confirmation.
My investigations shew that the germinal vesicle atrophies in the Skate before impregnation, and in this respect accord with very many recent observations. Of these the following may be mentioned.
(1) Oellacher (Bird, Osseous Fish). (2) Götte (Bombinator igneus). (3) Kupffer (Ascidia canina). (4) Strasburger (Phallusia mamillata). (5) Kleinenberg (Hydra). (6) Metschnikoff (Geryonia, Polyzenia leucostyla, Epibulia aurantiaca, and other Hydrozoa).
This list is sufficient to shew that the disappearance of the germinal vesicle before impregnation is very common, and I am unacquainted with any observations tending to shew that its disappearance is due to impregnation.
In some cases, e.g. Asterocanthion[78], the germinal vesicle vanishes after the spermatozoa have begun to surround the egg; but I do not know that its disappearance in these cases has been shewn to be due to impregnation. To do so it would be necessary to prove that in ripe eggs let loose from the ovary, but not fertilized, the germinal vesicle did not undergo the same changes as in the case of fertilized eggs; and this, as far as I [Pg 221] know, has not been done. After the disappearance of the germinal vesicle, and before the first act of division, a fresh nucleus frequently appears [—vide—Auerbach (Ascaris nigrovenosa), Fol (Geryonia), Kupffer (Ascidia canina), Strasburger (Phallusia mamillata), Flemming (Anodon), Götte (Bombinator igneus)], which is generally stated to vanish before the appearance of the first furrow; but in some cases (Kupffer and Götte, and as studied with especial care, Strasburger) it is stated to divide. Upon the second nucleus, or upon its relation to the germinal vesicle, I have no observations; but it appears to me of great importance to determine whether this fresh nucleus arises absolutely de novo, or is formed out of the matter of the germinal vesicle.
The germinal vesicle is situated in a bed of finely divided yolk-particles. These graduate insensibly into the coarser yolk-spherules around them, though the band of passage between the coarse and the finer yolk-particles is rather narrow. The mass of fine yolk-granules may be called the germinal disc. It is not to be looked upon as diverging in any essential particular from the remainder of the yolk, for the difference between the two is one of degree only. It contains in fact a larger bulk of active protoplasm, as compared with yolk-granules, than does the remainder of the ovum. The existence of this agreement in kind has been already strongly insisted on in my preliminary paper; and Schultz (loc. cit.) has arrived at an entirely similar conclusion, from his own independent observations.
One interesting feature about the germinal disc at this period is its size.
My observations upon it have been made with the eggs of the Skate (Raja) alone; but I think that it is not probable that its size in the Skate is greater than in Scyllium or Pristiurus. If its size is the same in all these genera, then the germinal disc of the unimpregnated ovum is very much greater than that portion of the ovum which undergoes segmentation, and which is usually spoken of as the germinal disc in impregnated ova.
I have no further observation on the ripe ovarian ovum; and my next observations concern an ovum in which two furrows have already appeared.
[70] Archiv für Micro. Anat. Vol. XI. 1875.
[71] Quart. Journ. Micro. Science, Oct. 1874. [This edition, No. V.]
[72] Archiv für Micr. Anat. Vol. VIII. p. 1.
[73] Entwicklungsgeschichte der Unke.
[74] Recherches sur la Composition et la Signification de l'Œuf.
[75]
Studien in der Entwicklungsgeschichte der Najaden,
Sitz. d. k. Akad. Wien, Bd. LXXI. 1875.
[76] Hydra. Leipzig, 1872.
[77]
Die Eier von Raja quadrimaculata,
Sitz. der
k. Akad. Wien, Bd. LXVIII.
[78] Agassiz, Embryology of the Star-Fish.
I have not been fortunate enough to obtain an absolutely complete series of eggs during segmentation.
In the cases of Pristiurus and Scyllium only have I had any considerable number of eggs in this condition, though one or two eggs of Raja in which the process was not completed have come into my hands.
In the youngest impregnated Pristiurus eggs, which I have obtained, the germinal disc was already divided into four segments.
The external appearance of the blastoderm, which remains nearly constant during segmentation, has been already well described by Leydig[79].
The yolk has a pale greenish tinge which, on exposure to the air, acquires a yellower hue. The true germinal disc appears as a circular spot of a bright orange colour, and is, according to Leydig's measurements, 1½m. in diameter. Its colour renders it very conspicuous, a feature which is further increased by its being surrounded by a narrow dark line (Pl. 6, fig. 2), the indication of a shallow groove. Surrounding this line is a concentric space which is lighter in colour than the remainder of the yolk, but whose outer border passes by insensible gradations into the yolk. As was mentioned in my preliminary paper (loc. cit.), and as Leydig (loc. cit.) had before noticed, the germinal disc is always situated at the pole of the yolk which is near the rounded end of the Pristiurus egg. It occupies a corresponding position in the eggs of both species of Scyllium (stellare and canicula) near the narrower end of the egg to which the shorter pair of strings is attached. The germinal disc in the youngest egg [Pg 223] examined, exhibited two furrows which crossed each other at right angles in the centre of the disc, but neither of which reached its edge. These furrows accordingly divided the disc into four segments, completely separated from each other at the centre of the disc, but united near its circumference.
I made sections, though not very satisfactorily, of this germinal disc. The sections shewed that the disc was composed of a protoplasmic basis, in which were imbedded innumerable minute spherical yolk-globules so closely packed as to constitute nearly the whole mass of the germinal disc.
In passing from the coarsest yolk-spheres to the fine spherules of the germinal disc, three bands of different-sized yolk-particles have to be traversed. These bands graduate into one another and are without sharp lines of demarcation. The outer of the three is composed of the largest-sized yolk-spherules which constitute the greater part of the ovum. The middle band forms a concentric layer around the germinal disc, and is composed of yolk-spheres considerably smaller than those outside it. Where it cuts the surface it forms the zone of lighter colour immediately surrounding the germinal disc. The innermost band is formed by the germinal disc itself and is composed of spherules of the smallest size. These features are shewn in Pl. 6, fig. 6, which is the section of a germinal disc with twenty-one segments; in it however the outermost band of spherules is not present.
From this description it is clear, as has already been mentioned in the description of the ripe unimpregnated ovum, that the germinal disc is not to be looked upon as a body entirely distinct from the remainder of the ovum, but merely as a part of the ovum in which the protoplasm is more concentrated and the yolk-spherules smaller than elsewhere. Sections shew that the furrows visible on the surface end below, as indeed they do on the surface, before they reach the external limit of the finely granular matter of the germinal disc. There are therefore at this stage no distinct segments: the otherwise intact germinal disc is merely grooved by two furrows.
I failed to observe any nuclei in the germinal disc just described, but it by no means follows that they were not present.
[Pg 224] In the next youngest of the eggs[80] examined the germinal disc was already divided into twenty-one segments. When viewed from the surface (Pl. 6, fig. 3), the segments appeared divided into two distinct groups—an inner group of eleven smaller segments, and an outer group of segments surrounding the former. The segments of both the inner and the outer group were very irregular in shape and varied considerably in size. The amount of irregularity is far from constant and many germinal discs are more regular than the one figured.
In this case the situation of the germinal disc and its relations to the yolk were precisely the same as in the earlier stage.
In sections of this germinal disc (Pl. 6, fig. 6), the groove which separates it from the yolk is well marked on one side, but hardly visible at the other extremity of the section.
Passing from the external features of this stage to those which are displayed by sections, the striking point to be noticed is the persisting continuity of the segments, marked out on the surface, with the floor of the germinal disc.
The furrows which are visible on the surface merely form a pattern, but do not isolate a series of distinct segments. They do not even extend to the limit of the finely granular matter of the germinal disc.
The section represented, Pl. 6, fig. 6, bears out the statements about the segments as seen on the surface. There are three smaller segments in the middle of the section, and two larger at the two ends. These latter are continuous with the coarser yolk-spheres surrounding the germinal disc and are not separated from them by a segmentation furrow.
In a slightly older embryo than the one figured I met with a few completely isolated segments at the surface. These segments were formed by the apparent bifurcation of furrows as they neared the surface of the germinal disc. The segments thus produced are triangular in form. They probably owe their origin to the meeting of two oblique furrows. The last-formed of these furrows apparently ceases to be prolonged after meeting the first-formed furrow. I have not in any case [Pg 225] observed an example of two furrows crossing one another at this stage.
The furrows themselves for the most part are by no means simple slits with parallel sides. They exhibit a beaded structure, shewn imperfectly in Pl. 6, fig. 6, but better in Pl. 6, fig. 6a, which is executed on a larger scale. They present intervals of dilatations where the protoplasms of the segments on the two sides of the furrow are widely separated, alternating with intervals where the protoplasms of the two segments are almost in contact and are only separated from one another by a very narrow space.
A closer study of the germinal disc at this period shews that the cavities which cause the beaded structure of the furrows are not only present along the lines of the furrows but are also found scattered generally through the germinal disc, though far more thickly in the neighbourhood of the furrows. Their appearance is that of vacuoles, and with these they are probably to be compared. There can be little question that in the living germinal disc they are filled with fluid. In some cases, they are collected in very large numbers in the region of a furrow. Such a case as this is shewn in Pl. 6, fig. 6b. In numerous other cases they occur, roughly speaking, alternately on each side of a furrow. Some furrows, though not many, are entirely destitute of these structures. The character of their distribution renders it impossible to overlook the fact that these vacuole-like bodies have important relations with the formation of the segmentation furrows.
Lining the two sides of the segmentation furrows there is present in sections a layer which stains deeply with colouring reagents; and the surface of the blastoderm is stained in the same manner. In neither case is it permissible to suppose that any membrane-like structure is present. In many cases a similar very delicate, but deeply-stained line, invests the vacuolar cavities, but the fluid filling these remains quite unstained. When distinct segments are formed, each of these is surrounded by a similarly stained line.
The yolk-spherules are so numerous, and render even the thinnest section so opaque, that I have failed to make satisfactory observations on the behaviour of the nucleus. I find [Pg 226] nuclei in many of the segments, though it is very difficult even to see them, and only in very favourable specimens can their structure be studied. In some cases, two of them lie one on each side of a furrow; and in one case at the extreme end of a furrow I could see two peculiar aggregations of yolk-spherules united by a band through which the furrow, had it been continued, would have passed. The connection (if any exists) between this appearance and the formation of the fresh nuclei in the segments, I have been unable to elucidate.
The peculiar appearances attending the formation of fresh nuclei in connection with cell-division, which have recently been described by so many observers, have hitherto escaped my observation at this stage of the segmentation, though I shall describe them in a later stage. A nucleus of this stage is shewn on Pl. 6, fig. 6c. It is lobate in form and is divided by lines into areas in each of which a deeply-stained granule is situated.
The succeeding stages of segmentation present from the surface no fresh features of great interest. The somewhat irregular (Pl. 6, figs. 4 and 5) circular line, which divides the peripheral larger from the central smaller segments, remains for a long time conspicuous. It appears to be the representative of the horizontal furrow which, in the Batrachian ovum, separates the smaller pigmented spheres from the larger spheres of the lower pole of the egg.
As the segments become smaller and smaller, the distinction between the peripheral and the central segments becomes less and less marked; but it has not disappeared by the time that the segments become too small to be seen with the simple lens. When the spheres become smaller than in the germinal disc represented on Pl. 6, fig. 5, the features of segmentation can be more easily and more satisfactorily studied by means of sections.
To the features presented in sections, both of the latter and of the earlier blastoderms, I now return. A section of one of the earlier germinal discs, of about the age of the one represented on Pl. 6, fig. 4, is shewn in Pl. 6, fig. 7.
It is clear at a glance that we are now dealing with true segments completely circumscribed on all sides. The peripheral [Pg 227] segments are, as a rule, larger than the more central ones, though in this respect there is considerable irregularity. The segments are becoming smaller by repeated division; but, in addition to this mode of increase, there is now going on outside the germinal disc a segmentation of the yolk, by which fresh segments are being formed from the yolk and added to those which already exist in the germinal disc. One or two such segments are seen in the act of being formed (Pl. 6, fig. 7, f); and it is to be noticed that the furrows which will eventually mark out the segments, do so at first in a partial manner only, and do not circumscribe the whole circumference of the segment in the act of being formed. These fresh furrows are thus repetitions on a small scale of the earliest segmentation furrows.
It deserves to be noticed that the portion of the germinal disc which has already undergone segmentation, is still surrounded by a broad band of small-sized yolk-spherules. It appears to me probable that owing to changes taking place in the spherules of the yolk, which result in the formation of fresh spherules of a small size, this band undergoes a continuous renovation.
The uppermost row of segmentation spheres is now commencing to be distinguished from the remainder as a separate layer which becomes progressively more distinct as segmentation proceeds.
The largest segments in this section measure about the 1/100th of an inch in diameter, and the smallest about 1/300th of an inch.
The nuclei at this stage present points of rather a special interest. In the first place, though visible in many, and certainly present in all the segments[81], they are not confined to these: they are also to be seen, in small numbers, in the band of fine spherules which surrounds the already segmented part of the germinal disc. Those found outside the germinal disc are not confined to the spots where fresh segments are appearing, [Pg 228] but are also to be seen in places where there are no traces of fresh segments.
This fact, especially when taken in connection with the formation of fresh segments outside the germinal disc and with other facts which I shall mention hereafter, is of great morphological interest as bearing upon the nature and homologies of the food-yolk. It also throws light upon the behaviour and mode of increase of the nuclei. All the nuclei, both those of the segments and those of the yolk, have the peculiar structure I described in the last stage.
In specimens of this stage I have been able to observe certain points which have an important bearing upon the behaviour of the nucleus during cell-division.
Three figures, illustrating the behaviour of the nucleus, as I have seen it in sections of blastoderms hardened in chromic acid, are shewn in Pl. 6, figs. 7a, 7b and 7c.
In the place of the nucleus is to be seen a sharply defined figure (Fig. 7a) stained in the same way as the nucleus or more deeply. It has the shape of two cones placed base to base. From the apex of each cone there diverge towards the base a series of excessively fine striæ. At the junction between the two cones is an irregular linear series of small deeply stained granules which form an apparent break between the two. The line of this break is continued very indistinctly beyond the edge of the figure on each side.
From the apex of each cone there diverge outwards into the protoplasm of the cell a series of indistinct markings. They are rendered obscure by the presence of yolk-spherules, which completely surround the body just described, but which are not arranged with any reference to these markings. These latter striæ, diverging from the apex of the cone, are more distinctly seen when the apex points to the observer (Fig. 7b), than when a side of the cone is in view.
The striæ diverging outwards from the apices of the cones must be carefully distinguished from the striæ of the cones themselves. The cones are bodies quite as distinctly differentiated from the protoplasm of the cell as nuclei, while the striæ which diverge from their apices are merely structures in the general protoplasm of the cell.
[Pg 229] In some cells, which contain these bodies, no trace of a commencing line of division is visible. In other cases (Fig. 7c), such a line of division does appear and passes through the junction of the two cones. In one case of this kind I fancied I could see (and have represented) a coloured circular body in each cone. I do not feel any confidence that these two bodies are constantly present; and even where visible they are very indistinct.
Instead of an ordinary nucleus a very indistinctly marked vesicular body sometimes appears in a segment; but whether it is to be looked on as a nucleus not satisfactorily stained, or as a nucleus in the act of being formed, I cannot decide.
With reference to the situation of the cone-like bodies I have described I have made an observation which appears to me to be of some interest. I find that bodies of this kind are found in the yolk completely outside the germinal disc. I have made this observation, in at least two cases which admitted of no doubt (vide Fig. 7, nx´).
We have therefore the remarkable fact, that whatever connection these bodies may have with cell-division, they can occur in cases where this is altogether out of the question and where an increase in the number of nuclei can be their only product.
These are the main facts which I have been able to determine with reference to the nuclei of this stage; but it will conduce to clearness if I now finish what I have to say upon this subject.
At a still later stage of segmentation the same peculiar bodies are to be seen as during the stage just described, but they are rarer; and, in addition to them, other bodies are to be seen of a character intermediate between ordinary nuclei and the former bodies.
Three such are represented in Pl. 6, figs. 8a, 8b, 8c. In all of these there can be traced out the two cones, which are however very irregular. The striation of the cones is still present, but is not nearly so clear as it was in the earlier stage.
In addition to this, there are numerous deeply stained granules scattered about the two figures which resemble exactly the granules of typical nuclei.
[Pg 230] All these bodies occupy the place of an ordinary nucleus, they stain like an ordinary nucleus and are as sharply defined as an ordinary nucleus.
There is present around some of these, especially those situated in the yolk, the network of lines of the yolk described by me in a preliminary paper[82], and I feel satisfied that there is in some cases an actual connection between the network and the nuclei. This network I shall describe more fully hereafter.
Further points about these figures and the nuclei of this stage I should like to have been able to observe more completely than I have done, but they are so small that with the highest powers I possess (Zeiss, Immersion No. 2 = 1/15 in.) their complete and satisfactory investigation is not possible.
Most of the true nuclei of the cells of the germinal disc are regularly rounded; those however of the yolk are frequently irregular in shape and often provided with knob-like processes. The gradations are so complete between typical nuclei and bodies like that shewn (Pl. 6, fig. 8c) that it is impossible to refuse the name of nucleus to the latter.
In many cases two nuclei are present in one cell.
In later stages knob-like nuclei of various sizes are scattered in very great numbers in the yolk around the blastoderm (vide Pl. 7). In some cases it appears to me that several of these are in close juxtaposition, as if they had been produced by the division of one primitive nucleus. I do not feel absolutely confident that this is the case, owing to the fact that in the investigation of a knobbed body there is great difficulty in ascertaining that the knobs, which appear separate in one plane, are not in reality united in another.
I have, in spite of careful search, hitherto failed to find amongst these later nuclei cone-like figures, similar to those I found in the yolk during segmentation. This is the more remarkable since in the early stages of segmentation, when very few nuclei are present in the yolk, the cone-like figures are not uncommon; whereas, in the latter stages of development when the nuclei of the yolk are very common and obviously increasing rapidly, such figures are not to be met with.
[Pg 231] In no case have I been able to see a distinct membrane round any of the nuclei.
I have hitherto attempted to describe the appearances bearing on the behaviour of the nuclei in as objective a manner as possible.
My observations are not as complete as could be desired; but, taken in conjunction with those of other investigators, they appear to me to point towards certain definite conclusions with reference to the behaviour of the nucleus in cell-division.
The most important of these conclusions may be stated as follows. In the act of cell-division the nuclei of the resulting cells are formed from the nucleus of the primitive cell.
This may occur:—
(1) By the complete solution of the old nucleus within the protoplasm of the mother cell and the subsequent reaggregation of its matter to form the nuclei of the freshly formed daughter cells,
(2) By the simple division of the nucleus,
(3) Or by a process intermediate between these two where part of the old nucleus passes into the general protoplasm and part remains always distinguishable and divides; the fresh nucleus being in this case formed from the divided parts as well as from the dissolved parts of the old nucleus.
Included in this third process it is permissible to suppose that we may have a series of all possible gradations between the extreme processes 1 and 2. If it be admitted, and the evidence we have is certainly in favour of it, that in some cases, both in animal and vegetable cells, the nucleus itself divides during cell division, and in others the nucleus completely vanishes during the cell-division, it is more reasonable to suspect the existence of some connection between the two processes, than to suppose that they are entirely different in kind. Such a connection is given by the hypothesis I have just proposed.
The evidence for this view, derived both from my own observations and those of other investigators, may be put as follows.
The absolute division of the nucleus has been stated to occur in animal cells, but the number of instances where the [Pg 232] evidence is quite conclusive are not very numerous. Recently F. E. Schultze[83] appears to have observed it in the case of an Amœba in an altogether satisfactory manner. The instance is quoted by Flemming[84]. Schultze saw the nucleus assume a dumb-bell shape, divide, and the two halves collect themselves together. The whole process occupied a minute and a half and was shortly followed by the division of the Amœba, which occupied eight minutes. Amongst vegetable cells the division of the nucleus seems to be still rarer than with animal cells. Sachs[85] admits the division of the nucleus in the case of the parenchyma cells of certain Dicotyledons (Sambucus, Helianthus, Lysimachia, Polygonum, Silene) on the authority of Hanstein.
The division of the nucleus during cell-division, though seemingly not very common, must therefore be considered as a thoroughly well authenticated occurrence.
The frequent disappearance of the nucleus during cell-division is now so thoroughly recognised, both for animal and vegetable cells, as to require no further mention.
In many cases the partial or complete disappearance of the nucleus is accompanied by the formation of two peculiar star-like figures. Appearances of the kind have been described by Fol[86], Flemming[87], Auerbach[88] and possibly also Oellacher[89] as well as other observers.
These figures[90] are possibly due to the streaming out of the [Pg 233] protoplasm of the nucleus into that of the cell[91]. The appearance of striation may on this hypothesis be explained as due to the presence of granules in the protoplasm. When the streaming out of the protoplasm of a nucleus into that of a cell takes place, any large granule which cannot be moved by the stream will leave behind it a slack area where there is no movement of the fluid. Any granules which are carried into this area will remain there, and by the continuation of a process of this kind a row of granules may be formed, and a series of such rows would produce an appearance of striation. In many cases, e.g. Anodon, vide Flemming[92], even the larger yolk-spherules are arranged in this fashion.
On the supposition that the striation of these figures is due to the outflow from the nucleus, the appearances presented in Elasmobranchii admit of the following explanation.
The central body consisting of two cones (figs. 7a, 7c) is almost without question the remnant of the primitive nucleus. This is shewn by its occupying the same position as the primitive nucleus, staining in the same way, and by there being a series of insensible gradations between it and a typical nucleus. The contents must be supposed to be streaming out from the two apices of the cones, as appears from the striæ in the body converging on each side towards the apex, and then diverging again from it. In my specimens the yolk-spherules are not arranged with any reference to the radiating striation.
It is very likely that in the cases of the disappearance of the nucleus, its protoplasm streams out in two directions, towards the two parts of the cell which will eventually become separated from each other; and probably, after the division, the matter of the old nucleus is again collected to form two fresh nuclei.
In some cases of cell-division a remnant of the old nucleus is stated to be visible after the fresh nuclei have appeared. These cases, of which I have not seen full accounts, are perhaps analogous to what occasionally happens with the germinal [Pg 234] vesicle of an ovum. The whole of the contents of the germinal vesicle become at its disappearance mingled with the protoplasm of the ovum, but the resistant membrane remains and is eventually ejected from the egg, vide p. 215 et seq. If the remnant of the old nucleus in the cases described is nothing more than its membrane, no difficulty is offered to the view that the constituents of the old nucleus may help to form the new ones.
In many cases the total bulk of the new nuclei is greater than that of the old one; in such instances part of the protoplasm of the cell necessarily has a share in forming the new nuclei.
Although, in instances where the nucleus vanishes, an absolute demonstration of the formation of the fresh nuclei from the matter of the old one is not possible; yet, if cases of the division of the old nucleus to form the new ones be admitted to exist, the derivation in the first process of the fresh nuclei from the old ones must be postulated in order to maintain a continuity between the two processes of formation; and, as I have attempted to shew, all the circumstantial evidence is in favour of it.
Admitting the existence of the two extreme processes of nuclear formation, I wish to shew that my results in Elasmobranchii tend to demonstrate the existence of intermediate steps between them. The first figures I described of two opposed cones, appear to me almost certainly to represent nuclei in the act of dissolution; but though a portion of the nucleus may stream out into the yolk, I think it impossible that the whole of it does[93].
I described these bodies in two states. An earlier one, in which the two cones were separated by an irregular row of deeply stained granules; and a later one in which a furrow had already appeared dividing the cones as well as the cell. In neither of these conditions could I see any signs of the body vanishing completely. It was as clearly defined and as deeply stained as an ordinary nucleus, and in its later condition the signs of the streaming out of material from its pointed extremities were less marked than in the earlier stage.
[Pg 235] All these facts, to my mind, point to the view that these cone-like bodies do not disappear, but form the basis for the new nuclei. Possibly the body visible in each cone in the later stage, was the commencement of this new nucleus. Götte[94] has figured structures somewhat similar to these bodies, but I hardly understand either his figure or his account sufficiently clearly to be able to pronounce upon the identity of the two. In case they are identical, Götte gives a very different explanation of them from my own[95].
A second of my results, which points to a series of intermediate steps between division and solution of the nucleus, is the distribution in time of the peculiar cone-like bodies. These are present in fair abundance at an early period of segmentation, when there are but few nuclei either in the blastoderm or the yolk. But at later periods, when there are both more nuclei, especially in the yolk, and they are also increasing in numbers more rapidly than before, no bodies of this kind are to be seen. This fact becomes the more striking from the lobate appearance of the later nuclei of the yolk, an appearance which exactly suits the hypothesis of the rapid budding off of fresh nuclei.
The observations of R. Hertwig[96] on the gemmation of Podophrya gemmipara, support my interpretation of the knobbed condition of the nuclei. Hertwig finds (p. 47) that
The horse-shoe shaped nucleus grows out into numerous anastomosing projections. Over the free ends of the projections little knobs appear on the surface of the body, into which the lengthening ends of the processes of the nucleus grow up. Here they bend themselves into a horse-shoe form. The newly-formed nucleus then separates from the original nucleus, and afterwards the bud containing it from the body.
From the peculiar arrangement of the net-work of lines of the yolk around these knobbed nuclei, it is reasonable to conclude that interchange of material between the protoplasm of [Pg 236] the yolk and the nuclei is still taking place, even during the later periods.
These facts about the distribution in time of the cone-like bodies afford a strong presumptive evidence of a change in the manner of nuclear increase.
The last argument I propose urging on this head is derived from the bodies (Pl. 6, fig. 8a, b, c) which I have described as intermediate between the true cone-like bodies and typical nuclei. They appear to afford evidence of less and less of the matter of the nucleus streaming out into the yolk and of a large proportion of it becoming divided.
The conclusion to be derived from all these facts is that for Elasmobranchii in the earlier stages of segmentation, and during the formation of fresh segments, a partial solution of the old nucleus takes place, but all its constituents serve for the reconstruction of the fresh nuclei.
In later periods of development a still smaller part of the nucleus becomes dissolved, and the rest divides; but the two fresh nuclei are still derived from the two sources. After the close of segmentation the fresh nuclei are formed by a simple division of the older ones.
The appearance of the cone-like bodies in the yolk outside the germinal disc is a point of some interest. It demonstrates in a conclusive manner that whatever influence (if any) the nucleus may have in ordinary cases of cell division, yet it may undergo changes of a precisely similar character to those which it experiences during cell division, without exerting any influence on the surrounding protoplasm[97]. If the lobate nuclei are also nuclei undergoing division, we have in the egg of an Elasmobranch examples of all the known forms of nuclear increase unaccompanied by cell division.
The next stage in the segmentation does not present so many features of interest as the last one. The segments are [Pg 237] now so small, as to be barely visible from the surface with a simple lens. A section of an embryo of this stage is represented in Pl. 6, fig. 8. The section, which is drawn on the same scale as the section belonging to the last stage, serves to shew the relative size of the segments in the two cases.
The epiblast is now more distinct than it was. The segments composing it are markedly smaller than the remainder of the cells of the germinal disc, but possess nuclei of an absolutely larger size than do the other cells. They are irregular in shape, with a slight tendency to be columnar. An average segment of this layer measures about 1/700 inch.
The cells of the lower layer are more polygonal than those of the epiblast, and are decidedly larger. An average specimen of the larger cells of the lower layer measures about 1/400 in. in diameter, and is therefore considerably smaller than one of the smallest cells of the last stage. The formation of fresh segments from the yolk still continues with fair rapidity, but nearly comes to an end shortly after this.
Of the nuclei of the lower layer cells, there is not much to add to what has already been said. Not infrequently two nuclei may be observed in a single cell.
The nuclei in the yolk which surrounds the germinal disc are more numerous than in the earlier periods, and are now to be met with in fair numbers in every section (fig. 8, n´).
These are the main features which characterise the present stage, they are in all essential points similar to those of the last stage, and the two germinal discs hardly differ except in the size of the segments of which they are composed.
In the last stage which I consider as belonging to the segmentation, the cells of the whole blastoderm have become smaller (Pl. 6, fig. 9).
The epiblast (ep) now consists of a very marked layer of columnar cells. It is, as far as I have been able to observe, never more than one cell deep. The cells of the lower layer are of an approximately uniform size, though a few of those at the circumference of the blastoderm considerably exceed the remainder in the bulk.
There are two fresh features of importance in germinal discs of this age.
[Pg 238] Instead of being but indistinctly separated from the surrounding yolk, the blastoderm has now very clearly defined limits.
This is an especially marked feature of preparations made with osmic acid. In these there may frequently be seen a deeply stained doubly contoured line, which forms the limit of the yolk, where it surrounds the germinal disc. Lines of this kind are often to be seen on the surface of the yolk, or even of the blastoderm, but are probably to be regarded as products of reagents, rather than as organised structures. The outline of the germinal disc is well rounded, though it is occasionally broken, from the presence of a larger cell in the act of being formed from the yolk.
It is not probable that any great importance is to be attached to the comparative distinctness of the outline of the germinal disc at this stage, which is in a great measure due to a cessation in the formation of fresh cells in the surrounding yolk, and in part to the small and comparatively uniform size of the cells of the germinal disc.
The formation of fresh cells from the yolk nearly comes to an end during this period, but it still continues on a small scale.
The number of the nuclei around the germinal disc has increased.
Another feature of interest which first becomes apparent during this stage is the asymmetry of the germinal disc. If a section were made through the germinal disc, as it lay in situ in the egg capsule, parallel or nearly so to the long axis of the capsule, one end of the section would be found to be much thicker than the other. There would in fact be a far larger collection of cells at one extremity of the germinal disc than at the other. The end at which this collection of cells is formed points towards the end of the egg capsule opposite to that near which the yolk is situated. This collection of cells is the first trace of the embryo; and with its appearance the segmentation may be supposed to terminate.
The section I have represented, though not quite parallel to the long axis of the egg, is sufficiently nearly so to shew the greater mass of cells at the embryonic end of the germinal disc.
[Pg 239] This very early appearance of a distinction in the germinal disc between the extremity at which the embryo appears and the non-embryonic part of the disc, besides its inherent interest, has a further importance from the fact that in Osseous Fishes a similar occurrence takes place. Oellacher[98] and Götte[99] both agree as to the very early period at which a thickening of one extremity of the blastoderm in Osseous Fishes is formed, which serves to indicate the position at which the embryo will appear. There are many details of development in which Osseous Fish and Elasmobranchii agree, which, although if taken individually are without any great importance, yet serve to shew how long even insignificant features in development may be retained.
* * * * *
The segmentation of the Elasmobranch egg presents in most of its features great regularity, and exhibits in its mode of occurrence the closest resemblance to that in other meroblastic vertebrate ova.
There is, nevertheless, one point with reference to which a slight irregularity may be observed. In almost all eggs segmentation commences by, what for convenience may be called, a vertical furrow which is followed by a second vertical furrow at right angles to the first. The third furrow however is a horizontal one, and cuts the other two at right angles. This method of segmentation must be looked on as the normal one, in almost all the important groups of the animal kingdom, both for the so-called holoblastic and meroblastic eggs, and the gradations intermediate between the two. The Frog amongst vertebrates exhibits a most typical instance of this form of segmentation.
In Elasmobranchii the first two furrows are formed in a perfectly normal manner, but though I have not observed the actual formation of the next furrow, yet from the later stages, which I have observed, I conclude that it is parallel to one of the first formed furrows; and it is fairly certain that, not till a considerably later period, is a furrow homologous with the horizontal furrow of the Batrachian egg formed. This furrow appears to be represented in the Elasmobranch segmentation [Pg 240] by the irregular circumscription of a body of central smaller spheres from a ring of peripheral larger ones (vide Pl. 6, figs. 3, 4 and 5).
In the Bird the representative of the horizontal furrow appears relatively much earlier. It is formed when there are eight segments marked out on the surface of the germinal disc[100]. From Oellacher's[101] account of the segmentation in the fowl[102] it seems certain, as might be anticipated, that this furrow is nearly parallel to the surface of the disc, so that it cuts the earlier formed vertical furrows and causes the segments of the germinal disc to be completely circumscribed below as well as at the surface. In the Elasmobranch egg this is not the case; so that, even after the smaller central segments have become separated from the outer ring of larger ones, none of the segments of the disc are completely circumscribed, and only appear to be so in surface views (vide Pl. 6, fig. 6). Segmentation in the Elasmobranch egg differs in the following particulars from that in the Bird's egg:
(1) The equivalent of the horizontal furrow of the Batrachian egg appears much later than in the Bird.
(2) When it has appeared it travels inwards much more slowly.
As a result of these differences, the segments of the germinal disc of the Birds' eggs are much earlier circumscribed on all sides than those of the Elasmobranch egg.
As might be expected, the segmentation of the Elasmobranch egg resembles in many points that of Osseous Fishes (vide Oellacher[103] and Klein[104]). It may be noticed, that with Osseous as with Elasmobranch Fishes, the furrow corresponding with the horizontal furrow of the Amphibian's egg does not appear at as early a period as is normal. The third furrow of an Osseous Fish egg is parallel to one of the first formed pair.
In Oellacher's[105] figures, Pl. 23, figs. 19-21, peculiar beadings [Pg 241] of the sides of the earlier formed furrows are distinctly shewn. No mention of these is made in the text, but they are unquestionably similar to those I have described in the Elasmobranch furrows. In the case of Elasmobranchii I pointed out that not only were the sides of the furrow beaded, but that there appeared in the protoplasm, close to the furrows, peculiar vacuole-like cavities, precisely similar to the cavities which were the cause of the beadings of the furrows.
The presence of these seems to shew that the molecular cohesion of the protoplasm becomes, as compared with other parts, much diminished in the region where a furrow is about to appear, so that before the protoplasm finally gives way along a particular line to form a furrow, its cohesion is broken at numerous points in this region, and thus a series of vacuole-like spaces is formed.
If this is the true explanation of the formation of these spaces, their presence gives considerable support to the views of Dr Kleinenberg upon the causes of segmentation, so clearly and precisely stated in his monograph upon Hydra; and is opposed to any view which regards the forces which come into play during segmentation as resident in the nucleus.
I have not observed the peculiar threads of protoplasm which Oellacher[106] describes as crossing the commencing segmentation furrows. I have also failed to discover any signs of a concentration of the yolk-spherules, round one or two centres, in the segmentation spheres, similar to that observed by Oellacher in the segmenting eggs of Osseous Fish. The appearances observed by him are probably connected with the behaviour of the nucleus during segmentation, and are related to the curious bodies I have already described.
With reference to the nuclei which Oellacher[107] has described as occurring in the eggs of Osseous Fish during segmentation, there can, I think, be little doubt that they are identical with the peculiar nuclei in the Elasmobranch eggs.
He[108] says:
In an unsegmented germ there occurred at a certain point in the section ... a small aggregation of round bodies. I do not feel satisfied whether these aggregations represent one or more nuclei.
[Pg 242] Fig. 29 shews such aggregation; by focusing at its optical section eleven unequally large rounded bodies measuring from 0.004 - 0.009 mm. may be distinguished. They lay as if in a multilocular gap in the germ mass, which however they did not quite fill. In each of these bodies there appeared another but far smaller body. These aggregations were distinguished from the germ by an especially beautiful intense violet gold chloride colouration of their elements. The smaller elements contained in the larger were still more intensely coloured than the larger.
He further states that these aggregations equal the segments in number, and that the small bodies within the elements are not always to be seen with the same distinctness.
Oellacher's description as well as his figures of these bodies leaves no doubt in my mind that they are exactly similar bodies to those which I have already spoken of as nuclei, and the characteristic features of which I have shortly mentioned, and shall describe more fully at a later stage. A moderately full description of them is to be found in my preliminary paper[109].
Their division into a series of separate areas each with a deeply-stained body, as well as the staining of the whole of them, exactly corresponds to what I have found. That each is a single nucleus is quite certain, though their knobbed form might occasionally lead to the view of their being divided. This knobbed condition, observed by Oellacher as well as myself, certainly supports the view, that they are in the act of budding off fresh nuclei. Oellacher conceives, that the areas into which these nuclei are divided represent a series of separate bodies—this according to my observations is not the case. Nuclei of the same form have already been described in Nephelis, and are probably not very rare. They pass by insensible gradations into ordinary nuclei with numerous granules.
One marked feature of the segmentation of the Elasmobranch egg is the continuous advance of the process of segmentation into the yolk and the assimilation of this into the germ by the direct formation of fresh segments out of it. Into the significance of this feature I intend to enter fully hereafter; but it is interesting to notice that Oellacher's descriptions point to a similar feature in the segmentation of Osseous Fish. This however consists chiefly in the formation of fresh segments [Pg 243] from the lower parts of the germinal disc which in Osseous Fish is more distinctly marked off from the food-yolk than in Elasmobranchii.
I conclude my description of the segmentation by a short account of what other investigators have written about its features in these fishes. One of the earliest descriptions of this process was given by Leydig[110]. To his description of the germinal disc, I have already done full justice.
In the first stage of segmentation which he observed 20-30 segments were already visible on the surface. In each of these he recognized a nucleus but no nucleolus.
He rightly states that the segments have no membrane, and describes the yolk-spherules which fill them.
The next investigator is Gerbe[111]. I have unfortunately been unable to refer to this elaborate paper, but I gather from an abstract that M. Gerbe has given a careful description of the external features of segmentation.
Schenk[112] has also made important investigations on the subject. He considers that the ovum is invested with a very delicate membrane. This membrane I have failed to find a trace of, and agree with Leydig[113] in denying its existence. Schenk further found that after impregnation, but before segmentation, the germinal disc divided itself into two layers, an upper and a lower. Between the two a cavity made its appearance which Schenk looks upon as the segmentation cavity. Segmentation commences in the upper of the two layers, but Schenk does not give a precise account of the fate of the lower. I have had no opportunity of investigating the impregnated ovum before the commencement of segmentation, but my observations upon the early stages of this process render it clear that no division of the germinal disc exists subsequently [Pg 244] to the commencement of segmentation, and that the cavity discovered by Schenk can have no connection whatever with the segmentation cavity. I am indeed inclined to look upon this cavity as an artificial product. I have myself met with somewhat similar appearances, after the completion of segmentation, which were caused by the non-penetration of my hardening reagent beyond a certain point.
Without attempting absolutely to explain the appearances described by Professor Schenk, I think that his observations ought to be repeated, either by himself or some other competent observer.
Several further facts are recorded by Professor Schenk in his interesting paper. He states that immediately after impregnation, the germinal disc presents towards the yolk a strongly convex surface, and that at a later period, but still before the commencement of segmentation, this becomes flattened out. He has further detected amœboid movements in the disc at the same period. As to the changes of the germinal disc during segmentation, his paper contains no facts of importance.
Next in point of time to the paper of Schenk, is my own preliminary account of the development of the Elasmobranch Fishes[114]. In this a large number of the facts here described in full are briefly alluded to.
The last author who has investigated the segmentation in Elasmobranchii, is Dr Alexander Schultz[115]. He merely states that he has observed the segmentation, and confirms Professor Schenk's statements about the amœboid movements of the germinal disc.
EXPLANATION OF PLATE 6.
Fig. 1. Section through the germinal disc of a ripe ovarian ovum of the Skate. gv. germinal vesicle.
Fig. 2. Surface-view of a germinal disc with two furrows.
Figs. 3, 4, 5. Surface-views of three germinal discs in different stages of segmentation.
[Pg 245] Fig. 6. Section through the germinal disc represented in fig 3. n. nucleus; x. edge of germinal disc. The engraver has not accurately copied my original drawings in respect to the structure of the segmentation furrows.
Figs. 6a and 6b. Two furrows of the same germinal disc more highly magnified.
Fig. 6c. A nucleus from the same germinal disc highly magnified.
Fig. 7. Section through a germinal disc of the same age as that represented in fig. 4. n. nucleus; nx. modified nucleus; nx´. modified nucleus of the yolk; f. furrow appearing in the yolk around the germinal disc.
Figs. 7a, 7b, 7c. Three segments with modified nuclei from the same germinal disc.
Fig. 8. Section through a somewhat older germinal disc. ep. epiblast; n´. nuclei of yolk.
Figs. 8a, 8b, 8c. Modified nuclei from the yolk from the same germinal disc.
Fig. 8d. Segment in the act of division from the same germinal disc.
Fig. 9. Section through a germinal disc in which the segmentation is completed. It shews the larger collection of cells at the embryonic end of the germinal disc than at the non-embryonic. ep. epiblast.
[79] Rochen und Haie.
[80] The germinal disc figured was from the egg of a Scyllium stellare and not Pristiurus, but I have also sections of a Pristiurus egg of the same age, which do not differ materially from the Scyllium sections.
[81] In the figure of this stage, I have inserted nuclei in all the segments. In the section from which the figure was taken, nuclei were not to be seen in many of the segments, but I have not a question that they were present in all of them. The difficulty of seeing them is, in part, due to the yolk-spherules and in part to the thinness of the section as compared with the diameter of a segmentation sphere.
[82] Loc. cit.
[83] Archiv f. Micr. Anat. XI. p. 592.
[84]
Entwicklungsgeschicte der Najaden,
LXXI. Bd. der Sitz. der k. Acad. Wien, 1875.
[85] Text-Book of Botany, English trans. p. 19.
[86]
Entw. d. Geryonideneies.
Jenaische
Zeitschrift, Bd. VII.
[87] Loc. cit.
[88] Organologische Studien, Zweites Heft.
[89]
Beiträge z. Entwicklungsgeschichte der
Knochenfischen.
Zeit. für Wiss. Zoologie. Bd.
XXII. 1872.
[90] The memoirs of Auerbach and Strasburger (Zellbildung u. Zelltheilung) have unfortunately come into my hands too late for me to take advantage of them. Especially in the magnificent monograph of Strasburger I find drawings precisely resembling those from my specimens already in the hands of the engraver. Strasburger comes to the conclusion from his investigations that the modified nucleus always divides and never vanishes as is usually stated. If his views on this point are correct part of the hypothesis I have suggested above is rendered unnecessary. The striæ of the protoplasm, which in accordance with Auerbach's view I have considered as being due to a streaming out of the matter of the nucleus, he regards as resulting from a polarity of the particles in the cell and the attraction of the nucleus. My own investigations though, as far as they go, quite in accordance with those of Strasburger, do not supply any grounds for deciding on the meaning of these striæ; and in some respects they support Strasburger's views against those of other observers, since they demonstrate that in Elasmobranchii the modified nucleus does actually divide.
[91] This is the view which has been taken by Auerbach (Organologische Studien).
[92] Loc. cit.
[93] After Strasburger's observation it must be considered very doubtful whether the streaming out of the contents of the nucleus, in the manner implied in the text, really takes place.
[94] Entwicklungsgeschite der Unke, Pl. 1, fig. 18.
[95] As I before mentioned, Strasburger (Zellbildung u. Zelltheilung) has represented bodies precisely similar to those I have described, which appear during the segmentation in the egg of Phallusia mammillata as well as similar figures observed by Butschli in eggs of Cucullanus elegans and Blatta Germanica. The figures in this monograph are the only ones I have seen, which are identical with my own.
[96] Morphologisches Jahrbuch, Bd. 1. pp. 46, 47.
[97] Strasburger's (loc. cit.) arguments about the influence of the nucleus in cell division are not to my mind conclusive; though not without importance. It is difficult to reconcile his views with the facts of cell division observable during the Elasmobranch segmentation; but even if their truth be admitted they do not bring us much nearer to a satisfactory understanding of cell division, unless accompanied (and at present they are not so) by a rational explanation of the forces which produce the division of the nucleus.
[98] Zeitschrift für Wiss. Zoologie, Bd. XXIII. 1873.
[99] Archiv für Micr. Anat. Bd. IX. 1873.
[100] Vide Elements of Embryology, p. 23.
[101] Stricker's Studien, 1869, Pt. I, Pl. II. fig. 4.
[102] Unfortunately Professor Oellacher gives no account of the surface appearance of the germinal discs of which he describes the sections. It is therefore uncertain to what period his sections belong.
[103] Zeitschrift für Wiss. Zool. Bd. XXII. 1872.
[104] Monthly Microscopical Journal, March, 1872.
[105] Loc. cit.
[106] Loc. cit.
[107] Loc. cit.
[108] Loc. cit. pp. 410, 411, &c.
[109] Loc. cit. p. 415. [This Edition, p. 64.]
[110] Rochen u. Haie. It is here mentioned that Coste observed the segmentation in these fishes.
[111]
Recherches sur la segmentation des products adventifs
de l'œuf des Plagiostomes et particulièrement des Raies.
Robin,
Journal de l'Anatomie et de la Physiologie,
p. 609, 1872.
[112]
Die Eier von Raja quadrimaculata innerhalb der
Eileiter.
Sitz. der k. Akad. Wien. Vol. LXXIII. 1873.
[113] Loc. cit. My denial of the existence of this membrane naturally applies only to the egg after impregnation, and to the genera Scyllium and Pristiurus.
[114] Loc. cit.
[115]
Die Embryonal Anlage der Selachier. Vorläufige
Mittheilung,
Centralblatt f. Med. Wiss. No. 33, 1875.
In the last chapter the blastoderm was left as a solid lens-shaped mass of cells, thicker at one end than at the other, its uppermost row of cells forming a distinct layer. There very soon appears in it a cavity, the well-known segmentation cavity, or cavity of von Baer, which arises as a small space in the midst of the blastoderm, near its non-embryonic end (Pl. 7, fig. 1.).
This condition of the segmentation cavity, though already[116] described, has nevertheless been met with in one case only. The circumstance of my having so rarely met with this condition is the more striking because I have cut sections of a considerable number of blastoderms in the hope of encountering specimens similar to the one figured, and it can only be explained on one of the two following hypotheses. Either the stage is very transitory, and has therefore escaped my notice except in the one instance; or else the cavity present in this instance is not the true segmentation cavity, but merely some abnormal structure. That this latter explanation is a possible one, appears from the fact that such cavities do at times occur in other parts of the blastoderm. Dr Schultz[117] does not mention having found any stage of this kind.
The position of the cavity in question, and its general appearance, incline me to the view that it is the segmentation cavity[118]. If this is the true view of its nature the fact should be [Pg 247] noted that at first its floor is formed by the lower layer cells and not by the yolk, and that its roof is constituted by both the lower layer cells and the epiblast cells. The relations of the floor undergo considerable modifications in the course of development.
The other features of the blastoderm at this stage are very much those of the previous stage.
The embryonic swelling is very conspicuous. The cells of the blastoderm are still disposed in two layers: an upper one of slightly columnar cells one deep, which constitutes the epiblast, and a lower one consisting of the remaining cells of the blastoderm.
An average cell of the lower layer has a diameter of about 1/900 inch, but the cells at the periphery of the layer are in some cases considerably larger than the more central ones. All the cells of the blastoderm are still completely filled with yolk spherules. In the yolk outside the peculiar nuclei, before spoken of, are present in considerable numbers. They seem to have been mistaken by Dr Schultz[119] for cells: there can however be no question that they are true nuclei.
In the next stage the relations of the segmentation cavity undergo important modifications.
The cells which form its floor disappear almost completely from that position, and the floor becomes formed by the yolk.
The stage, during which the yolk serves as the floor of the segmentation cavity, extends over a considerable period of time, but during it I have been unable to detect any important change in the constitution of the blastoderm. It no doubt gradually extends over the yolk, but even this growth is not nearly so rapid as in the succeeding stage. Although therefore the stage I proceed to describe is of long continuance, a blastoderm at the beginning of it exhibits, both in its external and in its internal features, no important deviations from one at the end of it.
Viewed from the surface (Pl. 8, fig. A) the blastoderm [Pg 248] at this stage appears slightly oval, but the departure from the circular form is not very considerable. The long axis of the oval corresponds with what eventually becomes the long axis of the embryo. From the yolk the blastoderm is still well distinguished by its darker colour; and it is surrounded by a concentric ring of light-coloured yolk, the outer border of which shades insensibly into the normal yolk.
At the embryonic portion of the blastoderm is a slight swelling, clearly shewn in Plate 8, fig. A, which can easily be detected in fresh and in hardened embryos. This swelling is to be looked upon as a local exaggeration of a slightly raised rim present around the whole circumference of the blastoderm. The roof of the segmentation cavity (fig. A, s.c.) forms a second swelling; and in the fresh embryo this region appears of a darker colour than other parts of the blastoderm.
It is difficult to determine the exact shape of the blastoderm, on account of the traction exercised upon it in opening the egg; and no reliance can be placed on the forms assumed by hardened blastoderms. This remark also applies to the sections of blastoderms of this stage. There can be no doubt that the minor individual variations exhibited by almost every specimen are produced in the course of manipulations while the objects are fresh. These variations may affect even the relative length of a particular region and certainly the curvature of it. The roof of the segmentation cavity is especially apt to be raised into a dome-like form.
The main internal feature of this stage is the disappearance of the layer of cells which, during the first stage, formed the floor of the segmentation cavity. This disappearance is nevertheless not absolute, and it is doubtful whether there is any period in which the floor of the cavity is quite without cells.
Dr Schultz supposes[120] that the entire segmentation cavity is, in the living animal, filled with a number of loose cells. Though it is not in my power absolutely to deny this, the point being one which cannot be satisfactorily investigated in sections, yet no evidence has come under my notice which would lead to the conclusion that more cells are present in the segmentation cavity than are represented on Pl. 13, fig. 1, of [Pg 249] my preliminary paper[121], an illustration which is repeated on Pl. 7, fig. 2.
The number of cells on the floor of the cavity differs considerably in different cases, but these cases come under the category of individual variations, and are not to be looked upon as indications of different states of development.
In many cases especially large cells are to be seen on the floor of the cavity (Pl. 7, fig. 2, bd). In my preliminary paper[122] the view was expressed that these are probably cells formed around the nuclei of the yolk. This view I am inclined to abandon, and to substitute for it the suggestion made by Dr Schultz, that they are remnants of the larger segmentation cells which were to be seen in the previous stages.
Plate 7, figs. 2, 3, 4 (all sections of this stage) shew the different appearances presented by the floor of the segmentation cavity. In only one of these sections are there any large number of cells upon the floor; and in no case have cells been observed imbedded in the yolk forming this floor, as described by Dr Schultz[123], but in all cases the cells simply rested upon it.
Passing from the segmentation cavity to the blastoderm itself, the first feature to be noticed is the more decided differentiation of the epiblast. This now forms a distinct layer composed of a single row of columnar cells. These are slightly more columnar in the region of the embryonic swelling than elsewhere, and become less elongated at the edge of the blastoderm. In my specimens this layer was never more than one cell deep, but Dr Schultz[124] states that, in the Elasmobranch embryos investigated by him, the epiblast was composed of more than a single row of cells.
Each epiblast cell is filled with yolk-spherules and contains a nucleus. Very frequently the nuclei in the layer are arranged in a regular row (vide Pl. 7, fig. 3). In the later blastoderms of this stage there is a tendency in the cells to assume a wedge-like form with their thin ends pointing alternately in opposite [Pg 250] directions. This arrangement is, however, by no means strictly adhered to, and the regularity of it is exaggerated in Plate 7, fig. 4.
The nuclei of the epiblast cells have the same characters as those of the lower layer cells to be presently described, but their intimate structure can only be successfully studied in certain exceptionally favourable sections. In most cases the yolk-spherules around them render the finer details invisible.
There is at this stage no such obvious continuity as in the succeeding stage between the epiblast and the lower layer cells; and this statement holds good more especially with the best conserved specimens which have been hardened in osmic acid (Pl. 7, fig. 4). In these it is very easy to see that the epiblast simply thins out at the edge of the blastoderm without exhibiting the slightest tendency to become continuous with the lower layer cells[125].
The lower layer cells form a mass rather than a layer, and constitute the whole of the blastoderm not included in the epiblast. The shape of this mass in a longitudinal section may be gathered from an examination of Plate 7, figs. 3 and 4.
It presents an especially thick portion forming the bulk of the embryonic swelling, and frequently contains one or two cavities, which from their constancy I regard as normal and not as artificial products.
In addition to the mass forming the embryonic swelling there is seen in sections another mass of lower layer cells at the opposite extremity of the blastoderm, connected with the [Pg 251] former by a bridge of cells, which constitutes the roof of the segmentation cavity. The lower layer cells may thus be divided into three distinct parts:
(1) The embryo swelling.
(2) The thick rim of cells round the edge of the remainder of the blastoderm.
(3) The cells which form the roof of the segmentation cavity.
These three parts form a continuous whole, but in addition to these there exist the previously mentioned cells, which rest on the floor of the segmentation cavity.
With the exception of these latter, the lower layer is composed of cells having a fairly uniform size, and exhibits no trace of a division into two layers.
The cells are for the most part irregularly polygonal from mutual pressure; and in their shape and arrangement, exhibit a marked contrast to the epiblast cells. A few of the lower layer cells, highly magnified, are represented in Pl. 7, fig. 2a. An average cell measures about 1/800 to 1/900 of an inch, but some of the larger ones on the floor attain to the 1/475 of an inch.
Owing to my having had the good fortune to prepare some especially favourable specimens of this stage, it has been possible for me to make accurate observations both upon the nuclei of the cells of the blastoderm, and upon the nuclei of the yolk.
The nuclei of the blastoderm cells, both of the epiblast and lower layer, have a uniform structure. Those of the lower layer cells are about 1/1600 of an inch in diameter. Roughly speaking each consists of a spherical mass of clear protoplasm refracting more highly than the protoplasm of its cell. The nucleus appears in sections to be divided by deeply stained lines into a number of separate areas, and in each of these a deeply stained granule is placed. In some cases two or more of such granules may be seen in a single area. The whole of the nucleus stains with the colouring reagents more deeply than the protoplasm of the cells; but this is especially the case with the granules and lines.
Though usually spherical the nuclei not infrequently have a somewhat lobate form.
Very similar to these nuclei are the nuclei of the yolk.
[Pg 252] One of the most important differences between the two is that of size. The majority of the nuclei present in the yolk are as large or larger than an ordinary blastoderm cell; while many of them reach a size very much greater than this. The examples I have measured varied from 1/500 to 1/250 of an inch in diameter.
Though they are divided, like the nuclei of the blastoderm, with more or less distinctness into separate areas by a network of lines, their greater size frequently causes them to present an aspect somewhat different from the nuclei of the blastoderm. They are moreover much less regular in outline than these, and very many of them have lobate projections (Pl. 7, figs. 2a and 2c and 3), which vary from simple knobs to projections of such a size as to cause the nucleus to present an appearance of commencing constriction into halves. When there are several such projections the nucleus acquires a peculiar knobbed figure. With bodies of this form it becomes in many cases a matter of great difficulty to decide whether or no a particular series of knobs, which appear separate in one plane, are united in a lower plane, whether, in fact, there is present a single knobbed nucleus or a number of nuclei in close apposition. A nucleus in this condition is represented in Pl. 7, fig. 2b.
The existence of a protoplasmic network in the yolk has already been mentioned. This in favourable cases may be observed to be in special connection with the nuclei just described. Its meshes are finer in the vicinity of the nuclei, and its fibres in some cases almost appear to start from them (Pl. 7, fig. 12). For reasons which I am unable to explain the nuclei of the yolk and the surrounding meshwork present appearances which differ greatly according to the reagent employed. In most specimens hardened in osmic acid the protoplasm of the nuclei is apparently prolonged in the surrounding meshwork (Pl. 7, fig. 12). In other specimens hardened in osmic acid (Pl. 7, fig. 11), and in all hardened in chromic acid (Pl. 7, fig. 2a and 2c), the appearances are far clearer than in the previous case, and the protoplasmic meshwork merely surrounds the nuclei, without shewing any signs of becoming continuous with them.
There is also around each nucleus a narrow space in which the spherules of the yolk are either much smaller than elsewhere or completely absent, vide Pl. 7, fig. 2b.
[Pg 253] It has not been possible for me to satisfy myself as to the exact meaning of the lines dividing these nuclei into a number of distinct areas. My observations leave the question open as to whether they are to be looked upon as lines of division, or as protoplasmic lines such as have been described in nuclei by Flemming[126], Hertwig[127] and Van Beneden[128]. The latter view appears to me to be the more probable one.
Such are the chief structural features presented by these nuclei, which are present during the whole of the earlier periods of development and retain throughout the same appearance. There can be little doubt that their knobbed condition implies that they are undergoing a rapid division. The arguments for this view I have already insisted on, and, in spite of the observations of Dr Kleinenberg shewing that similar nuclei of Nephelis do not undergo division, the case for their doing so in the Elasmobranch eggs is to my mind a very strong one.
During this stage the distribution of these nuclei in the yolk becomes somewhat altered from that in the earlier stages. Although the nuclei are still scattered generally throughout the finer yolk-matter around the blastoderm, yet they are especially aggregated at one or two points. In the first place a special collection of them may be noticed immediately below the floor of the segmentation cavity. They here form a distinct row or even layer. If the presence of this layer is coupled with the fact that at this period cells are beginning to appear on the floor of the segmentation cavity, a strong argument is obtained for the supposition that around these nuclei cells are being produced, which pass into the blastoderm to form the floor. Of the actual formation of cells at this period I have not been able to obtain any satisfactory example, so that it remains a matter of deduction rather than of direct observation.
Another special aggregation of nuclei is generally present at the periphery of the blastoderm, and the same amount of doubt hangs over the fate of these as over that of the previously mentioned nuclei.
[Pg 254] The next stage is the most important in the whole history of the formation of the layers. Not only does it serve to shew, that the process by which the layers are formed in Elasmobranchii can easily be derived from a simple gastrula type like that of Amphioxus, but it also serves as the key by which other meroblastic types of development may be explained. At the very commencement of this stage the embryonic swelling becomes more conspicuously visible than it was. It now projects above the level of the yolk in the form of a rim. At one point, which eventually forms the termination of the axis of the embryo, this projection is at its greatest; while on either side of this it gradually diminishes and finally vanishes. This projection I propose calling, as in my preliminary paper[129], the embryonic rim.
The segmentation cavity can still be seen from the surface, and a marked increase in the size of the blastoderm may be noticed. During the stage last described, the growth was but very slight; hence the rather sudden and rapid growth which now takes place becomes striking.
Longitudinal sections at this stage, as at the earlier stages, are the most instructive. Such a section on the same scale as Pl. 7, fig. 4, is represented in Pl. 7, fig. 5. It passes parallel to the long axis of the embryo, through the point of greatest development of the embryonic ring.
The three fresh features of the most striking kind are (1) the complete envelopment of the segmentation cavity within the lower layer cells, (2) the formation of the embryonic rim, (3) the increase in distance between the posterior end of the blastoderm and the segmentation cavity. The segmentation cavity has by no means relatively increased in size. The roof has precisely its earlier constitution, being composed of an internal lining of lower layer cells and an external one of epiblast. The thin lining of lower layer cells is, in the course of mounting the sections, very apt to fall off; but I am absolutely satisfied that it is never absent.
The floor of the cavity has undergone an important change, being now formed by a layer of cells instead of by the yolk. A [Pg 255] precisely similar but more partial change in the constitution of the floor takes place in Osseous Fishes[130].
The mode in which the floor is formed is a question of some importance. The nuclei, which during the last stage formed a row beneath it, probably, as previously pointed out, take some share in its formation. An additional argument to those already brought forward in favour of this view may be derived from the fact that during this stage such a row of nuclei is no longer present.
This argument may be stated as follows:
Before the floor of cells for the segmentation cavity is formed a number of nuclei are present in a suitable situation to supply the cells for the floor; as soon as the floor of cells makes its appearance these nuclei are no longer to be seen. From this it may be concluded that their disappearance arises from their having become the nuclei of the cells which form the floor.
It appears to me most probable that there is a growth inwards from the whole peripheral wall of the cavity, and that this ingrowth, as well as the cells derived from the yolk, assist in forming the floor of the cavity. In Osseous Fish there appears to be no doubt that the floor is largely formed by an ingrowth of this kind.
A great increase is observable in the distance between the posterior end of the segmentation cavity and the edge of the blastoderm. This is due to the rapid growth of the latter combined with the stationary condition of the former. The growth of the blastoderm at this period is not uniform, but is more rapid in the non-embryonic than in the embryonic parts.
The main features of the epiblast remain the same as during the last stages. It is still composed of a very distinct layer one cell deep. Over the segmentation cavity, and over the whole embryonic end of the blastoderm, the cells are very thin, columnar, and, roughly speaking, wedge-shaped with the thin ends pointing alternately in different directions. For this reason, the nuclei form two rows; but both the rows are situated near the upper surface of the layer (vide Pl. 7, fig. 5). Towards the [Pg 256] posterior end of the blastoderm the cells are flatter and broader; and the layer terminates at the non-embryonic end of the blastoderm without exhibiting the slightest tendency to become continuous with the lower layer cells. At the embryonic end of the blastoderm the relations of the epiblast and lower layer cells are very different. At this part, throughout the whole extent of the embryonic rim, the epiblast is reflected and becomes continuous with the lower layer cells.
The lower layer cells form, for the most part, a uniform stratum in which no distinction into mesoblast and hypoblast is to be seen.
Both the lower layer cells and the epiblast cells are still filled with yolk-spherules.
The structures at the embryonic rim, and the changes which are there taking place, unquestionably form the chief features of interest at this stage.
The general relations of these parts are very fairly shewn in Pl. 7, fig. 5, which represents a section passing through the median line of the embryonic region. They are however more accurately represented in Pl. 7, fig. 5a, taken from the same embryo, but in a lateral part of the embryonic rim; or in Pl. 7, fig. 6, from a slightly older embryo. In all of these figures the epiblast cells are reflected at the edge of the embryonic rim, and become perfectly continuous with the hypoblast cells. A few of the cells, immediately beyond the line of this reflection, precisely resemble in character the typical epiblast cells; but the remainder exhibit a gradual transition into typical lower layer cells. Adjoining these transitional cells, or partly enclosed in the corner formed between them and the epiblast, are a few unaltered lower layer cells (m), which at this stage are not distinctly separated from the transitional cells. The transitional cells form the commencement of the hypoblast (hy); and the cells (m) between them and the epiblast form the commencement of the mesoblast. The gradual conversion of lower layer cells into columnar hypoblast cells, is a very clear and observable phenomenon in the best specimens. Where the embryonic rim projects most, a larger number of cells have assumed a columnar form. Where it projects less clearly, a smaller number have done so. But in all cases there may be observed a series of gradations between [Pg 257] the columnar cells and the typical rounded lower layer cells[131].
In the last described embryo, although the embryonic rim had attained to a considerable development, no trace of the medullary groove had made its appearance. In an embryo in the next stage of which I propose describing sections, this structure has become visible.
A surface view of a blastoderm of this age, with the embryo, is represented on Pl. 8, fig. B; and I shall, for the sake of convenience, in future speak of embryos of this age as belonging to period B.
The blastoderm is nearly circular. The embryonic rim is represented by a darker shading at the edge. At one point in this rim may be seen the embryo, consisting of a somewhat raised area with an axial groove (mg). The head end of the embryo is that which points towards the centre of the blastoderm, and its free peripheral extremity is at the edge of the blastoderm.
A longitudinal section of an embryo of the same age as the one figured[132] is represented on Pl. 7, fig. 7. The general growth has been very considerable, though as before explained, it is mainly confined to that part of the blastoderm where the embryonic rim is absent.
A fresh feature of great importance is the complete disappearance of the segmentation cavity, the place which was previously occupied by it being now filled up by an irregular network of cells. There can be little question that the obliteration of the segmentation cavity is in part due to the entrance into the blastoderm of fresh cells formed around the nuclei of the yolk. The formation of these is now taking place with great rapidity and can be very easily followed.
Since the segmentation cavity ceases to play any further part in the history of the blastoderm, it will be well shortly to review the main points in its history.
[Pg 258] Its earliest appearance is involved in some obscurity, though it probably arises as a simple cavity in the midst of the lower layer cells (Pl. 7, fig. 1). In its second phase the floor ceases to be formed of lower layer cells, and the place of these is taken by the yolk, on which however a few scattered cells still remain (Pl. 7, figs. 2, 3, 4). During the third period of its history, a distinct cellular floor is again formed for it, so that it comes a second time into the same relations with the blastoderm as at its earliest appearance. The floor of cells which it receives is in part due to a growth inwards from the periphery of the blastoderm, and in part to the formation of fresh cells from the yolk. Coincidently with the commencing differentiation of hypoblast and mesoblast the segmentation cavity grows smaller and vanishes.
One of the most important features of the segmentation cavity in the Elasmobranchii which I have studied, is the fact that throughout its whole existence its roof is formed of lower layer cells. There is not the smallest question that the segmentation cavity of these fishes is the homologue of that of Amphioxus, Batrachians, etc., yet in the case of all of these animals, the roof of the segmentation cavity is formed of epiblast only. How comes it then to be formed of lower layer cells in Elasmobranchii?
To this question an answer was attempted in my paper, Upon the Early
Stages of the Development of Vertebrates[133].
It was there pointed out, that as the food
material in the ovum increases, the bulk of the lower layer cells
necessarily also increases; since these, as far as the blastoderm is
concerned, are the chief recipients of food material. This causes the lower
layer cells to encroach upon the segmentation cavity, and to close it in
not only on the sides, but also above; from the same cause it results that
the lower layer cells assume, from the first, a position around the spot
where the future alimentary cavity will be formed, and that this cavity
becomes formed by a simple split in the midst of the lower layer cells, and
not by an involution.
All the most recent observations[134] on Osseous Fishes tend [Pg 259] to shew that in them, the roof of the segmentation cavity is formed alone of epiblast; but on account of the great difficulty which is experienced in distinguishing the layers in the blastoderms of these animals, I still hesitate to accept as conclusive the testimony on this point.
In the formation a second time of a cellular floor for the segmentation cavity in the third stage, the Elasmobranch embryo seems to resemble that of the Osseous Fish[135]. Upon this feature great stress is laid both by Dr Götte[136] and Prof. Haeckel[137]: but I am unable to agree with the interpretation of it offered by them. Both Dr Götte and Prof. Haeckel regard the formation of this floor as part of an involution to which the lower layer cells owe their origin, and consider the involution an equivalent to the alimentary involution of Batrachians, Amphioxus, &c. To this question I hope to return, but it may be pointed out that my observations prove that this view can only be true in a very modified sense; since the invagination by which hypoblast and alimentary canal are formed in Amphioxus is represented in Elasmobranchii by a structure quite separate from the ingrowth of cells to form the floor of the segmentation cavity.
The eventual obliteration of the segmentation cavity by cells derived from the yolk is to be regarded as an inherited remnant of the involution by which this obliteration was primitively effected. The passage upwards of cells from the yolk, may possibly be a real survival of the tendency of the hypoblast cells to grow inwards during the process of involution.
The last feature of the segmentation cavity which deserves notice is its excentric position. It is from the first situated in much closer proximity to the non-embryonic than to the embryonic end of the blastoderm. This peculiarity in position is also characteristic of the segmentation cavity of Osseous Fishes, as is shewn by the concordant observations of Oellacher[138] and Götte[139]. Its meaning becomes at once intelligible by referring to the diagrams in my paper[140] on the Early Stages in the Development of Vertebrates. It in fact arises from the asymmetrical character [Pg 260] of the primitive alimentary involution in all anamniotic vertebrates with the exception of Amphioxus.
Leaving the segmentation cavity I pass on to the other features of my sections.
There is still to be seen a considerable aggregation of cells at the non-embryonic end of the blastoderm. The position of this, and its relations with the portion of the blastoderm which at an earlier period contained the segmentation cavity, indicate that the growth of the blastoderm is not confined to its edge, but that it proceeds at all points causing the peripheral parts to glide over the yolk.
The main features of the cells of this blastoderm are the same as they were in the one last described. In the non-embryonic region the epiblast has thinned out, and is composed of a single row of cells, which, in the succeeding stages, become much flattened.
The lower layer cells over the greater part of their extent, have not undergone any histological changes of importance. Amongst them may frequently be seen a few exceptionally large cells, which without doubt have been derived directly from the yolk.
The embryonic rim is now a far more considerable structure than it was. Vide Pl. 7, fig. 7. Its elongation is mainly effected by the continuous conversion of rounded lower layer cells into columnar hypoblast cells at its central or anterior extremity.
This conversion of the lower layer cells into hypoblast cells is still easy to follow, and in every section cells intermediate between the two are to be seen. The nature of the changes which are taking place requires for its elucidation transverse as well as longitudinal sections. Transverse sections of a slightly older embryo than B are represented on Pl. 7, fig. 8a, 8b and 8c.
Of these sections a is the most peripheral or posterior, and c the most central or anterior. By a combination of transverse and longitudinal sections, and by an inspection of a surface view, it is rendered clear that, though the embryonic rim is a far more considerable structure in the region of the embryo than elsewhere (compare fig. 6 and fig. 7 and 7a), yet that this gain in size is not produced by an outgrowth of the embryo beyond [Pg 261] the rest of the germ, but by the conversion of the lower layer cells into hypoblast having been carried far further towards the centre of the germ in the axial line than in the lateral regions of the rim.
The most anterior of the series of transverse sections (Pl. 7, fig. 8c) I have represented, is especially instructive with reference to this point. Though the embryonic rim is cut through at the sides of the section, yet in these parts the rim consists of hardly more than a continuity between epiblast and lower layer cells, and the lower layer cells shew no trace of a division into mesoblast and hypoblast. In the axis of the embryo, however, the columnar hypoblast is quite distinct; and on it a small cap of mesoblast is seen on each side of the medullary groove. Had the embryonic rim resulted from a projecting growth of the blastoderm, such a condition could not have existed. It might have been possible to find the hypoblast formed at the sides of the section and not at the centre; but the reverse, as in these sections, could not have occurred. Indeed it is scarcely necessary to have recourse to sections to prove that the growth of the embryonic rim is towards the centre of the blastoderm. The inspection of a surface view of a blastoderm at this period demonstrates it beyond a doubt (Pl. 8, fig. B). The embryo, close to which the embryonic rim is alone largely developed, does not project outwards beyond the edge of the germ, but inwards towards its centre.
The space between the embryonic rim and the yolk (Pl. 7, fig. 7, al.) is the alimentary cavity. The roof of this is therefore primitively formed of hypoblast and the floor of yolk. The external opening of this space at the edge of the blastoderm is the exact morphological homologue of the anus of Rusconi, or blastopore of Amphioxus, the Amphibians, &c. The importance of the mode of growth in the embryonic rim depends upon the homology of the cavity between it and the yolk, with the alimentary cavity of Amphioxus and Amphibians. Since this homology exists, the direction of the growth of this cavity ought to be, as it in fact is, the same as in Amphioxus, etc., viz. towards the centre of the germ and original position of the segmentation cavity. Thus though a true invagination is not present as in the other cases, yet this is represented in Elasmobranchii by the [Pg 262] continuous conversion of lower layer cells into hypoblast along a line leading towards the centre of the blastoderm.
In the parts of the rim adjoining the embryo, the lower layer cells, on becoming continuous with the epiblast cells, assume a columnar form. At the sides of the rim this is not strictly the case, and the lower layer cells retain their rounded form, though quite continuous with the epiblast cells. One curious feature of the layer of epiblast in these lateral parts of the rim is the great thickness it acquires before being reflected and becoming continuous with the hypoblast (Pl. 7, fig. 8c). In the vicinity of the point of reflection there is often a rather large formation of cells around the nuclei of the yolk. The cells formed here no doubt pass into the blastoderm, and become converted into columnar hypoblast cells. In some cases the formation of these cells is very rapid, and they produce quite a projection on the under side of the hypoblast. Such a case is represented in Pl. 7, fig. 8b, n.al. The cells constituting this mass eventually become converted into the lateral and ventral walls of the alimentary canal.
The formation of the mesoblast has progressed rapidly. While many of the lower layer cells become columnar and form the hypoblast, others, between these and the epiblast, remain spherical. The latter do not at once become separated as a layer distinct from the hypoblast, and, at first, are only to be distinguished from them through their different character, vide Plate 7, figs. 6 and 7. They nevertheless constitute the commencing mesoblast.
Thus much of the mode of formation of the mesoblast can be easily made out in longitudinal sections, but transverse sections throw still further light upon it.
From these it may at once be seen that the mesoblast is not formed in one continuous sheet, but as two lateral masses, one on each side of the axial line of the embryo[141]. In my [Pg 263] preliminary account[142] it was stated that this was a condition of the mesoblast at a very early period, and that it was probably its condition from the beginning. Sections are now in my possession which satisfy me that, from the very first, the mesoblast arises as two distinct lateral masses, one on each side of the axial line.
In the embryo from which the sections Pl. 7, fig. 8a, 8b, 8c were taken, the mesoblast had, in most parts, not yet become separated from the hypoblast. It still formed with this a continuous layer, though the mesoblast cells were distinguishable by their shape from the hypoblast. In only one section (b) was any part of the mesoblast quite separated from the hypoblast.
In the hindermost part of the embryo the mesoblast is at its maximum, and forms, on each side, a continuous sheet extending from the median line to the periphery (fig. 8a). The rounder form of the mesoblast cells renders the line of junction between the layer constituted by them and the hypoblast fairly distinct; but towards the periphery, where the hypoblast cells have the same rounded form as the mesoblast, the fusion between the two layers is nearly complete.
In an anterior section the mesoblast is only present as a cap on both sides of the medullary groove, and as a mass of cells at the periphery of the section (fig. 8b); but no continuous layer of it is present. In the foremost of the three sections (fig. 8c) the mesoblast can scarcely be said to have become in any way separated from the hypoblast except at the summit of the medullary folds (m).
From these and similar sections it may be certainly concluded, that the mesoblast becomes first separated from the hypoblast as a distinct layer in the posterior region of the embryo, and only at a later period in the region of the head.
In an embryo but slightly more developed than B, the formation of the layer is quite completed in the region of the embryo. To this embryo I now pass on.
In the non-embryonic parts of the blastoderm no fresh features of interest have appeared. It still consists of two layers. The epiblast is composed of flattened cells, and the lower layer of a network of more rounded cells, elongated in a lateral [Pg 264] direction. The growth of the blastoderm has continued to be very rapid.
In the region of the embryo (Pl. 7, fig. 9) more important changes have occurred. The epiblast still remains as a single row of columnar cells. The hypoblast is no longer fused with the mesoblast, and forms a distinct dorsal wall for the alimentary cavity. Though along the axis of the embryo the hypoblast is composed of a single row of columnar cells, yet in the lateral part of the embryo its cells are less columnar and are one or two deep.
Owing to the manner in which the mesoblast became split off from the hypoblast, a continuity is maintained between the hypoblast and the lower layer cells of the blastoderm (Pl. 7, fig. 9), while the two plates of mesoblast are isolated and disconnected from any other masses of cells.
The alimentary cavity is best studied in transverse sections. (Vide Pl. 7, fig. 10a, 10b and 10c, three sections from the same embryo.) It is closed in above and at the sides by the hypoblast, and below by the yolk. In its anterior part a floor is commencing to be formed by a growth of cells from the walls of the two sides. The cells for this growth are formed around the nuclei of the yolk; a feature which recalls the fact that in Amphibians the ventral wall of the alimentary cavity is similarly formed in part from the so-called yolk cells.
We left the mesoblast as two masses not completely separated from the hypoblast. During this stage the separation between the two becomes complete, and there are formed two great lateral plates of mesoblast cells, one on each side of the medullary groove. Each of these corresponds to a united vertebral and lateral plate of the higher Vertebrates. The plates are thickest in the middle and posterior regions (Pl. 7, fig. 10a and 10b), but thin out and almost vanish in the region of the head. The longitudinal section of this stage represented in Pl. 7, fig. 9, passes through one of the lateral masses of mesoblast cells, and shews very distinctly its complete independence of all the other cells in the blastoderm.
From what has been stated with reference to the development of the mesoblast, it is clear that in Elasmobranchii this layer is derived from the same mass of cells as the hypoblast, [Pg 265] and receives none of its elements from the epiblast. In connection with its development, as two independent lateral masses, I may observe, as I have previously done[143], that in this respect it bears a close resemblance to mesoblast in Euaxes, as described by Kowalevsky[144]. This resemblance is of some interest, as bearing on a probable Annelid origin of Vertebrata. Kowalevsky has also shewn[145] that the mesoblast in Ascidians is similarly formed as two independent masses, one on each side of the middle line.
It ought, however, to be pointed out that a similar bilateral origin of the mesoblast had been recently met with in Lymnæus by Carl Rabl[146]. A fact which somewhat diminishes the genealogical value of this feature in the mesoblast in Elasmobranchii.
During the course of this stage the spherules of food-yolk immediately beneath the embryo are used up very rapidly. As a result of this the protoplasmic network, so often spoken of, comes very plainly into view. Considerable areas may sometimes be seen without any yolk-spherule whatever.
On Pl. 7, fig. 7a, and figs. 11 and 12, I have attempted to reproduce the various appearances presented by this network: and these figures give a better idea of it than any description. My observations tend to shew that it extends through the whole yolk, and serves to hold it together. It has not been possible for me to satisfy myself that it had any definite limits, but on the other hand, in many parts all my efforts to demonstrate its presence have failed. When the yolk-spherules are very thickly packed, it is difficult to make out for certain whether it is present or absent, and I have not succeeded in removing the yolk-spherules from the network in cases of this kind. In medium-sized ovarian eggs this network is very easily seen, and extends through the whole yolk. Part of such an egg is shewn in Pl. 7, [Pg 266] fig. 14. In full-sized ovarian eggs, according to Schultz[147], it forms, as was mentioned in the first chapter, radiating striæ, extending from the centre to the periphery of the egg. When examined with the highest powers, the lines of this network appear to be composed of immeasurably small granules arranged in a linear direction. These granules are more distinct in chromic acid specimens than in those hardened in osmic acid, but are to be seen in both. There can be little doubt that these granules are imbedded in a thread or thin layer of protoplasm.
I have already (p. 252) touched upon the relation of this network to the nuclei of the yolk[148].
During the stages which have just been described specially favourable views are frequently to be obtained of the formation of cells in the yolk and their entrance into the blastoderm. Two representations of these are given, in Pl. 7, fig. 7a, and fig. 13. In both of these distinctly circumscribed cells are to be seen in the yolk (c), and in all cases are situated near to the typical nuclei of the yolk. The cells in the yolk have such a relation to the surrounding parts, that it is quite certain that their presence is not due to artificial manipulation, and in some cases it is even difficult to decide whether or no a cell area is circumscribed round a nucleus (Pl. 7, fig. 13). Although it would be possible for cells in the living state to pass from the blastoderm into the yolk, yet the view that they have done so in the cases under consideration has not much to recommend it, if the following facts be taken into consideration. (1) That the cells [Pg 267] in the yolk are frequently larger than those in the blastoderm. (2) That there are present a very large number of nuclei in the yolk which precisely resemble the nuclei of the cells under discussion. (3) That in some cases (Pl. 7, fig. 13) cells are seen indistinctly circumscribed as if in the act of being formed.
Between the blastoderm and the yolk may frequently be seen a membrane-like structure, which becomes stained with hæmatoxylin, osmic acid etc. It appears to be a layer of coagulated albumen and not a distinct membrane.
Summary.
At the close of segmentation, the blastoderm forms a somewhat lens-shaped disc, thicker at one end than at the other; the thicker end being termed the embryonic end.
It is divided into two layers—an upper one, the epiblast, formed by a single row of columnar cells; and a lower one, consisting of the remaining cells of the blastoderm.
A cavity next appears in the lower layer cells, near the non-embryonic end of the blastoderm, but the cells soon disappear from the floor of this cavity which then comes to be constituted by yolk alone.
The epiblast in the next stage is reflected for a small arc at the embryonic end of the blastoderm, and becomes continuous with the lower layer cells; at the same time some of the lower layer cells of the embryonic end of the blastoderm assume a columnar form, and constitute the commencing hypoblast. The portion of the blastoderm, where epiblast and hypoblast are continuous, forms a projecting structure which I have called the embryonic rim. This rim increases rapidly by growing inwards more and more towards the centre of the blastoderm, through the continuous conversion of lower layer cells into columnar hypoblast.
While the embryonic rim is being formed, the segmentation cavity undergoes important changes. In the first place, it receives a floor of lower layer cells, partly from an ingrowth from the two sides, and partly from the formation of cells around the nuclei of the yolk.
[Pg 268] Shortly after the floor of cells has appeared, the whole segmentation cavity becomes obliterated.
When the embryonic rim has attained to some importance, the position of the embryo becomes marked out by the appearance of the medullary groove at its most projecting part. The embryo extends from the edge of the blastoderm inwards towards the centre.
At about the time of the formation of the medullary groove, the mesoblast becomes definitely constituted. It arises as two independent plates, one on each side of the medullary groove, and is entirely derived from lower layer cells.
The two plates of mesoblast are at first unconnected with any other cells of the blastoderm, and, on their formation, the hypoblast remains in connection with all the remaining lower layer cells. Between the embryonic rim and the yolk is a cavity,—the primitive alimentary cavity. Its roof is formed of hypoblast, and its floor of yolk. Its external opening is homologous with the anus of Rusconi, of Amphioxus and the Amphibians. The ventral wall of the alimentary cavity is eventually derived from cells formed in the yolk around the nuclei which are there present.
* * * * *
Since the important researches of Gegenbaur[149] upon the meroblastic vertebrate eggs, it has been generally admitted that the ovum of every vertebrate, however complicated may be its apparent constitution, is nevertheless to be regarded as a simple cell. This view is, indeed, opposed by His[150] and to a very modified extent by Waldeyer[151], and has recently been attacked from an entirely new standpoint by Götte[152]; but, to my mind, the objections of these authors do not upset the well founded conclusions of previous observations.
[Pg 269] As soon as the fact is recognised that both meroblastic and holoblastic eggs have the same fundamental constitution, the admission follows, naturally, though not necessarily, that the eggs belonging to these two classes differ solely in degree, not only as regards their constitution, but also as regards the manner in which they become respectively converted into the embryo. As might have been anticipated, this view has gained a wide acceptance.
Amongst the observations, which have given a strong objective support to this view, may be mentioned those of Professor Lankester upon the development of Cephalopoda[153], and of Dr Götte[154] upon the development of the Hen's egg. In Loligo Professor Lankester shewed that there appeared, in the part of the egg usually considered as food-yolk, a number of bodies, which eventually developed a nucleus and became cells, and that these cells entered into the blastoderm. These observations demonstrate that in the eggs of Loligo the so-called food-yolk is merely equivalent to a part of the egg which in other cases undergoes segmentation.
The observations of Dr Götte have a similar bearing. He made out that in the eggs of the Hen no sharp line is to be found separating the germinal disc from the yolk, and that, independently of the normal segmentation, a number of cells are derived from that part of the egg hitherto regarded as exclusively food-yolk. This view of the nature of the food-yolk was also advanced in my preliminary account of the development of Elasmobranchii[155], and it is now my intention to put forward the positive evidence in favour of this view, which is supplied from a knowledge of the phenomena of the development of the Elasmobranch ovum; and then to discuss how far the facts of the growth of the blastoderm in Elasmobranchii accord with the view that their large food-yolk is exactly equivalent to part of the ovum, which in Amphibians undergoes segmentation, rather than some fresh addition, which has no equivalent in the Amphibian or other holoblastic ovum.
Taking for granted that the ripe ovum is a single cell, the [Pg 270] question arises whether in the case of meroblastic ova the cell is not constituted of two parts completely separated from one another.
Is the meroblastic ovum, before or after impregnation, composed of a germinal disc in which all the protoplasm of the cell is aggregated, and of a food-yolk in which no protoplasm is present? or is the protoplasm present throughout, being simply more concentrated at the germinal pole than elsewhere? If the former alternative is accepted, we must suppose that the mass of food-yolk is a something added which is not present in holoblastic ova. If the latter alternative is accepted, it may then be maintained that holoblastic and meroblastic ova are constituted in the same way and differ only in the proportions of their constituents.
My own observations in conjunction with the specially interesting observations of Dr Schultz[156] justify the view which regards the protoplasm as present throughout the whole ovum, and not confined to the germinal disc. Our observations shew that a fine protoplasmic network, with ramifications extending throughout the whole yolk, is present both before and after impregnation.
The presence of this network is, in itself, only sufficient to prove that the yolk may be equivalent to part of a holoblastic ovum; to demonstrate that it is so requires something more, and this link in the chain of evidence is supplied by the nuclei of the yolk, which have been so often referred to.
These nuclei arise independently in the yolk, and become the nuclei of cells which enter the germ and the bodies of which are derived from the protoplasm of the yolk. Not only so, but the cells formed around these nuclei play the same part in the development of Elasmobranchii as do the largest so-called yolk cells in the development of Amphibians. Like the homologous cells in Amphibians, they mainly serve to form the ventral wall of the alimentary canal and the blood-corpuscles. The identity in the fate of the so-called yolk cells of Amphibians with the cells derived from the yolk in Elasmobranchii, must be considered as a proof of the homology of the yolk cells in the first case [Pg 271] with the yolk in the second; the difference between the yolk in the two cases arising from the fact that in the Elasmobranch ovum the yolk-spherules bear a larger proportion to the protoplasm than they do in the Amphibian ovum. As I have suggested elsewhere[157], the segmentation or non-segmentation of a particular part of the ovum depends solely upon the proportion borne by the protoplasm to the yolk particles; so that, when the latter exceed the former in a certain fixed proportion, segmentation is no longer possible; and, as this limit is approached, segmentation becomes slower, and the resulting segments larger and larger.
The question how far the facts in the developmental history of the various vertebrate blastoderms accord with the view of the nature of the yolk just propounded is one of considerable interest. An answer to it has already been attempted from a general point of view in my paper[158] entitled 'The Comparison of the early stages of development in Vertebrates'; but the subject may be conveniently treated here in a special manner for Elasmobranch embryos.
In the woodcut, fig. 1, A, B, C[159], are represented three diagrammatic longitudinal sections of an Elasmobranch embryo. A nearly corresponds with the longitudinal section represented on Pl. 7, fig. 4, and B with Pl. 7, fig. 7. In Pl. 7, fig. 7, the segmentation cavity has however completely disappeared, while it is still represented as present in the diagram of the same period. If these diagrams, or better still, the woodcuts fig. 2 A, B, C (which only differ from those of the Elasmobranch fish in the smaller amount of food-yolk), be compared with the corresponding ones of Bombinator, fig. 3, A, B, C, they will be found to be in fundamental agreement with them. First let fig. 1, A, or fig. 2, A, or Pl. 7, fig. 4, be compared with fig. 3, A. In all there is present a segmentation cavity situated not centrally but near the surface of the egg. The roof of the cavity is thin in all, being composed in the Amphibian of epiblast alone, and in[Pg 272] the Elasmobranch of epiblast and lower layer cells. The floor of the cavity is, in all, formed of so-called yolk (vide Pl. 7, fig. 4), which in all forms the main mass of the egg. In the Amphibian the yolk is segmented, and, though it is not segmented in the Elasmobranch, it contains in compensation the nuclei so often mentioned. In all, the sides of the segmentation cavity are formed by lower layer cells. In the Amphibian the sides are enclosed by smaller cells (in the diagram) which correspond exactly in function and position with the lower layer cells of the Elasmobranch blastoderm.
Fig. 1.
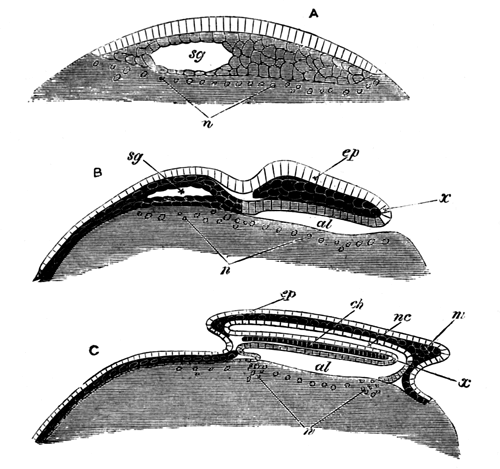
Diagrammatic longitudinal sections of an Elasmobranch embryo.
Epiblast without shading. Mesoblast black with clear outlines to the cells. Lower layer cells and hypoblast with simple shading.
ep. epiblast. m. mesoblast. al. alimentary cavity. sg. segmentation cavity. nc. neural canal. ch. notochord. x. point where epiblast and hypoblast become continuous at the posterior end of the embryo. n. nuclei of yolk.
A. Section of young blastoderm, with segmentation cavity in the middle of the lower layer cells.
B. Older blastoderm with embryo in which hypoblast and mesoblast are distinctly formed, and in which the alimentary slit has appeared. The segmentation cavity is still represented as being present, though by this stage it has in reality disappeared.
C. Older blastoderm with embryo in which neural canal has become formed, and is continuous posteriorly with alimentary canal. The notochord, though shaded like mesoblast, belongs properly to the hypoblast.
Fig. 2.
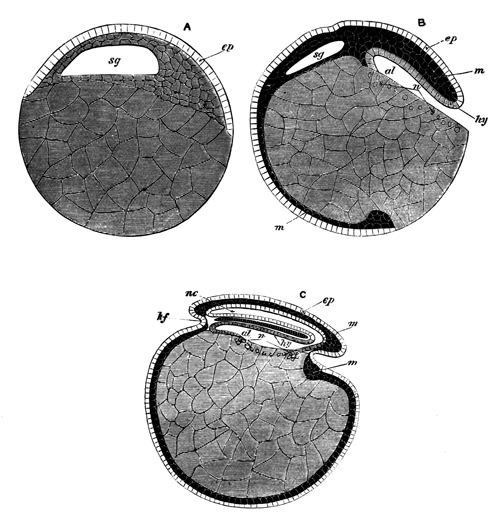
Diagrammatic longitudinal sections of embryo, which develops in the same manner as the Elasmobranch embryo, but in which the ovum contains far less food-yolk than is the case with the Elasmobranch ovum.
Epiblast without shading. Mesoblast black with clear outlines to the cells. Lower layer cells and hypoblast with simple shading.
ep. epiblast. m. mesoblast. hy. hypoblast. sg. segmentation cavity. al. alimentary cavity. nc. neural canal. hf. head fold. n. nuclei of the yolk.
The stages A, B and C are the same as in figure .[TN9]
Fig. 3.
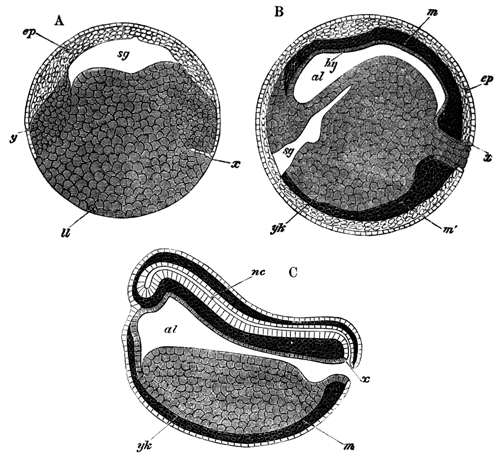
Diagrammatic longitudinal sections of Bombinator igneus. Reproduced with modifications from Götte.
Epiblast without shading. Mesoblast black with clear outlines to the cells. Lower layer cells and hypoblast with simple shading.
ep. epiblast. l.l. lower layer cells. y. smaller lower layer cells at the sides of the segmentation cavity. m. mesoblast. hy. hypoblast. al. alimentary cavity. sg. segmentation cavity. nc. neural cavity. yk. yolk-cells.
A is the youngest stage in which the alimentary involution has not yet appeared. x is the point from which the involution will start to form the dorsal wall of the alimentary tract. The line on each side of the segmentation cavity, which separates the smaller lower layer cells from the epiblast cells, is not present in Götte's original figure. The two shadings employed in the diagram render it necessary to have some line, but at this stage it is in reality not possible to assert which cells belong to the epiblast and which to the lower layer.
B. In this stage the alimentary cavity has become formed, but the segmentation cavity is not yet obliterated.
x. point where epiblast and hypoblast become continuous.
C. The neural canal is already formed, and communicates posteriorly with the alimentary.
x. point where epiblast and hypoblast become continuous.
[Pg 275] The relation of the yolk to the blastoderm in the Elasmobranch embryo at this stage of development very well suits the view of its homology with the large cells of the Amphibian ovum. The only essential difference between the two ova arises from the roof of the segmentation cavity being in the Elasmobranch embryo formed of lower layer cells, which are absent in the Amphibian embryo. This difference no doubt depends upon the greater quantity of yolk particles present in the Elasmobranch ovum. These increase the bulk of the lower layer cells, which are thus compelled to creep up the sides of the segmentation cavity till they close it in above.
In the next stage for the Elasmobranch, fig. 1 and 2 B and Pl. 7, fig. 7, and for the Amphibian, fig. 3, B, the agreement between the two types is again very close. In both for a small portion (x) of the edge of the blastoderm the epiblast and hypoblast become continuous, while at all other parts the epiblast, accompanied by lower layer cells, grows round the yolk or round the large cells which correspond to it. The yolk cells of the Amphibian ovum form a comparatively small mass, and are therefore rapidly enveloped; while in the case of the Elasmobranch ovum, owing to the greater mass of the yolk, the same process occupies a long period. In both ova the portion of the blastoderm, where epiblast and hypoblast become continuous, forms the dorsal lip of an opening—the anus of Rusconi—which leads into the alimentary cavity. This cavity has the same relation in both ova. It is lined dorsally by lower layer cells, and ventrally by yolk or what corresponds with yolk; the ventral epithelium of the alimentary canal being in both cases eventually supplied by the yolk cells.
As in the earlier stage, so in the present one, the anatomical relations of the yolk to the blastoderm in the one case (Elasmobranch) are nearly identical with those of the yolk cells to the blastoderm in the other (Amphibian). The main features in which the two embryos differ, during the stage under consideration, arise from the same cause as the solitary point of difference during the preceding stage.
[Pg 276] In Amphibians, the alimentary cavity is formed coincidently with a true ingrowth of cells from the point where epiblast and hypoblast become continuous, and from this ingrowth the dorsal wall of the alimentary cavity is formed. The same ingrowth causes the obliteration of the segmentation cavity.
In the Elasmobranchii, owing to the larger bulk of the lower layer cells caused by the food-yolk, these have been compelled to arrange themselves in their final position during segmentation, and no room is left for a true invagination; but instead of this there is formed a simple split between the blastoderm and the yolk. The homology of this with the primitive invagination is nevertheless proved by the survival of a number of features belonging to the ancestral condition in which a true invagination was present. Amongst the more important of these are the following:—(1) The continuity of epiblast and hypoblast at the dorsal lip of the anus of Rusconi. (2) The continuous conversion of indifferent lower layer cells into hypoblast, which gradually extends backwards towards the segmentation cavity, and exactly represents the course of the invagination whereby in Amphibians the dorsal wall of the alimentary cavity is formed. (3) The obliteration of the segmentation cavity during the period when the pseudo-invagination is occurring.
The asymmetry of the gastrula or pseudo-gastrula in Cyclostomes, Amphibians, Elasmobranchii and, I believe, Osseous Fishes, is to be explained by the form of the vertebrate body. In Amphioxus, where the small amount of food-yolk present is distributed uniformly, there is no reason why the invagination and resulting gastrula should not be symmetrical. In other vertebrates, where more food-yolk is present, the shape and structure of the body render it necessary for the food-yolk to be stored away on the ventral side of the alimentary canal. This, combined with the unsymmetrical position of the anus, which primitively corresponds in position with the blastopore or anus of Rusconi, causes the asymmetry of the gastrula invagination, since it is not possible for the part of the ovum which will become the ventral wall of the alimentary canal, and which is loaded with food-yolk, to be invaginated in the same fashion as the dorsal wall. From the asymmetry, so caused, follow a large number of features in vertebrate development, [Pg 277] which have been worked out in some detail in my paper already quoted[160].
Prof. Haeckel, in a paper recently published[161], appears to imply that because I do not find absolute invagination in Elasmobranchii, I therefore look upon Elasmobranchii as militating against his Gastræa theory. I cannot help thinking that Prof. Haeckel must have somewhat misunderstood my meaning. The importance of the Gastræa theory has always appeared to me to consist not in the fact that an actual ingrowth of certain cells occurs—an ingrowth which might have many different meanings[162]—but in the fact that the types of early development of all animals can be easily derived from that of the typical gastrula. I am perfectly in accordance with Professor Haeckel in regarding the type of Elasmobranch development to be a simple derivative from that of the gastrula, although believing it to be without any true ingrowth or invagination of cells.
Professor Haeckel[163] in the paper just referred to published his view upon the mutual relationships of the various vertebrate blastoderms. In this paper, which appeared but shortly after my own[164] on the same subject, he has put forward views which differ from mine in several important details. Some of these bear upon the nature of food-yolk; and it appears to me that Professor Haeckel's scheme of development is incompatible with the view that the food-yolk in meroblastic eggs is the homologue of part of the hypoblast of the holoblastic eggs.
The following is Professor Haeckel's own statement of the scheme or type, which he regards as characteristic of meroblastic eggs, pp. 98 and 99.
Jetzt folgt der höchst wichtige und
interessante Vorgang, den ich als Einstülpung der Blastula auffasse und der
zur Bildung der Gastrula führt (Fig. 63, 64)[165]. Es
schlägt sich nämlich der verdickte Saum der Keimscheibe, der Randwulst
oder das Properistom, nach innen um und eine dünne Zellenschicht
wächst als directe Fortsetzung desselben, wie ein immer [Pg 278] enger werdendes
Diaphragma, in die Keimhöhle hinein. Diese Zellenschicht ist das
entstehende Entoderm (Fig. 64 i, 74 i). Die Zellen, welche
dieselbe zusammensetzen und aus dem innern Theile des Randwulstes
hervorwachsen, sind viel grösser aber flacher als die Zellen der
Keimhöhlendecke und zeigen ein dunkleres grobkörniges Protoplasma. Auf dem
Boden der Keimhöhle, d. h. also auf der Eiweisskugel des Nahrungsdotters,
liegen sie unmittelbar auf und rücken hier durch centripetale Wanderung
gegen dessen Mitte vor, bis sie dieselbe zuletzt erreichen und nunmehr eine
zusammenhängende einschichtige Zellenlage auf dem ganzen Keimhöhlenboden
bilden. Diese ist die erste vollständige Anlage des Darmblatts, Entoderms
oder Hypoblasts
, und von nun an können wir, im Gegensatz dazu den
gesammten übrigen Theil des Blastoderms, nämlich die mehrschichtige Wand
der Keimhöhlendecke als Hautblatt, Exoderm oder Epiblast
bezeichnen. Der
verdickte Randwulst (Fig. 64 w, 74 w), in welchem beide
primäre Keimblätter in einander übergehen, besteht in seinem oberen und
äusseren Theile aus Exodermzellen, in seinem unteren und inneren Theile aus
Entodermzellen.
In diesem Stadium entspricht unser
Fischkeim einer Amphiblastula, welche mitten in der Invagination begriffen
ist, und bei welcher die entstehende Urdarmhöhle eine grosse Dotterkugel
aufgenommen hat. Die Invagination wird nunmehr dadurch vervollständigt und
die Gastrulabildung dadurch abgeschlossen, dass die Keimhöhle verschwindet.
Das wachsende Entoderm, dem die Dotterkugel innig anhängt, wölbt sich in
die letztere hinein und nähert sich so dem Exoderm. Die klare Flüssigkeit
in der Keimhöhle wird resorbirt und schliesslich legt sich die obere
convexe Fläche des Entoderms an die untere concave des Exoderms eng an: die
Gastrula des discoblastischen Eies oder die Discogastrula
ist fertig (Fig. 65, 76;
Meridiandurchschnitt Fig. 66, 75).
Die Discogastrula unsers Knochenfisches in
diesem Stadium der vollen Ausbildung stellt nunmehr eine kreisrunde Kappe
dar, welche wie ein gefüttertes Mützchen fast die ganze obere Hemisphäre
der hyalinen Dotterkugel eng anliegend bedeckt (Fig. 65). Der Ueberzug des
Mützchens entspricht dem Exoderm (e), sein Futter dem Entoderm
(i). Ersteres besteht aus drei Schichten von kleineren Zellen,
letzteres aus einer einzigen Schicht von grösseren Zellen. Die
Exodermzellen (Fig. 77) messen 0.006 - 0.009 Mm., und haben ein klares, sehr feinkörniges
Protoplasma. Die Entodermzellen (Fig. 78) messen 0.02 - 0.03 Mm. und ihr Protoplasma ist mehr grobkörnig und trüber.
Letztere bilden auch den grössten Theil des Randwulstes, den wir nunmehr
als Urmundrand der Gastrula, als
Properistoma
oder auch als Rusconi'schen After
bezeichnen können. Der letztere
umfasst die Dotterkugel, welche die ganze Urdarmhöhle ausfüllt und weit aus
der dadurch verstopften Urmund-Oeffnung vorragt.
My objections to the view so lucidly explained in the passage just quoted, fall under two heads.
[Pg 279] (1) That the facts of development of the meroblastic eggs of vertebrates, are not in accordance with the views here advanced.
(2) That even if these views be accepted as representing the actual facts of development, the explanation offered of these facts would not be satisfactory.
* * * * *
Professor Haeckel's views are absolutely incompatible with the facts of Elasmobranch development, if my investigations are correct.
The grounds of the incompatibility may be summed up under the following heads:
(1) In Elasmobranchii the hypoblast cells occupy, even before the close of segmentation, the position which, on Professor Haeckel's view, they ought only eventually to take up after being involuted from the whole periphery of the blastoderm.
(2) There is no sign at any period of an invagination of the periphery of the blastoderm, and the only structure (the embryonic rim) which could be mistaken for such an invagination is confined to a very limited arc.
(3) The growth of cells to form the floor of the segmentation cavity, which ought to be part of this general invagination from the periphery, is mainly due to a formation of cells from the yolk.
It is this ingrowth of cells for the floor of the segmentation cavity which, I am inclined to think, Professor Haeckel has mistaken for a general invagination in the Osseous Fish he has investigated.
(4) Professor Haeckel fails to give an account of the asymmetry of the blastoderm; an asymmetry which is unquestionably also present in the blastoderm of most Osseous Fishes, though not noticed by Professor Haeckel in the investigations recorded in his paper.
The facts of development of Osseous Fishes, upon which Professor Haeckel rests his views, are too much disputed, for their [Pg 280] discussion in this place to be profitable[166]. The eggs of Osseous Fishes appear to me unsatisfactory objects for the study of this question, partly on account of all the cells of the blastoderm being so much alike, that it is a very difficult matter to distinguish between the various layers, and, partly, because there can be little question that the eggs of existing Osseous Fishes are very much modified, through having lost a great part of the food-yolk possessed by the eggs of their ancestors[167]. This disappearance of the food-yolk must, without doubt, have produced important changes in development, which would be especially marked in a pelagic egg, like that investigated by Professor Haeckel.
The Avian egg has been a still more disputed object than even the egg of the Osseous Fishes. The results of my own investigations on this subject do not accord with those of Dr Götte, or the views of Professor Haeckel[168].
Apart from disputed points of development, it appears to me that a comparative account of the development of the meroblastic [Pg 281] vertebrate ova ought to take into consideration the essential differences which exist between the Avian and Piscian blastoderms, in that the embryo is situated in the centre of the blastoderm in the first case and at the edge in the second[169].
This difference entails important modifications in development, and must necessarily affect the particular points under discussion. As a result of the different positions of the embryo in the two cases, there is present in Elasmobranchii and Osseous Fishes a true anus of Rusconi, or primitive opening into the alimentary canal, which is absent in Birds. Yet in neither Elasmobranchii[170] nor Osseous Fishes does the anus of Rusconi correspond in position with the point where the final closing in of the yolk takes place, but in them this point corresponds rather with the blastopore of Birds[171].
Owing also to the respective situations of the embryo in the [Pg 282] blastoderm, the alimentary and neural canals communicate posteriorly in Elasmobranchii and Osseous Fishes, but not in Birds. Of all these points Professor Haeckel makes no mention.
The support of his views which Prof. Haeckel attempts to gain from Götte's researches in Mammalia is completely cut away by the recent discoveries of Van Beneden[172] and Hensen[173].
It thus appears that Professor Haeckel's views but ill accord with the facts of vertebrate development; but even if they were to do so completely it would not in my opinion be easy to give a rational explanation of them.
Professor Haeckel states that no sharp and fast line can be drawn between the types of 'unequal' and 'discoidal' segmentation[174]. In the cases of unequal segmentation he admits, as is certainly the case, that the larger yolk cells (hypoblast) are simply enclosed by a growth of the epiblast around them; which is to be looked on as a modification of the typical gastrula invagination, necessitated by the large size of the yolk cells (vide Professor Haeckel's paper, Taf. II. fig. 30). In these instances there is no commencement of an ingrowth in the manner supposed for meroblastic ova.
When the food-yolk becomes more bulky, and the hypoblast does not completely segment, it is not easy to understand why an ingrowth, which had no existence in the former case, should occur; nor where it is to come from. Such an ingrowth as is supposed to exist by Professor Haeckel would, in fact, break the continuity of development between meroblastic and holoblastic ova, and thus destroy one of the most important results of the Gastræa theory.
It is quite easy to suppose, as I have done, that in the cases of discoidal segmentation, the hypoblast (including the yolk) becomes enclosed by the epiblast in precisely the same manner as in the cases of unequal segmentation.
But even if Professor Haeckel supposes that in the unsegmented food-yolk a fresh element is added to the ovum, it [Pg 283] remains quite unintelligible to me how an ingrowth of cells from a circumferential line, to form a layer which had no previous existence, can be equivalent to, or derived from, the invagination of a layer, which exists before the process of invagination begins, and which remains continuous throughout it.
If Professor Haeckel's views should eventually turn out to be in accordance with the facts of vertebrate development, it will, in my opinion, be very difficult to reduce them into conformity with the Gastræa theory.
Although some space has been devoted to an attempt to refute the views of Professor Haeckel on this question, I wish it to be clearly understood that my disagreement from his opinions concerns matters of detail only, and that I quite accept the Gastræa theory in its general bearings.
* * * * *
Observations upon the formation of the layers in Elasmobranchii have hitherto been very few in number. Those published in my preliminary account of these fishes are, I believe, the earliest[175].
Since then there has been published a short notice on the subject by Dr Alex. Schultz[176]. His observations in the main accord with my own. He apparently speaks of the nuclei of the yolk as cells, and also of the epiblast being more than one cell deep. In Torpedo alone, amongst the genera investigated by me, is the layer of epiblast, at about the age of the last described embryo, composed of more than a single row of cells.
[Pg 284] EXPLANATION OF PLATE 7.
Complete List of Reference Letters.
c. Cells formed in the yolk around the nuclei of the yolk. ep. Epiblast. er. Embryonic ring. es. Embryo swelling. hy. Hypoblast. ll. Lower layer cells. ly. Line separating the yolk from the blastoderm. m. Mesoblast. mg. Medullary groove. n´. Nuclei of yolk. na. Cells to form ventral wall of alimentary canal which have been derived from the yolk. nal. Cells formed around the nuclei of the yolk which have entered the hypoblast. sc. Segmentation cavity. vp. Combined lateral and vertebral plate of mesoblast.
Fig. 1. Longitudinal section of a blastoderm at the first appearance of the segmentation cavity.
Fig. 2. Longitudinal section through a blastoderm after the layer of cells has disappeared from the floor of the segmentation cavity. bd. Large cell resting on the yolk, probably remaining over from the later periods of segmentation. Magnified 60 diameters. (Hardened in chromic acid.)
The section is intended to illustrate the fact that the nuclei form a layer in the yolk under the floor of the segmentation cavity. The roof of the segmentation cavity is broken.
Fig. 2a. Portion of same blastoderm highly magnified, to shew the characters of the nuclei of the yolk n´ and the nuclei in the cells of the blastoderm.
Fig. 2b. Large knobbed nucleus from the same blastoderm, very highly magnified.
Fig. 2c. Nucleus of yolk from the same blastoderm.
Fig. 3. Longitudinal section of blastoderm of same stage as fig. 2. (Hardened in chromic acid.)
Fig. 4. Longitudinal section of blastoderm slightly older than fig. 2. Magnified 45 diameters. (Hardened in osmic acid.)
It illustrates (1) the characters of the epiblast; (2) the embryonic swelling; (3) the segmentation cavity.
Fig. 5. Longitudinal section through a blastoderm at the time of the first appearance of the embryonic rim, and before the formation of the medullary groove. Magnified 45 diameters.
Fig. 5a. Section through the periphery of the embryonic rim of the blastoderm of which fig. 5 represents a section.
Fig. 6. Section through the embryonic rim of a blastoderm somewhat younger than that represented on Pl. 8, fig. B.
Fig. 7. Section through the most projecting portion of the embryonic rim of a blastoderm of the same age as that represented on Pl. 8, fig. B. The section is drawn on a very considerably smaller scale than that on fig. 5. It is intended to illustrate the growth of the embryonic rim and the disappearance of the segmentation cavity.
Fig. 7a. Section through peripheral portion of the embryonic rim of the same blastoderm, highly magnified. It specially illustrates the formation of a cell (c) around a nucleus in the yolk. The nuclei of the blastoderm have been inaccurately rendered by the artist.
[Pg 285] Figs. 8a, 8b, 8c. Three sections of the same embryo. Inserted mainly to illustrate the formation of the mesoblast as two independent lateral masses of cells; only half of each section is represented. 8a is the most posterior of the three sections. In it the mesoblast forms a large mass on each side, imperfectly separated from the hypoblast. In 8b, from the anterior part of the embryo, the main mass of mesoblast is far smaller, and only forms a cap to the hypoblast at the highest point of the medullary fold. In 8c a cap of mesoblast is present, similar to that in 8b, though much smaller. The sections of these embryos were somewhat oblique, and it has unfortunately happened that while in 8a one side is represented, in 8b and 8c the other side is figured, had it not been for this the sections 8b and 8c would have been considerably longer than 8a.
Fig. 9. Longitudinal section of an embryo belonging to a slightly later stage than B.
This section passes through one of the medullary folds. It illustrates the continuity of the hypoblast with the remaining lower layer cells of the blastoderm.
Figs. 10a, 10b, 10c. Three sections of the same embryo belonging to a stage slightly later than B, Pl. 8. The space between the mesoblast and the hypoblast has been made considerably too great in the figures of the three sections.
10a. The most posterior of the three sections. It shews the posterior flatness of the medullary groove and the two isolated vertebral plates.
10b. This section is taken from the anterior part of the same embryo and shews the deep medullary groove and the commencing formation of the ventral wall of the alimentary canal from the nuclei of the yolk.
10c shews the disappearance of the medullary groove and the thinning out of the mesoblast plates in the region of the head.
Fig. 11. Small portion of the blastoderm and the subjacent yolk of an embryo at the time of the first appearance of the medullary groove × 300. It shews two large nuclei of the yolk (n) and the protoplasmic network in the yolk between them; the network is seen to be closer round the nuclei than in the intervening space. There are no areas representing cells around the nuclei.
Fig. 12. Nucleus of the yolk in connection with the protoplasmic network hardened in osmic acid.
Fig. 13. Portion of posterior end of a blastoderm of stage B, shewing the formation of cells around the nuclei of the yolk.
Fig. 14. Section through part of a young Scyllium egg, about 1/15th of an inch in diameter.
nl. Protoplasmic network in yolk. zp. Zona pellucida. ch. Structureless chorion. fep. Follicular epithelium. x. Structureless membrane external to this.
[116] Qy. Journal of Microsc. Science, Oct. 1874. [This Edition, No. V.]
[117] Centr. f. Med. Wiss. No. 38, 1875.
[118]
Professor Bambeke (Poissons Osseux,
Mém.
Acad. Belgique 1875) describes a cavity in the blastoderm of
Leuciscus rutilus, which he regards as the true segmentation cavity, but
not as identical with the segmentation cavity of Osseous Fishes, usually
so called. Its relations are the same as those of my segmentation cavity
at this stage. This paper came into my hands at too late a period for me
to be able to do more than refer to it in this place.
[119] Loc. cit.
[120] Loc. cit.
[121] Loc. cit.
[122] Qy. Journal of Micros. Science, Oct. 1874. [This Edition, No. V.]
[123] Loc. cit. Probably Dr Schultz, here as in other cases, has mistaken nuclei for cells.
[124] Loc. cit.
[125]
Prof. Haeckel (Die Gastrula u. die Eifurchung d.
Thiere,
Jenaische Zeitschrift, Vol. IX.) has unfortunately
copied a figure from my preliminary paper (loc. cit.)
(repeated now), which I had carefully avoided using for the purpose of
describing the formation of the layers on account of the epiblast cells in
the original having been much altered by the chromic acid, as a result of
which the whole section gives a somewhat erroneous impression of the
condition of the blastoderm at this stage. I take this opportunity of
pointing out that the colouration employed by Professor Haeckel to
distinguish the layers in this section is not founded on my statements,
but is, on the contrary, in entire opposition to them. From the section as
represented by Professor Haeckel it might be gathered that I considered
the lower layer cells to be divided into two parts, one derived from the
epiblast, while the other constituted the hypoblast. Not only is no such
division present at this period, but no part of the lower layer cells, or
the mesoblast cells into which they become converted, can in any sense
whatever be said to be derived from the epiblast.
[126]
Entwicklungsgeschichte der Najaden,
Sitz. d.
k. Akad. Wien, 1875.
[127] Morphologische Jahrbuch, Vol. 1. Heft 3.
[128]
Développement des Mammifères,
Bul. de l'Acad.
de Belgique, XL. No. 12, 1875.
[129] Qy. Journal Microsc. Science, Oct. 1874. [This Edition, No. V.]
[130]
Götte, Der Keim d. Forelleneies,
Arch. f.
Mikr. Anat. Vol. IX.; Haeckel, Die Gastrula u. die Eifurchung d. Thiere,
Jenaische
Zeitschrift, Bd. IX.
[131] When writing my earlier paper I did not feel so confident about the mode of formation of the hypoblast as I now do, and even doubted the possibility of determining it from sections. The facts now brought forward are I hope sufficient to remove all scepticism on this point.
[132] Owing to the small size of the plates this section has been drawn on a considerably smaller scale than that represented in fig. 5.
[133] Quart. Journ. of Microscop. Science, July, 1875. [This Edition, No. VI.]
[134] Oellacher, Zeit. f. Wiss. Zoologie, Bd. XXIII. Götte, Archiv f. Mikr. Anat. Vol. IX. Haeckel, loc. cit.
[135] This floor appears in most Osseous Fish to be only partially formed. Vide Götte, loc. cit.
[136] Loc. cit.
[137] Loc. cit.
[138] Loc. cit.
[139] Loc. cit.
[140] Loc. cit.
[141] Professor Lieberkühn (Gesellschaft zu Marburg, Jan. 1876) finds in Mammalia a bilateral arrangement of the mesoblast, which he compares with that described by me in Elasmobranchii. In Mammalia, however, he finds the two masses of mesoblast connected by a very thin layer of cells, and is apparently of opinion that a similar thin layer exists in Elasmobranchii though overlooked by me. I can definitely state that, whatever may be the condition of the mesoblast in Mammalia, in Elasmobranchii at any rate no such layer exists.
[142] Loc. cit.
[143] Quart. Journ. of Microsc. Science, Oct., 1874. [This Edition, No. V.]
[144]
Embryologische Studien an Würmern u. Arthropoden.
Mémoires de l'Acad. S. Pétersbourg. Vol. XIV. 1873.
[145] Archiv für Mikr. Anat. Vol. VII.
[146] Jenaische Zeitschrift, Vol. IX. 1875. A bilateral development of mesoblast, according to Professor Haeckel (loc. cit.), occurs in some Osseous Fish. Hensen, Zeit. für Anat. u. Entw. Vol. 1., has recently described the mesoblast in Mammalia as consisting of independent lateral masses.
[147] Archiv für Mikr. Anat. Vol. XI.
[148]
A protoplasmic network resembling in its essential features the one just
described has been noticed by many observers in other ova. Fol has figured
and described a network or sponge-like arrangement of the protoplasm in
the eggs of Geryonia. (Jenaische Zeitschrift, Vol. VII.) Metschnikoff (Zeitschrift f.
Wiss. Zoologie, 1874) has demonstrated its presence in the ova of
many Siphonophoriæ and Medusæ. Flemming (Entwicklungsgeschichte der Najaden,
Sitz. der k.
Akad. Wien, 1875) has found it in the ovarian ova of fresh-water
mussels (Anodonta and Unio), but regards it as due to the action of
reagents, since he fails to find it in the fresh condition. Amongst
vertebrates it has been carefully described by Eimer (Archiv für Mikr. Anat., Vol.
VIII.) in
the ovarian ova of Reptiles. Eimer moreover finds that it is continuous
with prolongations from cells of the epithelium of the follicle in which
the ovum is contained. According to him remnants of this network are to be
met with in the ripe ovum, but are no longer present in the ovum when
taken from the oviduct.
[149]
Wirbelthiereier mit partieller Dottertheilung.
Müller's Arch. 1861.
[150] Erste Anlage des Wirbelthierleibes.
[151] Eierstock u. Ei.
[152] Entwicklungsgeschichte der Unke. The important researches of Götte on the development of the ovum, though meriting the most careful attention, do not admit of discussion in this place.
[153] Annals and Magaz. of Natural History, Vol. XI. 1873, p. 81.
[154] Archiv f. Mikr. Anat. Vol. X.
[155] Quart. Journ. of Micr. Science, Oct. 1874.
[156] Archiv f. Mikr. Anat. Vol. XXI.
[157]
Comparison,
&c., Quart. Journ. Micr.
Science, July, 1875. [This Edition, No. VI.]
[158] Loc. cit.
[159] This figure, together with figs. 2 and 3, are reproduced from my paper upon the comparison of the early stages of development in vertebrates.
[160] Quart. Journ. of Micr. Science, July, 1875. [This Edition, No. VI.]
[161]
Die Gastrula u. Eifurchung d. Thiere,
Jenaische Zeitschrift, Vol.
IX.
[162] For instance, in Crustaceans it does not in some cases appear certain whether an invagination is the typical gastrula invagination, or only an invagination by which, at a period subsequent to the gastrula invagination, the hind gut is frequently formed.
[163] Loc. cit.
[164] Loc. cit.
[165] The references in this quotation are to the figures in the original.
[166]
A short statement by Kowalevsky on this subject in a note to his account
of the development of Ascidians, would seem to indicate that the type of
development of Osseous Fishes is precisely the same as that of
Elasmobranchii. Kowalevsky says, Arch. f. Mikr.
Anat. Vol. VII. p. 114, note 5,
According to my observations on Osseous Fishes the germinal wall
consists of two layers, an upper and lower, which are continuous with one
another at the border. From the upper one develops skin and nervous
system, from the lower hypoblast and mesoblast.
This statement, which
leaves unanswered a number of important questions, is too short to serve
as a basis for supporting my views, but so far as it goes its agreement
with the facts of Elasmobranch development is undoubtedly striking.
[167] The eggs of the Osseous Fishes have, I believe, undergone changes of the same character, but not to the same extent, as those of Mammalia, which, according to the views expressed both by Professor Haeckel and myself, are degenerated from an ovum with a large food-yolk. The grounds on which I regard the eggs of Osseous Fishes as having undergone an analogous change, are too foreign to the subject to be stated here.
[168] I find myself unable without figures to understand Dr Rauber's (Centralblatt für Med. Wiss. 1874, No. 50; 1875, Nos. 4 and 17) views with sufficient precision to accord to them either my assent or dissent. It is quite in accordance with the view propounded in my paper (loc. cit.) to regard, with Dr Rauber and Professor Haeckel, the thickened edge of the blastoderm as the homologue of the lip of the blastopore in Amphioxus; though an invagination, in the manner imagined by Professor Haeckel, is no necessary consequence of this view. If Dr Rauber regards the whole egg of the bird as the homologue of that of Amphioxus, and the inclosure of the yolk by the blastoderm as the equivalent to the process of invagination in Amphioxus, then his views are practically in accordance with my own.
[169]
I have suggested in a previous paper (Comparison,
&c., Quart. Journal of Micr. Science,
July, 1875) that the position occupied by the embryo of Birds at the
centre, and not at the periphery, of the blastoderm may be due to an
abbreviation of the process by which the Elasmobranch embryos cease to be
situated at the edge of the blastoderm (vide
p. 296 and Pl. 9,
fig. 1, 2). Assuming this to be the real explanation of the position of
the embryo in Birds, I feel inclined to repeat a speculation which I made
some time ago with reference to the primitive streak in Birds (Quart. Journ. of Micr.
Science, 1873, p. 280). In Birds there is, as is well
known, a structure called the primitive streak, which has been shewn by
the observations of Dursy, corroborated by my observations (loc. cit.), to be situated behind the medullary groove, and
to take no part in the formation of the embryo. I further shewed that the
peculiar fusion of epiblast and mesoblast, called by His the axis cord,
was confined to this structure and did not occur in other parts of the
blastoderm. Nearly similar results have been recently arrived at by Hensen
with reference to the primitive streak in Mammals. The position of the
primitive streak immediately behind the embryo suggests the speculation
that it may represent the line along which the edges of the blastoderm
coalesced, so as to give to the embryo the central position which it has
in the blastoderms of Birds and Mammals, and that the peculiar fusion of
epiblast and mesoblast at this point may represent the primitive
continuity of epiblast and lower layer cells at the dorsal lip of the anus
of Rusconi in Elasmobranchii. I put this speculation forward as a mere
suggestion, in the hope of elucidating the peculiar structure of the
primitive streak, which not improbably may be found to be the keystone to
the nature of the blastoderm of the higher vertebrates.
[170]
Vide p. 296 and Plate
9, fig. 1 and 2, and Self, Comparison,
&c., loc. cit.
[171] The relation of the anus of Rusconi and blastopore in Elasmobranchii was fully explained in the paper above quoted. It was there clearly shewn that neither the one nor the other exactly corresponds with the blastopore of Amphioxus, but that the two together do so. Professor Haeckel states that in the Osseous Fish investigated by him the anus of Rusconi and the blastopore coincide. This is not the case in the Salmon.
[172]
Développement Embryonnaire des Mammifères,
Bulletin de l'Acad. r. d. Belgique, 1875.
[173] Loc. cit.
[174] For an explanation of these terms, vide Prof. Haeckel's original paper or the abstract in Quart. Journ. of Micr. Science for January, 1876.
[175] I omit all reference to a paper published in Russian by Prof. Kowalevsky. Being unable to translate it, and the illustrations being too meagre to be in themselves of much assistance, it has not been possible for me to make any use of it.
[176] Centralblatt f. Med. Wiss. No. 33, 1875.
No complete series of figures, representing the various stages in development of an Elasmobranch Embryo, has hitherto been published. With the view of supplying this deficiency Plate 8 has been inserted. The embryos represented in this Plate form a fairly complete series, but do not all belong to a single species. Figs. A, B, C, D, E, F, H, I represent embryos of Pristiurus; G being an embryo of Torpedo. The remaining figures, excepting K, which is a Pristiurus embryo, are embryos of Scyllium canicula. The embryos A-I were very accurately drawn from nature by my sister, Miss A. B. Balfour. Unfortunately the exceptional beauty and clearness of the originals is all but lost in the lithographs. To facilitate future description, letters will be employed in the remainder of these pages to signify that an embryo being described is of the same age as the embryo on this Plate to which the letter used refers. Thus an embryo of the same age as L will be spoken of hereafter as belonging to stage L.
A.
This figure represents a hardened blastoderm at a stage when the embryo-swelling (e.s.) has become obvious, but before the appearance of the medullary groove. The position of the segmentation cavity is indicated by a slight swelling of the blastoderm (s.c). The shape of the blastoderm, in hardened specimens, is not to be relied upon, owing to the traction which the blastoderm undergoes during the process of removing the yolk from the egg-shell.
B.
B is the view of a fresh blastoderm. The projecting part of this, already mentioned as the 'embryonic rim', is indicated [Pg 287] by the shading. At the middle of the embryonic rim is to be seen the rudiment of the embryo (m.g.). It consists of an area of the blastoderm, circumscribed on its two sides and at one end, by a slight fold, and whose other end forms part of the edge of the blastoderm. The end of the embryo which points towards the centre of the blastoderm is the head end, and that which forms part of the edge of the blastoderm is the tail end. To retain the nomenclature usually adopted in treating of the development of the Bird, the fold at the anterior end of the embryo may be called the head fold, and those at the sides the side folds. There is in Elasmobranchii no tail fold, owing to the position of the embryo at the periphery of the blastoderm, and it is by the meeting of the three above-mentioned folds only, that the embryo becomes pinched off from the remainder of the blastoderm. Along the median line of the embryo is a shallow groove (m.g.), the well-known medullary groove of vertebrate embryology. It flattens out both anteriorly and posteriorly, and is deepest in the middle part of its course.
C.
This embryo resembles in most of its features the embryo last described. It is, however, considerably larger, and the head fold and side folds have become more pronounced structures. The medullary groove is far deeper than in the earlier stage, and widens out anteriorly. This anterior widening is the first indication of a distinction between the brain and the remainder of the central nervous system, a distinction which arises long before the closure of the medullary canal.
D.
This embryo is far larger than the one last described, but the increase in length does not cause it to project beyond the edge of the blastoderm, but has been due to a growth inwards towards the centre of the blastoderm. The head is now indicated by an anterior enlargement, and the embryo also widens out posteriorly. The posterior widening (t.s.) is formed by a pair of rounded prominences, one on each side of the middle line. These are very conspicuous organs during the earlier stages of development, and consist of two large aggregations of mesoblast cells. [Pg 288] In accordance with the nomenclature adopted in my preliminary paper[177], they may be called 'tail-swellings'. Between the cephalic enlargements and the tail-swellings is situated the rudimentary trunk of the embryo. It is more completely pinched off from the blastoderm than in the last described embryo. The medullary groove is of a fairly uniform size throughout the trunk of the embryo, but flattens out and vanishes completely in the region of the head. The blastoderm in Pristiurus and Scyllium grows very rapidly, and has by this stage attained a very considerable size; but in Torpedo its growth is very slow.
E and F.
These two embryos may be considered together, for, although they differ in appearance, yet they are of an almost identical age; and the differences between the two are purely external. E appears to be a little abnormal in not having the cephalic region so distinctly marked off from the trunk as is usual. The head is proportionally larger than in the last stage, and the tail-swellings remain as conspicuous as before. The folding off from the blastoderm has progressed rapidly, and the head and tail are quite separated from it. The medullary groove has become closed posteriorly in both embryos, but the closing has extended further forwards in F than in E. In F the medullary folds have not only united posteriorly, but have very nearly effected a fresh junction in the region of the neck. At this point a second junction of the two medullary folds is in fact actually effected before the posterior closing has extended forwards so far. The later junction in the region of the neck corresponds in position with the point, where in the Bird the medullary folds first unite. No trace of a medullary groove is to be met with in the head, which simply consists of a wide flattened plate. Between the two tail-swellings surface views present the appearance of a groove, but this appearance is deceptive, since in sections no groove, or at most a very slight one, is perceptible.
G.
During the preceding stages growth in the embryo is very slow, and considerable intervals of time elapse before any [Pg 289] perceptible changes are effected. This state of things now becomes altered, and the future changes succeed each other with far greater rapidity. One of the most important of these, and one which first presents itself during this stage, is the disappearance of the yolk-spherules from the embryonic cells, and the consequently increased transparency of the embryo. As a result of this, a number of organs, which in the earlier stages were only to be investigated by means of sections, now become visible in the living embryo.
The tail-swellings (t.s.) are still conspicuous objects at the posterior extremity of the embryo. The folding off of the embryo from the yolk has progressed to such an extent that it is now quite possible to place the embryo on its side and examine it from that point of view.
The embryo may be said to be attached to the yolk by a distinct stalk or cord, which in the succeeding stages gradually narrows and elongates, and is known as the umbilical cord (so.s.). The medullary canal has now become completely closed, even in the region of the brain, where during the last stage no trace of a medullary groove had appeared. Slight constrictions, not perceptible in views of the embryo as a transparent object, mark off three vesicles in the brain. These vesicles are known as the fore, mid, and hind brain. From the fore-brain there is an outgrowth on each side, the first rudiment of the optic vesicle (op.).
The mesoblast on each side of the body is divided into a series of segments, known as protovertebræ or muscle-plates, the first of which lies a little behind the head. The mesoblast of the tail has not as yet undergone this segmentation. There are present in all seventeen segments. These first appeared at a much earlier date, but were not visible owing to the opacity of the embryo.
Another structure which became developed in even a younger embryo than C is now for the first time visible in the living embryo. This is the notochord: it extends from almost the extreme posterior to the anterior end of the embryo. It lies between the ventral wall of the spinal canal and the dorsal wall of the intestine; and round its posterior end these two walls become continuous with each other (vide fig.). Anteriorly the [Pg 290] termination of the notochord cannot be seen, it can only be traced into a mass of mesoblast at the base of the brain, which there separates the epiblast from the hypoblast. The alimentary canal (al.) is completely closed anteriorly and posteriorly, though still widely open to the yolk-sac in the middle part of its course. In the region of the head it exhibits on each side a slight bulging outwards, the rudiment of the first visceral cleft. This is represented in the figure by two lines (I v.c.). The visceral clefts at this stage consist of a pair of simple diverticula from the alimentary canal, and there is no communication between the throat and the exterior.
H.
The present embryo is far larger than the last, but it has not been possible to represent this increase in size in the drawings. Accompanying this increase in size, the folding off of the embryo from the yolk has considerably progressed, and the stalk which unites the embryo with the yolk is proportionately narrower and longer than before.
The brain is now very distinctly divided into the three lobes, whose rudiments appeared during the last stage. From the foremost of these, the optic vesicles now present themselves as well-marked lateral outgrowths, towards which there appears a growing in, or involution, from the external skin (op.) to form the lens. The opening of this involution is represented by the dark spot in the centre.
A fresh organ of sense, the auditory sac, now for the first time becomes visible as a shallow pit in the external skin on each side of the hind-brain (au.v.). The epiblast which is involuted to form this pit becomes much thickened, and thereby the opacity, indicated in the figure, is produced.
The muscle-plates have greatly increased in number by the formation of fresh segments in the tail. Thirty-eight of them were present in the embryo figured. The mesoblast at the base of the brain has increased in quantity, and there is still a certain mass of unsegmented mesoblast which forms the tail-swellings. The first rudiment of the heart becomes visible during this stage as a cavity between the mesoblast of the splanchnopleure and the hypoblast (ht.).
[Pg 291] The fore and hind guts are now longer than they were. A slight pushing in from the exterior to form the mouth has appeared (m.), and an indication of the future position of the anus is afforded by a slight diverticulum of the hind gut towards the exterior some little distance from the posterior end of the embryo (an.). The portion of the alimentary canal behind this point, though at this stage large, and even dilated into a vesicle at its posterior end (al.v.), becomes eventually completely atrophied. In the region of the throat the rudiment of a second visceral cleft has appeared behind the first; neither of them are as yet open to the exterior. The number of visceral clefts present in any given Pristiurus embryo affords a very easy and simple way of determining its age.
I.
A great increase in size is again to be noticed in the embryo, but, as in the case of the last embryo, it has not been possible to represent this in the figure. The stalk connecting the embryo with the yolk has become narrower and more elongated, and the tail region of the embryo proportionately far longer than in the last stage. During this stage the first spontaneous movements of the embryo take place, and consist in somewhat rapid excursions of the embryo from side to side, produced by a serpentine motion of the body.
The cranial flexure, which commenced in stage G, has now become very evident, and the mid-brain[178] begins to project in the same manner as in the embryo fowl on the third day, and will soon form the anterior termination of the long axis of the embryo. The fore-brain has increased in size and distinctness, and the anterior part of it may now be looked on as the unpaired rudiment of the cerebral hemispheres.
Further growths have taken place in the organs of sense, especially in the eye, in which the involution for the lens has made considerable progress. The number of the muscle-plates has again increased, but there is still a region of unsegmented [Pg 292] mesoblast in the tail. The thickened portions of mesoblast which caused the tail-swellings are still to be seen and would seem to act as the reserve from which is drawn the matter for the rapid growth of the tail, which occurs soon after this. The mass of the mesoblast at the base of the brain has again increased. No fresh features of interest are to be seen in the notochord. The heart is now much more conspicuous than before, and its commencing flexure is very apparent. It now beats actively. The hind gut especially is much longer than in the last specimen; and the point where the anus will appear is very easily detected by the bulging out of the gut towards the external skin at that point (an.). The alimentary vesicle, first observable during the last stage, is now a more conspicuous organ (al.v.). Three visceral clefts, none of which are as yet open to the exterior, may now be seen.
K.
The figures G, H, I are representations of living and transparent embryos, but the remainder of the figures are drawings of opaque embryos which were hardened in chromic acid.
The stalk connecting the embryo with the yolk is now, comparatively speaking, quite narrow, and is of sufficient length to permit the embryo to execute considerable movements.
The tail has grown immensely, but is still dilated terminally. This terminal dilatation is mainly due to the alimentary vesicle, but the tract of gut connecting this with the gut in front of the anus is now a solid rod of cells and very soon becomes completely atrophied.
The two pairs of limbs have appeared as elongated ridges of epiblast. The anterior pair is situated just at the front end of the umbilical stalk; and the posterior pair, which is the more conspicuous of the two, is situated some little distance behind the stalk.
The cranial flexure has greatly increased, and the angle between the long axis of the front part of the head and of the body is less than a right angle. The conspicuous mid-brain forms the anterior termination of the long axis of the body. The thin roof of the fourth ventricle may in the figure be noticed behind the mid-brain. The auditory sac is nearly closed and its [Pg 293] opening is not shewn in the figure. In the eye the lens is completely formed.
Owing to the opacity of the embryo, the muscle-plates are only indistinctly indicated, and no other features of the mesoblast are to be seen.
The mouth is now a deep pit, whose borders are almost completely formed by the thickening in front of the first visceral cleft, which may be called the first visceral arch or mandibular arch.
Four visceral clefts are now visible, all of which are open to the exterior, but in a transparent embryo one more, not open to the exterior, would have been visible behind the last of these.
L.
This embryo is considerably older than the one last described, but growth is not quite so rapid as might be gathered from the fact that L is nearly twice as long as K, since the two embryos belong to different genera; and the Scyllium embryos, of which L is an example, are larger than Pristiurus embryos. The umbilical stalk is now quite a narrow elongated structure, whose subsequent external changes are very unimportant, and consist for the most part merely in an increase in its length.
The tail has again grown greatly in length, and its terminal dilatation together with the alimentary vesicle contained in it, have both completely vanished. A dorsal and ventral fin are now clearly visible; they are continuous throughout their whole length. The limbs have grown and are more easily seen than in the previous stage.
Great changes have been effected in the head, resulting in a diminution of the cranial flexure. This diminution is nevertheless apparent rather than real, and is chiefly due to the rapid growth of the rudiment of the cerebral hemispheres. The three main divisions of the brain may still be clearly seen from the surface. Posteriorly is situated the hind-brain, now consisting of the medulla oblongata and cerebellum. At the anterior part of the medulla is to be seen the thin roof of the fourth ventricle, and anteriorly to this again the roof becomes thickened to form the rudiment of the cerebellum. In front of the hind-brain lies the mid-brain, the roof of which is formed by the [Pg 294] optic lobes, which are still situated at the front end of the long axis of the embryo.
Beyond the mid-brain is placed the fore-brain, whose growth is rapidly rendering the cranial flexure imperceptible.
The rudiments of the nasal sacs are now clearly visible as a pair of small pits. The pits are widely open to the exterior, and are situated one on each side, near the front end of the cerebral hemispheres. Five visceral clefts are open to the exterior, and in them the external gills have commenced to appear (L´).
The first cleft is no longer similar to the rest, but has commenced to be metamorphosed into the spiracle.
Accompanying the change in position of the first cleft, the mandibular arch has begun to bend round and enclose the front as well as the side of the mouth. By this change in the mandibular arch the mouth becomes narrowed in an antero-posterior direction.
M.
Of this embryo the head alone has been represented. Two views of it are given, one (M) from the side and the other (M´) from the under surface. The growth of the front part of the head has considerably diminished the prominence of the cranial flexure. The full complement of visceral clefts is now present—six in all. But the first has already atrophied considerably, and may easily be recognized as the spiracle. In Scyllium, there are present at no period more than six visceral clefts. The first visceral arch on each side has become bent still further round, to form the front border of the mouth. The opening of the mouth has in consequence become still more narrowed in an antero-posterior direction. The width of the mouth in this direction, serves for the present and for some of the subsequent stages as a very convenient indication of age.
N.
The limbs, or paired fins, have now acquired the general features and form which they possess in the adult.
The unpaired fins have now also become divided in a manner not only characteristic of the Elasmobranchii but even of the genus Scyllium.
[Pg 295] There is a tail fin, an anal fin and two dorsal fins, both the latter being situated behind the posterior paired fins.
In the head may be noticed a continuation of the rapid growth of the anterior part.
The mouth has become far more narrow and slit-like; and with many other of the organs of the period commences to approach the form of the adult.
The present and the three preceding stages shew the gradual changes by which the first visceral arch becomes converted into the rudiments of the upper and of the lower jaw. The fact of the conversion was first made known through the investigations of Messrs Parker and Gegenbaur.
O.
In this stage the embryo is very rapidly approaching the form of the adult.
This is especially noticeable in the fins, which project in a manner quite characteristic of the adult fish. The mouth is slit-like, and the openings of the nasal sacs no longer retain their primitive circular outline. The external gills project from all the gill-slits including the spiracle.
P.
The head is rapidly elongating by the growth of the snout, and the divisions of the brain can no longer be seen with distinctness from the exterior, and, with the exception of the head and of the external gills, the embryo almost completely resembles the adult.
Q.
The snout has grown to such an extent, that the head has nearly acquired its adult shape. In the form of its mouth the embryo now quite resembles the adult fish.
* * * * *
This part of the subject may be conveniently supplemented by a short description of the manner in which the blastoderm encloses the yolk. It has been already mentioned that the growth of the blastoderm is not uniform. The part of it in the immediate neighbourhood of the embryo remains comparatively stationary, while the growth elsewhere is very rapid. From [Pg 296] this it results that that part of the edge of the blastoderm where the embryo is attached forms a bay in the otherwise regular outline of the edge of the blastoderm. By the time that one-half of the yolk is enclosed the bay is a very conspicuous feature (Pl. 9, fig. 1). In this figure bl. points to the blastoderm, and yk. to the part of the yolk not yet enclosed by the blastoderm.
Shortly subsequent to this the bay becomes obliterated by its two sides coming together and coalescing, and the embryo ceases to lie at the edge of the yolk.
This stage is represented on Pl. 9, fig. 2. In this figure there is only a small patch of yolk not yet enclosed (yk), which is situated at some little distance behind the embryo. Throughout all this period the edge of the blastoderm has remained thickened, a feature which persists till the complete investment of the yolk, which takes place shortly after the stage last figured. In this thickened edge a circular vein arises, which brings back the blood from the yolk-sac to the embryo. The opening in the blastoderm (Pl. 9, fig. 2, yk.), exposing the portion of the yolk not yet enclosed, may be conveniently called the blastopore, according to Professor Lankester's nomenclature.
The interesting feature which characterizes the blastopore in Elasmobranchii is the fact of its not corresponding in position with the opening of the anus of Rusconi. We thus have in Elasmobranchii two structures, each of which corresponds in part with the single structure in Amphioxus which may be called either blastopore or anus of Rusconi, which yet do not in Elasmobranchii coincide in position. It is the blastopore of Elasmobranchii which has undergone a change of position, owing to the unequal growth of the blastoderm; while the anus of Rusconi retains its normal situation. In Osseous Fishes the blastopore undergoes a similar change of position. The possibility of a change in position of this structure is peculiarly interesting, in that it possibly serves to explain how the blastopore of different animals corresponds in different cases with the anus or the mouth, and has not always a fixed situation[179].
[Pg 297] EXPLANATION OF PLATES 8 and 9.
Complete List of Reference Letters.
a. Arteries of yolk sac (red). al. Alimentary cavity. alv. Alimentary vesicle at the posterior end of the alimentary canal. an. Point where anus will appear. auv. Auditory vesicle. bl. Blastoderm. ch. Notochord. es. Embryo-swelling. h. Head. ht. Heart. m. Mouth. mg. Medullary groove. mp. Muscle-plate or protovertebra. op. Eye. sc. Segmentation cavity. sos. Somatic stalk. ts. Tail-swelling. v. Veins of yolk sac (blue). vc. Visceral cleft. I. vc. 1st visceral cleft. x. Portion of blastoderm outside the arterial circle in which no blood-vessels are present. yk. Yolk.
Plate 8.
Fig. A. Surface view of blastoderm of Pristiurus hardened in chromic acid.
Fig. B. Surface view of fresh blastoderm of Pristiurus.
Figs. C, D, E, and F. Pristiurus embryos hardened in chromic acid.
Fig. G. Torpedo embryo viewed as a transparent object.
Figs. H, I. Pristiurus embryos viewed as transparent objects.
Fig. K. Pristiurus embryo hardened in chromic acid.
The remainder of the figures are representations of embryos of Scyllium canicula hardened in chromic acid. In every case, with the exception of the figures marked P and Q, two representations of the same embryo are given; one from the side and one from the under surface.
Plate 9.
Fig. 1. Yolk of a Pristiurus egg with blastoderm and embryo. About two-thirds of the yolk have been enveloped by the blastoderm. The embryo is still situated at the edge of the blastoderm, but at the end of a bay in the outline of this. The thickened edge of the blastoderm is indicated by a darker shading. Two arteries have appeared.
Fig. 2. Yolk of an older Pristiurus egg. The yolk has become all but enveloped by the blastoderm, and the embryo ceases to lie at the edge of the blastoderm, owing to the coalescence of the two sides of the bay which existed in the earlier stage. The circulation is now largely developed. It consists of an external arterial ring, and an internal venous ring, the latter having been developed in the thickened edge of the blastoderm. Outside the arterial ring no vessels are developed.
Fig. 3. The yolk has now become completely enveloped by the blastoderm. The arterial ring has increased in size. The venous ring has vanished, owing to the complete enclosure of the yolk by the blastoderm. The point where it existed is still indicated (y) by the brush-like termination of the main venous trunk in a number of small branches.
Fig. 4. Diagrammatic projection of the vascular system of the yolk sac of a somewhat older embryo.
The arterial ring has grown much larger and the portion of the yolk where no vessels exist is very small (x). The brush-like termination of the venous trunk is still to be noticed.
The two main trunks (arterial and venous) in reality are in close contact as in fig. 5, and enter the somatic stalk close together.
The letter a which points to the venous (blue) trunk should be v and not a.
Fig. 5. Circulation of the yolk sac of a still older embryo, in which the arterial circle has ceased to exist, owing to the space outside it having become smaller and smaller and finally vanished.
[177] Quart. Journ. Micr. Science, Oct. 1874. [This Edition, No. V.]
[178] The part of the brain which I have here called mid-brain, and which unquestionably corresponds to the part called mid-brain in the embryos of higher vertebrates, becomes in the adult what Miklucho-Maclay and Gegenbaur called the vesicle of the third ventricle or thalamencephalon. I shall always speak of it as the mid-brain.
[179]
For a fuller discussion of this question vide Self,
A comparison of the early stages of development in vertebrates.
Quart.
Journ. of Micr. Science, July, 1875. [This Edition, No. VI.]
The present chapter deals with the history of the development of the Elasmobranch embryo from the period when the medullary groove first arises till that in which it becomes completely closed, and converted into the medullary canal. The majority of the observations recorded were made on Pristiurus embryos, a few on embryos of Torpedo. Where nothing is said to the contrary the statements made apply to the embryos of Pristiurus only.
The general external features for this period have already been given in sufficient detail in the last chapter; and I proceed at once to describe consecutively the history of the three layers.
General Features of the Epiblast.
At the commencement of this period, during the stage intermediate between B and C, the epiblast is composed of a single layer of cells. (Pl. 10, fig. 1.)
These are very much elongated in the region of the embryo, but flattened in other parts of the blastoderm. Throughout they contain numerous yolk-spherules.
In a Torpedo embryo of this age (as determined by the condition of the notochord) the epiblast presents a very different structure. It is composed of small spindle-shaped cells several rows deep. The nuclei of these are very large in proportion to the cells containing them, and the yolk-spherules are far less numerous than in the cells of corresponding Pristiurus embryos.
During stage C the condition of the epiblast does not undergo any important change, with the exception of the layer [Pg 299] becoming much thickened, and its cells two or three deep in the anterior parts of the embryo. (Pl. 10, fig. 2.)
In the succeeding stages that part of the epiblast, which will form the spinal cord, gradually becomes two or three cells deep. This change is effected by a decrease in the length of the cells as compared with the thickness of the layer. In the earlier stages the cells are wedge-shaped with an alternate arrangement, so that a decrement in the length of the cells at once causes the epiblast to be composed of two rows of interlocking cells.
The lateral parts of the epiblast which form the epidermis of the embryo are modified in quite a different manner to the nervous parts of the layer, becoming very much diminished in thickness and composed of a single row of flattened cells. (Pl. 10, fig. 3.)
Till the end of stage F, the epiblast cells and indeed all the cells of the blastoderm retain their yolk-spherules, but the epiblast begins to lose them and consequently to become transparent in stage G.
Medullary Groove.
During stage B the medullary groove is shallow posteriorly, deeper in the middle part, and flattened out again at the extreme anterior end of the embryo. (Pl. 7, fig. 10a, b, c.)
A similar condition obtains in the stage between B and C, but the canal has now in part become deeper. Anteriorly no trace of it is to be seen. In stage C it exhibits the same general features. (Pl. 10, fig. 2a, 2b, 2c.)
By stage D we find important modifications of the canal.
It is still shallow behind and deep in the dorsal region, Pl. 10, figs. 3d, 3e, 3f; but the anterior flattened area in the last stage has grown into a round flat plate which may be called the cephalic plate, Pl. 8, D and Pl. 10, figs. 3a, 3b, 3c. This plate becomes converted into the brain. Its size and form give it a peculiar appearance, but the most remarkable feature about it is the ventral curvature of its edges. Its edges do not, as might be expected, bend dorsalwards towards each other, but become sharply bent in a ventral direction. This feature is for the first [Pg 300] time apparent at this stage, but becomes more conspicuous during the succeeding ones, and attains its maximum in stage F (Pl. 10, fig. 5), in which it might almost be supposed that the edges of the cephalic plate were about to grow downwards and meet on the ventral side of the embryo.
In the stages subsequent to D the posterior part of the canal deepens much more rapidly than the rest (vide Pl. 10, fig. 4, taken from the posterior end of an embryo but slightly younger than F), and the medullary folds unite and convert the posterior end of the medullary groove into a closed canal (Pl. 8, fig. F), while the groove is still widely open elsewhere[180]. The medullary canal does not end blindly behind, but simply forms a tube not closed at either extremity. The importance of this fact will appear later.
In a stage but slightly subsequent to F nearly the whole of the medullary canal becomes formed. This occurs in the usual way by the junction and coalescence of the medullary folds. In the course of the closing of the medullary groove the edges of the cephalic plate lose their ventral curvature and become bent up in the normal manner (vide Pl. 10, fig. 6, a section taken through the posterior part of the cephalic plate), and the enlarged plate merely serves to enclose a dilated cephalic portion of the medullary canal. The closing of the medullary canal takes place earlier in the head and neck than in the back. The anterior end of the canal becomes closed and does not remain open like the posterior end.
Elasmobranch embryos resemble those of the Sturgeon (Acipenser) and the Amphibians in the possession of a spatula-like cephalic expansion: but so far as I am aware a ventral flexure in the medullary plates of the head has not been observed in other groups.
The medullary canal in Elasmobranchii is formed precisely on the type so well recognised for all groups of vertebrates with the exception of the Osseous Fishes. The only feature in any respect peculiar to these fishes is the closing of their medullary canal first commencing behind, and then at a second point in the [Pg 301] cervical region. In those vertebrates in which the medullary folds do not unite at approximately the same time throughout their length, they appear usually to do so first in the region of the neck.
Mesoblast.
The separation from the hypoblast of two lateral masses of mesoblast has already been described. Till the close of stage C the mesoblast retains its primitive bilateral condition unaltered. Throughout the whole length of the embryo, with the exception of the extreme front part, there are present two plates of rounded mesoblast cells, one on each side of the medullary groove. These plates are in very close contact with the hypoblast, and also follow with fair accuracy the outline of the epiblast. This relation of the mesoblast plates to the epiblast must not however be supposed to indicate that the medullary groove is due to growth in the mesoblast: a view which is absolutely negatived by the manner of formation of the medullary groove in the head. Anteriorly the mesoblast plates thin out and completely vanish.
In stage D, the plates of mesoblast in the trunk undergo important changes. The cells composing them become arranged in two layers (Pl. 10, fig. 3), a splanchnic layer adjoining the hypoblast (sp), and a somatic layer adjoining the epiblast[181] (so). Although these two layers are distinctly formed, they do not become separated at this stage in the region of the trunk, and in the trunk no true body-cavity is formed.
By stage D the plates of mesoblast have ceased to be quite isolated, and are connected with the lower layer cells of the general blastoderm.
Moreover the lower layer cells outside the embryo now exhibit distinct traces of a separation into two layers, one continuous with the hypoblast, the other with the mesoblast. Both layers are composed of very flattened cells, and the mesoblast layer is often more than one cell deep, and sometimes exhibits a mesh-like arrangement of its elements.
[Pg 302] Coincidentally with the appearance of a differentiation into a somatic and splanchnic layer the mesoblast plates become partially split by a series of transverse lines of division into protovertebræ. Only the proximal regions of the plates become split in this way, while their peripheral parts remain quite intact. As a result of this each plate becomes divided into a proximal portion adjoining the medullary canal, which is divided into protovertebræ, and may be called the vertebral plate, and a peripheral portion not so divided, which may be called the lateral plate. These two parts are at this stage quite continuous with each other; and, as will be seen in the sequel, the body-cavity originally extends uninterruptedly to the summit of the vertebral plates.
By stage D at the least ten protovertebræ have appeared.
In Torpedo the mesoblast commences to be divided into two layers much earlier than in Pristiurus; and even before stage C this division is more or less clearly marked.
In the head and tail the condition of the mesoblast is by no means the same as in the body.
In the tail the plates of mesoblast become considerably thickened and give rise to two projections, one on each side, which have already been alluded to as caudal or tail-swellings; vide Pl. 8, figs. D, F, and Pl. 10, fig. 3f and fig. 4, ts.
These masses of mesoblast are neither divided into protovertebræ, nor do they exhibit any trace of a commencing differentiation into somatopleure and splanchnopleure.
In the head, so far as I have yet been able to observe, the mesoblastic plates do not at this stage become divided into protovertebræ. The other changes exhibited in the cephalic region are of interest, mainly from the fact that here appears a cavity in the mesoblast directly continuous with the body-cavity (when that cavity becomes formed), but which appears at a very much earlier date than the body-cavity. This cavity can only be looked on in the light of a direct continuation of the body or peritoneal cavity into the head. Theoretical considerations with reference to it I propose reserving till I have described the changes which it undergoes in the subsequent periods.
Pl. 10, figs. 3a, 3b and 3c exhibit very well the condition of the mesoblast in the head at this period. In fig. 3c, a section [Pg 303] taken through the back part of the head, the mesoblast plates have nearly the same form as in the sections immediately behind. The ventral continuation of the mesoblast formed by the lateral plate has, however, become much thinner, and the dorsal or vertebral portion has acquired a more triangular form than in the sections through the trunk (figs. 3d and 3e).
In the section (fig. 3b) in front of this the ventral portion of the plate is no longer present, and only that part exists which corresponds with the vertebral division of the primitive plate of mesoblast.
In this a distinct cavity, forming part of the body-cavity, has appeared.
In a still anterior section (fig. 3a) no cavity is any longer present in the mesoblast; whilst in sections taken from the foremost part of the head no mesoblast is to be seen (vide Pl. 10, fig. 5, taken from the front part of the head of the embryo represented in Pl. 8, fig. F).
A continuation of the body-cavity into the head has already been described by Oellacher[182] for the Trout: but he believes that the cavity in this part is solely related to the formation of the pericardial space.
The condition of the mesoblast undergoes no important change till the end of the period treated of in this chapter. The masses of mesoblast which form the tail-swellings become more conspicuous (Pl. 10, fig. 4); and indeed their convexity is so great that the space between them has the appearance of a median groove, even after the closure of the neural canal in the caudal region.
In embryos of stage G, which may be considered to belong to the close of this period, eighteen protovertebræ are present both in Pristiurus and Torpedo embryos.
The Alimentary Canal.
The alimentary canal at the commencement of this period (stage B) forms a space between the embryo and the yolk, ending blindly in front, but opening posteriorly by a widish slit-like aperture, which corresponds to the anus of Rusconi (Pl. 7, fig. 7).
[Pg 304] The cavity anteriorly has a more or less definite form, having lateral walls, as well as a roof and floor (Pl. 7, figs. 10b and 10c). Posteriorly it is not nearly so definitely enclosed (Pl. 7, fig. 10a). The ventral wall of the cavity is formed by yolk. But even in stage B there are beginnings of a cellular ventral wall derived from an ingrowth of cells from the two sides.
By stage C considerable progress has been made in the formation of the alimentary canal. Posteriorly it is as flattened and indefinite as during stage B (Pl. 10, figs. 2b and 2c). But in the anterior part of the embryo the cavity becomes much deeper and narrower, and a floor of cells begins to be formed for it (Pl. 10, fig. 2); and, finally, in front, it forms a definite space completely closed in on all sides by cells (Pl. 10, fig. 2a). Two distinct processes are concerned in effecting these changes in the condition of the alimentary cavity. One of these is a process of folding off the embryo from the blastoderm. The other is a simple growth of cells independent of any folding. To the first of these processes the depth and narrowness of the alimentary cavity is due; the second is concerned in forming its ventral wall. The combination of the two processes produces the peculiar triangular section which characterises the anterior closed end of the alimentary cavity at this stage. The process of the folding off of the embryo from the blastoderm resembles exactly the similar process in the embryo bird. The fold by which the constricting off of the embryo is effected is a perfectly continuous one, but may be conveniently spoken of as composed of a head fold and two lateral folds.
Of far greater interest than the nature of these folds is the formation of the ventral wall of the alimentary canal. This, as has been said, is effected by a growth of cells from the two sides to the middle line (Pl. 10, fig. 2). The cells for this are however not derived from pre-existing hypoblast cells, but are formed spontaneously around nuclei of the yolk. This fact can be determined in a large number of sections, and is fairly well shewn in Pl. 10, fig. 2, na. The cells are formed in the yolk, as has been already mentioned, by a simple aggregation of protoplasm around pre-existing nuclei.
The cells being described are in most cases formed close to the pre-existing hypoblast cells, but often require to undergo a [Pg 305] considerable change of position before attaining their final situation in the wall of the alimentary canal.
I have already alluded to this feature in the formation of the ventral wall of the alimentary cavity. Its interest, as bearing on the homology of the yolk, is considerable, owing to the fact that the so-called yolk-cells of Amphibians play a similar part in supplying the ventral epithelium of the alimentary cavity, as do the cells derived from the yolk in Elasmobranchii.
The fact of this feature being common to the yolk-cells of Amphibians and the yolk of Elasmobranchii, supplies a strong argument in favour of the homology of the yolk-cells in the one case with the yolk in the other[183].
[Pg 306] The history of the alimentary canal during the remainder of this period may be told briefly.
The folding off and closing of the alimentary canal in the anterior part of the body proceeds rapidly, and by stage D not only is a considerable tract of alimentary canal formed, but a great part of the head is completely folded off from the yolk (Pl. 10, fig. 3a). By stage F a still greater part is folded off. The posterior part of the alimentary canal retains for a long period its primitive condition. It is not until stage F that it begins to be folded off behind. After the folding has once commenced it proceeds with great rapidity, and before stage G the hinder part of the alimentary canal becomes completely closed in.
The folding in of the gut is produced by two lateral folds, and the gut is not closed posteriorly.
It may be remembered that the neural canal also remained open behind. Thus both the neural and alimentary canals are open behind; and, since both of them extend to the posterior [Pg 307] end of the body, they meet there, their walls coalesce, and a direct communication from the neural to the alimentary canal is instituted. The process may be described in another way by saying that the medullary folds are continuous round the end of the tail with the lateral walls of the alimentary canal; so that, when the medullary folds unite to form a canal, this canal becomes continuous with the alimentary canal, which is closed in at the same time. In whatever way this arrangement is produced, the result of it is that it becomes possible to pass in a continuously closed passage along the neural canal round the end of the tail and into the alimentary canal. A longitudinal section shewing this feature is represented on Pl. 10, fig. 7.
This communication between the neural and alimentary canals, which is coupled, as will be seen in the sequel, with the atrophy of a posterior segment of the alimentary canal, is a feature of great interest which ought to throw considerable light upon the meaning of the neural canal. So far as I know, no suggestion as to the origin of it has yet been made. It is by no means confined to Elasmobranchii, but is present in all the vertebrates whose embryos are situated at the centre and not at the periphery of the blastoderm. It has been described by Goette[184] in Amphibians and by Kowalevsky, Owsjannikow and Wagner[185] in the Sturgeon (Acipenser). The same arrangement is also stated by Kowalevsky[186] to exist in Osseous Fishes and Amphioxus. The same investigator has shewn that the alimentary and neural canals communicate in larval Ascidians, and we may feel almost sure that they do so in the Marsipobranchii.
The Reptilia, Aves, and Mammalia have usually been distinguished from other vertebrates by the possession of a well-developed allantois and amnion. I think that we may further say that the lower vertebrates, Pisces and Amphibia, are to be distinguished from the three above-mentioned groups of higher [Pg 308] vertebrates, by the positive embryonic character that their neural and alimentary canals at first communicate posteriorly. The presence or absence of this arrangement depends on the different positions of the embryo in the blastoderm. In Reptiles, Birds and Mammals, the embryo occupies a central position in the blastoderm, and not, as in Pisces and Amphibia, a peripheral one at its edge. We can, in fact, only compare the blastoderm of the Bird and the Elasmobranch, by supposing that in the blastoderm of the Bird there has occurred an abbreviation of the processes, by which the embryo Elasmobranch is eventually placed in the centre of the blastoderm: as a result of this abbreviation the embryo Bird occupies from the first a central position in the blastoderm[187].
The peculiar relations of the blastoderm and embryo, and the resulting relations of the neural and alimentary canal, appear to me to be features of quite as great an importance for classification as the presence or absence of an amnion and allantois.
General Features of the Hypoblast.
There are but few points to be noticed with reference to the histology of the hypoblast cells. The cells of the dorsal wall of the alimentary cavity are columnar and form a single row. Those derived from the yolk to form the ventral wall are at first roundish, but subsequently assume a more columnar form.
One of the most interesting features in the Elasmobranch development is the formation of the notochord from the hypoblast. All the steps in the process by which this takes place can be followed with great ease and certainty.
Up to stage B the hypoblast is in contact with the epiblast immediately below the medullary groove, but exhibits no trace of a thickening or any other formation at that point.
Between stage B and C the notochord first arises.
In the hindermost sections of this stage the hypoblast retains a perfectly normal structure and uniform thickness throughout. In the next few sections (Pl. 10, fig. 1c, ch´) a slight thickening is to be observed in the hypoblast, immediately below the medullary canal. The layer, which elsewhere is composed of a single row of cells, here becomes two cells deep, but no sign of a division into two layers exhibited.
In the next few sections the thickening of the hypoblast becomes much more pronounced; we have, in fact, a ridge projecting from the hypoblast towards the epiblast (Pl. 10, fig. 1b, ch´).
This ridge is pressed firmly against the epiblast, and causes in it a slight indentation. The hypoblast in the region of the ridge is formed of two layers of cells, the ridge being entirely due to the uppermost of the two.
In sections in front of this a cylindrical rod, which can at once be recognised as the notochord and is continuous with the ridge just described, begins to be split off from the hypoblast. It is difficult to say at what point the separation of this rod from the hypoblast is completed, since all intermediate gradations between complete separation and complete attachment are to be seen.
Where the separation first appears, a fairly thick bridge of hypoblast is left connecting the two lateral halves of the layer, but anteriorly this bridge becomes excessively delicate and thin (Pl. 10, fig. 1a), and in some cases is barely visible except with high powers.
From the series of sections represented, it is clear that the [Pg 310] notochord commences to be separated from the hypoblast anteriorly, and that the separation gradually extends backwards.
The posterior extremity of the notochord remains for a long time attached to the hypoblast; and it is not till the end of the period treated of in this chapter that it becomes completely free.
A sheath is formed around the notochord, very soon after its formation, at a stage intermediate between stages C and D. This sheath is very delicate, though it stains with both osmic acid and hæmatoxylin. I conclude from its subsequent history, that it is to be regarded as a product of the cells of the notochord, but at the same time it should be stated that it precisely resembles membrane-like structures, which I have already described as being probably artificial.
Towards the end of this period the cells of the notochord become very much flattened vertically, and cause the well-known stratified appearance which characterises the notochord in longitudinal sections. In transverse sections the outlines of the cells of the notochord appear rounded.
Throughout this period the notochord cells are filled with yolk-spherules, and near its close small vacuoles make their appearance in them.
An account of the development of the notochord, substantially similar to that I have just given, appeared in my preliminary paper[188] on the development of the Elasmobranch fishes.
To the remarks which were there made, I have little to add. There are two possible views, which can be held with reference to the development of the notochord from the hypoblast.
We may suppose that this is the primitive mode of development of the notochord, or we may suppose that the separation of the notochord from the hypoblast is due to a secondary process.
If the latter view is accepted, it will be necessary to maintain that the mesoblast becomes separated from the hypoblast as three separate masses, two lateral, and one median, and that the latter becomes separated much later than the two former.
We have, I think, no right to assume the truth of this view without further proof. The general admission of assumptions of this kind is apt to lead to an injurious form of speculation, in [Pg 311] which every fact presenting a difficulty in the way of some general theory is explained away by an arbitrary assumption, while all the facts in favour of it are taken for granted. It is however clear that no theory can ever be fairly tested so long as logic of this kind is permitted. If, in the present instance, the view is adopted that the notochord has in reality a mesoblastic origin, it will be possible to apply the same view to every other organ derived from the hypoblast, and to say that it is really mesoblastic, but has become separated at rather a late period from the hypoblast.
If, however, we provisionally reject this explanation, and accept the other alternative, that the notochord is derived from the hypoblast, we must be prepared to adopt one of two views with reference to the development of the notochord in other vertebrates. We must either suppose that the current statements as to the development of the notochord in other vertebrates are inaccurate, or that the notochord has only become secondarily mesoblastic.
The second of these alternatives is open to the same objections as the view that the notochord has only apparently a hypoblastic source in Elasmobranchii, and, provisionally at least, the first of them ought to be accepted. The reasons for accepting this alternative fall under two heads. In the first place, the existing accounts and figures of the development of the notochord exhibit in almost all cases a deficiency of clearness and precision. The exact stage necessary to complete the series never appears. It cannot, therefore, at present be said that the existing observations on the development of the notochord afford a strong presumption against its hypoblastic origin.
In the second place, the remarkable investigations of Hensen[189], on the development of the notochord in Mammalia, render it very probable that, in this group, the notochord is developed from the hypoblast.
Hensen finds that in Mammalia, as in Elasmobranchii, the mesoblast forms two independent lateral masses, one on each side of the medullary canal.
After the commencing formation of the protovertebræ the hypoblast becomes considerably thickened beneath the medullary [Pg 312] groove; and, though he has not followed out all the steps of the process by which this thickening is converted into the notochord, yet his observations go very far towards proving that it does become the notochord.
Against the observations of Hensen, there ought, however, to be mentioned those of Lieberkühn[190]. He believes that the two lateral masses of mesoblast, described by Hensen (in an earlier paper than the one quoted), are in reality united by a delicate layer of cells, and that the notochord is formed from a thickening of these.
Lieberkühn gives no further statements or figures, and it is clear that, even if there is present the delicate layer of mesoblast, which he fancies he has detected, yet this cannot in any way invalidate such a section as that represented on Pl. X. fig. 40, of Hensen's paper.
In this figure of Hensen's, the hypoblast cells become distinctly more columnar, and the whole layer much thicker immediately below the medullary canal than elsewhere, and this independently of any possible layer of mesoblast.
It appears to me reasonable to conclude that Lieberkühn's statements do not seriously weaken the certainty of Hensen's results.
In addition to the observations of Hensen's on Mammalia, those of Kowalevsky and Kuppfer on Ascidians may fairly be pointed to as favouring the hypoblastic origin of the notochord.
It is not too much to say that at the present moment the balance of evidence is in favour of regarding the notochord as a hypoblastic organ.
This conclusion is, no doubt, rather startling, and difficult to understand. The only feature of the notochord in its favour is the fact of its being unsegmented[191].
Should it eventually turn out that the notochord is developed in most vertebrates from the mesoblast, and only exceptionally from the hypoblast, the further question will have to be settled [Pg 313] as to whether it is primitively a hypoblastic or a mesoblastic organ; but, from whatever layer it has its source, an excellent example will be afforded of an organ changing from the layer in which it was originally developed into another distinct layer.
EXPLANATION OF PLATE 10.
Complete List of Reference Letters.
al. Alimentary canal. ch. Chorda dorsalis or notochord. ch´. Ridge of hypoblast, which will become separated off as the notochord. ep. Epiblast. hy. Hypoblast. lp. Coalesced lateral and vertebral plate of mesoblast. mg. Medullary groove. n. Nucleus of yolk. na. Cells formed around the nuclei of the yolk to enter into the ventral wall of the alimentary canal. nc. Neural or medullary canal. pv. Protovertebra. so. Somatopleure. sp. Splanchnopleure. ts. Mesoblast of tail-swelling. yk. Yolk-spherules.
Figs. 1a, 1b, 1c. Three sections from the same embryo belonging to a stage intermediate between B and C, of which fig. 1a is the most anterior. × 96 diameters.
The sections illustrate (1) The different characters of the medullary groove in the different regions of the embryo. (2) The structure of the coalesced lateral and vertebral plates. (3) The mode of formation of the notochord as a thickening of the hypoblast (ch´), which eventually becomes separated from the hypoblast as an elliptical rod (1a, ch).
Fig. 2. Section through the anterior part of an embryo belonging to stage C. The section is mainly intended to illustrate the formation of the ventral wall of the alimentary canal from cells formed around the nuclei of the yolk. It also shews the shallowness of the medullary groove in the anterior part of the body.
Figs. 2a, 2b, 2c. Three sections from the same embryo as fig. 2. Fig. 2a is the most anterior of the three sections and is taken through a point shortly in front of fig. 2. The figures illustrate the general features of an embryo of stage C, more especially the complete closing of the alimentary canal in front and the triangular section which it there presents.
Fig. 3. Section through the posterior part of an embryo belonging to stage D. × 86 diameters.
It shews the general features of the layers during the stage, more especially the differentiation of somatic and splanchnic layers of the mesoblast.
Figs. 3a, 3b, 3c, 3d, 3e, 3f. Sections of the same embryo as fig. 3 (× 60 diameters). Fig. 3 belongs to part of the embryo intermediate between figs. 3e and 3f.
The sections shew the features of various parts of the embryo. Figs. 3a, 3b and 3c belong to the head, and special attention should be paid to the presence of a cavity in the mesoblast in 3b and to the ventral curvature of the medullary folds.
Fig. 3d belongs to the neck, fig. 3e to the back, and fig. 3f to the tail.
Fig. 4. Section through the region of the tail at the commencement of stage F. × 60 diameters.
The section shews the character of the tail-swellings and the commencing closure of the medullary groove.
[Pg 314] Fig. 5. Transverse section through the anterior part of the head of an embryo belonging to stage F (× 60 diameters). It shews (1) the ventral curvature of the medullary folds next the head. (2) The absence of mesoblast in the anterior part of the head. hy points to the extreme front end of the alimentary canal.
Fig. 6. Section through the head of an embryo at a stage intermediate between F and G. × 86 diameters.
It shews the manner in which the medullary folds of the head unite to form the medullary canal.
Fig. 7. Longitudinal and vertical section through the tail of an embryo belonging to stage G.
It shews the direct communication which exists between the neural and alimentary canals.
The section is not quite parallel to the long axis of the embryo, so that the protovertebræ are cut through in its anterior part, and the neural canal passes out of the section anteriorly.
Fig. 8. Network of nuclei from the yolk of an embryo belonging to stage H.
[180] Vide Preliminary Account, etc. Q. Jl. Micros. Science, Oct. 1874, Pl. 14, 8a. [This Edition, No. V. Pl. 3, 8a.] This and the other section from the same embryo (stage F) may be referred to. I have not thought it worth while repeating them here.
[181] I underestimated the distinctness of this formation in my earlier paper, loc. cit., although I recognised the fact that the mesoblast cells became arranged in two distinct layers.
[182] Zeitschrift f. wiss. Zoologie, 1873.
[183]
Nearly simultaneously with Chapter III. of the present monograph on the
Development of Elasmobranchii, which dealt in a fairly complete manner with
the genesis of cells outside the blastoderm, there appeared two important
papers dealing with the same subject for Teleostei. One of these, by
Professor Bambeke, Embryologie des Poissons Osseux,
Mém. Cour. Acad. Belgique, 1875, which appeared some
little time before my paper, and a second by Dr Klein, Quart. Jour. of Micr.
Sci. April, 1876. In both of these papers a development of
nuclei and of cells is described as occurring outside the blastoderm in a
manner which accords fairly well with my own observations.
The conclusions of both these investigators differ however from my own. They regard the finely granular matter, in which the nuclei appear, as pertaining to the blastoderm, and morphologically quite distinct from the yolk. From their observations we can clearly recognise that the material in which the nuclei appear is far more sharply separated off from the yolk in Osseous Fish than in Elasmobranchii, and this sharp separation forms the main argument for the view of these authors. Dr Klein admits, however, that this granular matter (which he calls parablast) graduates into the typical food-yolk, though he explains this by supposing that the parablast takes up part of the yolk for the purpose of growth.
It is clear that the argument from a sharp separation of yolk and parablast cannot have much importance, when it is admitted (1) that in Osseous Fish there is a gradation between the two substances, while (2) in Elasmobranchii the one merges slowly and insensibly into the other.
The only other argument used by these authors is
stated by Dr Klein in the following way. The fact that the parablast
has, at the outset, been forming one unit with what represents the
archiblast, and, while increasing has spread i.e. grown over
the yolk which underlies the segmentation-cavity, is, I think, the
most absolute proof that the yolk is as much different from the parablast
as it is from the archiblast.
This argument to me merely demonstrates
that certain of the nutritive elements of the yolk become in the course of
development converted into protoplasm, a phenomenon which must necessarily
be supposed to take place on my own as well as on Dr Klein's view of the
nature of the yolk. My own views on the subject have already been fully
stated. I regard the so-called yolk as composed of a larger or smaller
amount of food-material imbedded in protoplasm, and the meroblastic ovum
as a body constituted of the same essential parts as a holoblastic ovum,
though divided into regions which differ in the proportion of protoplasm
they contain. I do not propose to repeat the positive arguments used by me
in favour of this view, but content myself with alluding to the
protoplasmic network found by Schultz and myself extending through the
whole yolk, and to the similar network described by Bambeke as being
present in the eggs of Osseous Fish after deposition but before
impregnation. The existence of these networks is to me a conclusive proof
of the correctness of my views. I admit that in Teleostei the 'parablast'
contains more protoplasm than the homologous material in the Elasmobranch
ovum, while it is probable that after impregnation the true yolk of
Teleostei contains little or no protoplasm; but these facts do not appear
to me to militate against my views.
I agree with Prof. Bambeke in regarding the cells derived from the sub-germinal matter as homologous with the so-called yolk-cells of the Amphibian embryo.
I have recently, in some of the later stages of development, met with very peculiar nuclei of the yolk immediately beneath the blastoderm at some little distance from the embryo, Pl. 10, fig. 8. They were situated not in finely sub-germinal matter, but amongst large yolk-spherules. They were very large, and presented still more peculiar forms than those already described by me, being produced into numerous long filiform processes. The processes from the various nuclei were sometimes united together, forming a regular network of nuclei quite unlike anything that I have previously seen described.
The sub-germinal matter, in which the nuclei are usually formed, becomes during the later stages of development far richer in protoplasm than during the earlier. It continually arises at fresh points, and often attains to considerable dimensions, no doubt by feeding on yolk-spherules. Its development appears to be determined by the necessities of growth in the blastoderm or embryo.
[184] Entwicklungsgeschichte der Unke.
[185] Mélanges Biologiques de l'Académie Pétersbourg, Tome VII.
[186] Archiv. f. mikros. Anat. Vol. VII. p. 114. In the passage on this point Kowalevsky states that in Elasmobranchii the neural and alimentary canals communicate. This I believe to be the first notice published of this peculiar arrangement.
[187] Vide Note on p. 281, also p. 295, and Pl. 9, figs. 1 and 2, and Comparison, &c., Qy. Jl. of Micros. Sci. July, 1875, p. 219. [This Edition, No. VI. p. 125.] These passages give an account of the change of position of the Elasmobranch embryo, and the Note on p. 281 contains a speculation about the nature of the primitive streak with its contained primitive groove. I have suggested that the primitive streak is probably to be regarded as a rudiment at the position where the edges of the blastoderm coalesced to give to the embryos of Birds and Mammals the central position which they occupy.
If my hypothesis should turn out to be correct, various, now unintelligible, features about the primitive streak would be explained: such as its position behind the embryo, the fusion of the epiblast and mesoblast in it, the groove it contains, &c.
The possibility of the primitive streak representing the blastopore, as it in fact does according to my hypothesis, ought also to throw light on E. Van Beneden's recent researches on the development of the Mammalian ovum.
In order clearly to understand the view here expressed, the reader ought to refer to the passages above quoted.
[188] Loc. cit.
[189] Zeitschrift f. Anat. u. Entwicklungsgeschichte, Vol. I. p. 366.
[190] Sitz. der Gesell. zu Marburg, Jan. 1876.
[191] In my earlier paper I suggested that the endostyle of Ascidians afforded an instance of a supporting organ being derived from the hypoblast. This parallel does not hold since the endostyle has been shewn to possess a secretory function. I never intended (as has been imagined by Professor Todaro) to regard the endostyle as the homologue of the notochord.
By the stage when the external gills have become conspicuous objects, the rudiments of the greater number of the important organs of the body are definitely established.
Owing to this fact the first appearance of the external gills forms a very convenient break in the Elasmobranch development; and in the present chapter the history is carried on to the period of this occurrence.
While the last chapter dealt for the most part with the formation of the main organic systems from the three embryonic layers, the present one has for its subject the gradual differentiation of these systems into individual organs. In treating of the development of the separate organs a divergence from the plan of the last chapter becomes necessary, and the following arrangement has been substituted for it. First of all an account is given of the development of the external epiblast, which is followed by a description of the organs derived from the mesoblast and of the notochord.
External Epiblast.
During stages G to I the epiblast[192] is formed of a single layer of flattened cells; and in this, as in the earlier stages, it deserves to be especially noticed that the epiblast is never more than one cell deep, and is therefore incapable of presenting any differentiation into nervous and epidermic layers. (Pl. 11, figs. 1-5.)
[Pg 316] The cells which compose it are flattened and polygonal in outline, but more or less spindle-shaped in section. They present a strong contrast to the remaining embryonic cells of the body in possessing a considerable quantity of clear protoplasm, which in most other cells is almost entirely absent. Their granular nucleus is rounded or oval, and typically contains a single nucleolus. Frequently, however, two nucleoli are present, and when this is the case an area free from granules is to be seen around each nucleolus, and a dark line, which could probably be resolved into granules by the use of a sufficiently high magnifying power, divides the nucleus into two halves. These appearances probably indicate that nuclei, in which two nucleoli are present, are about to divide.
The epiblast cells vary in diameter from .022 to .026 Mm. and their nuclei from .014 to .018 Mm. They present a fairly uniform character over the greater part of the body. In Torpedo they present nearly the same characters as in Pristiurus and Scyllium, but are somewhat more columnar. (Pl. 11, fig. 7.)
Along the summit of the back from the end of the tail to the level of the anus, or slightly beyond this, epiblast cells form a fold—the rudiment of the embryonically undivided dorsal fin—and the cells forming this, unlike the general epiblast cells, are markedly columnar; they nevertheless, here as elsewhere, form but a single layer. (Pl. 11, fig. 3 and 5, df.) Although at this stage the dorsal fin is not continued as a fold anteriorly to the level of the anus, yet a columnar thickening or ridge of epiblast, extending along the median dorsal line nearly to the level of the heart, forms a true morphological prolongation of the fin.
On the ventral side of the tail is present a rudiment of the ventral unpaired fin, which stops short of the level of the anus, but, though less prominent, is otherwise quite similar to the dorsal fin and continuous with it round the end of the tail. At this stage the mesoblast has no share in forming either fin.
In many sections of the tail there may be seen on each side two folds of skin, which are very regular, and strongly simulate the rudimentary fins just described. The cells composing them are, however, not columnar, and the folds themselves are merely artificial products due to shrinking.
[Pg 317] At a stage slightly younger than K an important change takes place in the epiblast.
From being composed of a single layer of cells it becomes two cells deep. The two layers appear first of all anteriorly, and subsequently in the remaining parts of the body. At first, both layers are formed of flattened cells (Pl. 11, figs. 8, 9); but at a stage slightly subsequent to that dealt with in the present chapter, the cells of the inner of the two layers become columnar, and thus are established the two strata always present in the epidermis of adult vertebrates, viz. an outer layer of flattened cells and an inner one of columnar cells[193].
The history of the epiblast in Elasmobranchii is interesting, from the light which it throws upon the meaning of the nervous and epidermic layers into which the epiblast of Amphibians and some other Vertebrates is divided. The Amphibians and Elasmobranchii present the strongest contrast in the development of their epiblast, and it is worth while shortly to review and compare the history of the layer in the two groups.
In Amphibians the epiblast is from the first divided into an outer stratum formed of a single row of flattened cells, and an inner stratum composed of several rows of more rounded cells. These two strata were called by Stricker the nervous and epidermic layers, and these names have been very generally adopted.
Both strata have a share in forming the general epiblast, and though eventually they partially fuse together, there can be but little doubt that the horny layer of the adult epiblast, where such can be distinguished[194], is derived from the epidermic layer of the embryo, and the mucous layer of the epiblast from the embryonic nervous layer. Both layers of the epiblast assist in the formation of the cerebro-spinal nervous system, and there also at first fuse together[195], though the epidermic layer probably separates itself again, as the central epithelium of the spinal canal. The lens and auditory sac are derived exclusively from the nervous layer [Pg 318] of the epidermis, while this layer also has the greater share in forming the olfactory sac.
In Elasmobranchii the epiblast is at first uniformly composed of a single row of cells. The part of the layer which will form the central nervous system next becomes two or three cells deep, but presents no distinction into two layers; the remaining portions of the layer remain, as before, one cell deep. Although the epiblast at first presents this simple structure, it eventually, as we have seen, becomes divided throughout into two layers, homologous with the two layers which arise so early in Amphibians. The outer one of the two forms the horny layer of the epidermis and the central epithelium of the neural canal. The inner one, the mucous layer of the epidermis and the nervous part of the brain and spinal cord. Both layers apparently enter into the formation of the organs of sense.
While there is no great difficulty in determining the equivalent parts of the epidermis in Elasmobranchii and Amphibians, it still remains an open question in which of these groups the epiblast retains its primitive condition.
Though it is not easy to bring conclusive proofs on the one side or the other, the balance of argument appears to me to be decidedly in favour of regarding the condition of the epiblast in Elasmobranchii, and most other Vertebrates, as the primitive one, and its condition in Amphibians as a secondary one, due to the throwing back of the differentiation of their epiblast into two layers to a very early period in their development.
In favour of this view are the following points: (1) That a primitive division of the epiblast into two layers is unknown in the animal kingdom, except amongst Amphibians and (?) Osseous Fish. (2) That it appears more likely for a particular feature of development to be thrown back to an earlier period, than for such an important feature as a distinction between two primary layers to be absolutely lost during an early period of development, and then to reappear again in later stages.
The fact of the epiblast of the neural canal being divided, like the remainder of the layer, into nervous and epidermic parts, cannot, I think, be used as an argument in favour of the opposite view to that here maintained.
It seems probable that the central canal of the nervous [Pg 319] system arose as an involution from the exterior, and therefore that the epidermis lining it is in reality merely a part of the external epidermis, and as such is naturally separated from the true nervous structures adjacent to it[196].
Leaving the general features of the external skin, I pass to the special organs derived from it during the stage just anterior to K.
The unpaired Fins. The unpaired fins have grown considerably, and the epiblast composing them becomes, like the remainder of the layer, divided into two strata, both however composed of more or less columnar cells. The ventral fin has now become more prominent than the dorsal fin; but the latter extends forward as a fold quite to the anterior part of the body.
The paired Fins. Along each side of the body there appears during this stage a thickened line of epiblast, which from the first exhibits two special developments: one of these just in front of the anus, and a second and better marked one opposite the front end of the segmental duct. These two special thickenings are the rudiments of the paired fins, which thus arise as special developments of a continuous ridge on each side, precisely like the ridges of epiblast which form the rudiments of the unpaired fins.
Similar thickenings to those in Elasmobranchii are found at the ends of the limbs in the embryos of both Birds and Mammals, in the form of caps of columnar epiblast[197].
The ridge, of which the limbs are special developments, is situated on a level slightly ventral to that of the dorsal aorta, and extends from just behind the head to the level of the anus. It is not noticeable in surface views, but appears in sections as a portion of the epiblast where the cells are more columnar than elsewhere; precisely resembling in this respect the forward continuation of the dorsal fin. At the present stage the posterior thickenings of this ridge which form the abdominal fins are so slight as to be barely visible, and their real nature can only be detected by a careful comparison between sections of this and the succeeding stages. The rudiments of the anterior pair of [Pg 320] limbs are more visible than those of the posterior, though the passage between them and the remainder of the ridges is most gradual. Thus at first the rudiments of both the limbs are nothing more than slight thickenings of the epiblast, where its cells are more columnar than elsewhere. During stage K the rudiments of both pairs of limbs, but especially of the anterior pair, grow considerably, while at the same time the thickened ridge of epiblast which connects them together rapidly disappears. The thoracic limbs develop into an elongated projecting fold of epiblast, in every way like the folds forming the unpaired fins; while at the same time the cells of the subjacent mesoblast become closely packed, and form a slight projection, at the summit of which the fold of the epiblast is situated (Pl. 11, fig. 9). The maximum projection of the thoracic fin is slightly in advance of the front end of the segmental duct. The abdominal fins do not, during stage K, develop quite so fast as the thoracic, and at its close are merely elongated areas where the epiblast is much thickened, and below which the mesoblast is slightly condensed. In the succeeding stages they develop into projecting folds of skin, precisely as do the thoracic fins.
The features of the development of the limbs just described, are especially well shewn in Torpedo; in the embryos of which the passage from the general linear thickening of epiblast into the but slightly better marked thickening of the thoracic fin is very gradual, and the fact of the limb being nothing else than a special development of the linear lateral thickening is proved in a most conclusive manner.
If the account just given of the development of the limbs is an accurate record of what really takes place, it is not possible to deny that some light is thrown by it upon the first origin of the vertebrate limbs. The facts can only bear one interpretation, viz.: that the limbs are the remnants of continuous lateral fins.
The unpaired dorsal fin develops as a continuous thickening, which then grows up into a projecting fold of columnar cells. The greater part of this eventually atrophies, but three separate lobes are left which form the two dorsal fins and the upper lobe of the caudal fin.
The development of the limbs is almost identically similar [Pg 321] to that of the dorsal fins. There appears a lateral linear thickening of epiblast, which however does not, like the similar thickening of the fins, grow into a distinct fold. Its development becomes confined to two special points, at each of which is formed a continuous elongated fold of columnar cells precisely like the fold of skin forming the dorsal fins. These two folds form the paired fins. If it be taken into consideration that the continuous lateral fin, of which the rudiment appears in Elasmobranchii, does not exist in any adult Vertebrate, and also that a continuous dorsal fin exists in many Fishes, the small differences in development between the paired fins and the dorsal fins will be seen to be exactly those which might have been anticipated beforehand. Whereas the continuous dorsal fin, which often persists in adult fishes, attains a considerable development before vanishing, the originally continuous lateral one has only a very ephemeral existence.
While the facts of development strongly favour a view which would regard the limbs as remnants of a primitively continuous lateral fin, there is nothing in the structure of the limbs of adult Fishes which is opposed to this view. Externally they closely resemble the unpaired fins, and both their position and nervous supply appear clearly to indicate that they do not belong to one special segment of the body. They appear rather to be connected with a varying number of segments; a fact which would receive a simple explanation on the hypothesis here adopted[198].
My researches throw no light on the nature of the skeletal parts of the limb, but the suggestion which has been made by Günther[199] with reference to the limb of Ceratodus (the most primitive known), that it is a modification of a series of parallel rays, would very well suit the view here proposed.
Dr Dohrn[200] in speaking of the limbs, points out the difficulties [Pg 322] in the way of supposing that they can have originated de novo, and not by the modification of some pre-existing organ, and suggests that the limbs are modified gill-arches; a view similar to which has been hinted at by Professor Gegenbaur[201].
Dr Dohrn has not as yet given the grounds for his determination, so that any judgment on his views is premature.
None of my observations on Elasmobranchii lends any support to these views; but perhaps, while regarding the limbs as the remains of a continuous fin, it might be permissible to suppose that the pelvic and thoracic girdles are altered remnants of the skeletal parts of some of the gill-arches which have vanished in existing Vertebrates.
The absence of limbs in the Marsipobranchii and Amphioxus, for reasons already insisted upon by Dr Dohrn[202], cannot be used as an argument against limbs having existed in still more primitive Vertebrates.
Though it does not seem probable that a dorsal and ventral fin can have existed contemporaneously with lateral fins (at least not as continuous fins), yet, judging from such forms as the Rays, there is no reason why small balancing dorsal and caudal fins should not have co-existed with fully developed lateral fins.
Mesoblast. G-K.
The mesoblast in stage F forms two independent lateral plates, each with a splanchnic and somatic layer, and divided, as before explained, into a vertebral portion and a parietal portion. At their peripheral edge these plates are continuous with the general mesoblastic tissue of the non-embryonic part of the blastoderm; except in the free parts of the embryo, where they are necessarily separated from the mesoblast of the yolk-sac, and form completely independent lateral masses of cells.
During the stages G and H, the two layers of which the mesoblast is composed cease to be in contact, and leave between them a space which constitutes the commencement of the body-cavity (Pl. 10, fig. 1). From the very first this cavity is more or less clearly divided into two distinct parts; one of them [Pg 323] in the vertebral portion of the plates of mesoblast, the other in the parietal. The cavity in the parietal part of the plates alone becomes the true body-cavity. It extends uninterruptedly through the anterior parts of the embryo, but does not appear in the caudal region, being there indicated only by the presence of two layers in the mesoblast plates. Though fairly wide below, it narrows dorsally before becoming continuous with the cavity in the vertebral plates. The line of junction of the vertebral and parietal plates is a little ventral to the dorsal summit of the alimentary canal (Pl. 10, fig. 5). Owing to the fact that the vertebral plates are split up into a series of segments (protovertebræ), the section of the body-cavity they enclose is necessarily also divided into a series of segments, one for each protovertebra.
Thus the whole body-cavity consists of a continuous parietal space which communicates by a series of apertures with a number of separate cavities enclosed in the protovertebræ. The cavity in each of the protovertebræ is formed of a narrowed dorsal and a dilated ventral segment, the latter on the level of the dorsal aorta (Pl. 11, fig. 5). Cavities are present in all the vertebral plates with the exception of a few far back in the tail; and exist in part of the caudal region posterior to that in which a cavity in the parietal plate is present.
Protovertebræ. Each protovertebra[203] or vertebral segment of the mesoblast plate forms a flattened rectangular body, ventrally continuous with the parietal plate of mesoblast. During stage G the dorsal edge of the protovertebræ is throughout on about a level with the ventral third of the spinal cord. Each vertebral plate is composed of two layers, a somatic and a splanchnic, and encloses the already-mentioned section of the body-cavity. The cells of both layers of the plate are columnar, and each consists of a very large nucleus, invested by a delicate layer of protoplasm.
Before the end of stage H the inner or splanchnic wall of the protovertebra loses its simple constitution, owing to the middle part of it, opposite the dorsal two-thirds of the notochord, undergoing [Pg 324] peculiar changes. These changes are indicated in transverse sections (Pl. 11, figs. 5 and 6, mp´), by the cells in the part we are speaking of acquiring a peculiar angular appearance, and becoming one or two deep; and the meaning of the changes is at once shewn by longitudinal horizontal sections. These prove (Pl. 12, fig. 10) that the cells in this situation have become elongated in a longitudinal direction, and, in fact, form typical spindle-shaped embryonic muscle-cells, each with a large nucleus. Every muscle-cell extends for the whole length of a protovertebra, and in the present stage, or at any rate in stage I, acquires a peculiar granulation, which clearly foreshadows transverse striation (Pl. 12, figs. 11-13).
Thus by stage H a small portion of the splanchnopleure which forms the inner layer of each protovertebra, becomes differentiated into a distinct band of longitudinal striated muscles; these almost at once become functional, and produce the peculiar serpentine movements of the embryo, spoken of in a previous chapter, p. 291.
It may be well to say at once that these muscles form but a very small part of the muscles which eventually appear; which latter are developed at a very much later period from the remaining cells of the protovertebræ. The band developed at this stage appears to be a special formation, which has arisen through the action of natural selection, to enable the embryo to meet its respiratory requirements, by continually moving about, and so subjecting its body to fresh oxydizing influences; and as such affords an interesting example of an important structure acquired during and for embryonic life.
Though the cavities in the protovertebræ are at first perfectly continuous with the general body-cavity, of which indeed they merely form a specialized part, yet by the close of stage H they begin to be constricted off from the general body-cavity, and this process is continued rapidly, and completed shortly after stage I, and considerably before the commencement of stage K (Pl. 11, figs. 6 and 8). While this is taking place, part of the splanchnic layer of each protovertebra, immediately below the muscle-band just described, begins to proliferate, and produce a number of cells, which at once grow in between the muscles and the notochord. These cells are very easily [Pg 325] seen both in transverse and longitudinal sections, and form the commencing vertebral bodies (Pl. 11, fig. 6, and Pl. 12, figs. 10 and 11, Vr).
At first the vertebral bodies have the same segmentation as the protovertebræ from which they sprang; that is to say, they form masses of embryonic cells separated from each other by narrow slits, continuous with the slits separating the protovertebræ. They have therefore at their first appearance a segmentation completely different from that which they eventually acquire (Pl. 12, fig. 11).
After the separation of the vertebral bodies from the protovertebræ, the remaining parts of the protovertebræ may be called muscle-plates; since they become directly converted into the whole voluntary muscular system of the trunk. At the time when the cavity of the muscle-plates has become completely separate from the body-cavity, the muscle-plates themselves are oblong structures, with two walls enclosing the cavity just mentioned, in which the original ventral dilatation is still visible. The outer or somatic wall of the plates retains its previous simple constitution. The splanchnic wall has however a somewhat complicated structure. It is composed dorsally and ventrally of a columnar epithelium, but in its middle portion of the muscle-cells previously spoken of. Between these and the central cavity of the plates the epithelium forming the remainder of the layer commences to insert itself; so that between the first-formed muscle and the cavity of the muscle-plate there appears a thin layer of cells, not however continuous throughout.
At the end of the period K the muscle-plates have extended dorsally two-thirds of the way up the sides of the spinal cord, and ventrally to the level of the segmental duct. Their edges are not straight, but are bent into an angular form, with the apex pointing forwards. Vide Pl. 12, fig. 17, mp.
Before the end of the period a number of connective-tissue cells make their appearance, and extend upwards from the dorsal summit of the muscle-plates around the top of the spinal cord. These cells are at first rounded, but become typical branched connective-tissue cells before the close of the period (Pl. 11, figs. 7 and 8).
Between stages I and K the bodies of the vertebræ rapidly [Pg 326] increase in size and send prolongations downwards and inwards to meet below the notochord.
These soon become indistinguishably fused with other cells which appear in the area between the alimentary cavity and the notochord, but probably serve alone to form the vertebral bodies, while the cells adjoining them form the basis for the connective tissue of the kidneys, &c.
The vertebral bodies also send prolongations dorsalwards between the sides of the spinal cord and the muscle-plates. These grow round till they meet above the spinal and enclose the dorsal nerve-roots. They soon however become fused with the dorsal prolongations from the muscle-plates, at least so far as my methods of investigation enable me to determine; but it appears to me probable that they in reality remain distinct, and become converted into the neural arches, while the connective-tissue cells from the muscle-plates form the adjoining subcutaneous and inter-muscular connective tissue.
All the cells of the vertebral rudiments become stellate and form typical embryonic connective-tissue. The rudiments however still retain their primitive segmentation, corresponding with that of the muscle-plates, and do not during this period acquire their secondary segmentation. Their segmentation is however less clear than it was at an earlier period, and in the dorsal part of the vertebral rudiments is mainly indicated by the dorsal nerve-roots, which always pass out in the interval between two vertebral rudiments. Vide Pl. 12, fig. 12, pr.
Intermediate Cell-mass. At about the period when the muscle-plates become completely free, a fusion takes place between the somatopleure and splanchnopleure immediately above the dorsal extremity of the true body-cavity (Pl. 11, fig. 6). The cells in the immediate neighbourhood of this fusion form a special mass, which we may call the intermediate cell-mass—a name originally used by Waldeyer for the homologous cells in the Chick. Out of it are developed the urinogenital organs and the adjoining tissues. At first it forms little more than a columnar epithelium, but by the close of the period is divided into (1) An epithelium on the free surface; from this are derived the glandular parts of the kidneys and functional parts of the genital glands; and (2) a subjacent stroma which forms the [Pg 327] basis for the connective-tissue and vascular parts of these organs.
To the history of these parts a special section is devoted; and I now pass to the description of the mesoblast which lines the body-cavity and forms the connective tissue of the body-wall, and the muscular and connective tissue of the wall of the alimentary canal.
Body-cavity and Parietal Plates. By the close of stage H, as has been already mentioned, a cavity is formed between the somatopleure and splanchnopleure in the anterior part of the trunk, which rapidly widens during the succeeding stages. Anteriorly, it invests the heart, which arises during stage G, as a simple space between the ventral wall of the throat and the splanchnopleure (Pl. 11, fig. 4). Posteriorly it ends blindly.
This cavity forms in the region of the heart the rudiment of the pericardial cavity. The remainder of the cavity forms the true body-cavity.
Immediately behind the heart the alimentary canal is still open to the yolk-sac, and here naturally the two lateral halves of the body-cavity are separated from each other. In the tail of the embryo no body-cavity has appeared by stage I, although the parietal plates of mesoblast are distinctly divided into somatic and splanchnic layers. In the caudal region the lateral plates of mesoblast of the two sides do not unite ventrally, but are, on the contrary, quite disconnected. Their ventral edge is moreover much swollen (Pl. 11, fig. 1). At the caudal swelling the mesoblast plates cease to be distinctly divided into somatopleure and splanchnopleure, and more or less fuse with the hypoblast of the caudal vesicle (Pl. 11, fig. 2).
Between stages I and K the body-cavity extends backwards behind the point where the anus is about to appear, though it never reaches quite to the extreme end of the tail. The backward extension of the body-cavity, as is primitively the case everywhere, is formed of two independent lateral halves (Pl. 11, fig. 9a). Anteriorly, opposite the hind end of the small intestine, these two lateral halves unite ventrally to form a single cavity in which hangs the small intestine (Pl. 11, fig. 8) suspended by a very short mesentery.
[Pg 328] The most important change which takes place in the body-cavity during this period is the formation of a septum which separates off a pericardial cavity from the true body-cavity.
Immediately in front of the liver the splanchnic and somatic walls of the body come into very close contact, and I believe unite over the greater part of their extent. The septum so formed divides the original body-cavity into an anterior section or pericardial cavity, and a posterior section or true body-cavity. There is left, however, on each side dorsally a rather narrow passage which serves to unite the pericardial cavity in front with the true body-cavity behind.
In Pl. 11, fig. 8a, there is seen on one side a section through this passage, while on the other side the passage is seen to be connected with the pericardial cavity.
It is not possible from transverse sections to determine for certain whether the septum spoken of is complete. An examination of longitudinal horizontal sections from an embryo belonging to the close of the stage K has however satisfied me that this septum, by that stage at any rate, is fully formed.
The two lateral passages spoken of above probably unite in the adult to form the passage connecting the pericardial with the peritoneal cavity, which, though provided with but a single orifice into the pericardial cavity, divides into two limbs before opening into the peritoneal cavity.
The body-cavity undergoes no further changes of importance till the close of the period.
Somatopleure and Splanchnopleure. Both the somatic and splanchnic walls of the body-cavity during stage I exhibit a simple uniform character throughout their whole extent. They are formed of columnar cells where they line the dorsal part of the body-cavity, but ventrally of more rounded and irregular cells (Pl. 11, fig. 5).
In them may occasionally be seen aggregations of very peculiar and large cells with numerous highly refracting spherules; the cells forming these are not unlike the primitive ova to be described subsequently, but are probably large cells derived from the yolk.
It is during the stage intermediate between I and K that the first changes become visible which indicate a distinction between [Pg 329] an epithelium (endothelium) lining the body-cavity and the connective tissue adjoining this.
There are at first but very few connective-tissue cells between the epithelium of the somatic layer of the mesoblast and the epiblast, but a connection between them is established by peculiar protoplasmic processes which pass from the one to the other (Pl. 11, fig. 8). Towards the end of stage K, however, there appears between the two a network of mesoblastic cells connecting them together. In the rudimentary outgrowth to form the limbs the mesoblast cells of the somatic layer are crowded in an especially dense manner.
From the first the connective-tissue cells around the hypoblastic epithelium of the alimentary tract are fairly numerous (Pl. 11, fig. 8), and by the close of this period become concentrically arranged round the intestinal epithelium, though not divided into distinct layers. A special aggregation of them is present in the hollow of the rudimentary spiral valve.
Behind the anal region the two layers of the mesoblast (somatic and splanchnic) completely fuse during stage K, and form a mass of stellate cells in which no distinction into two layers can be detected (Pl. 11, figs. 9c, 9d).
The alimentary canal, which at first lies close below the aorta, becomes during this period gradually carried further and further from this, remaining however attached to the roof of the body-cavity by a thin layer of the mesoblast of the splanchnopleure formed of an epithelium on each side, and a few interposed connective-tissue cells. This is the mesentery, which by the close of stage K is of considerable length in the region of the stomach, though shorter elsewhere.
* * * * *
The above account of the protovertebræ and body-cavity applies solely to the genera Pristiurus and Scyllium. The changes of these parts in Torpedo only differ from those of Pristiurus in unimportant though fairly noticeable details. Without entering into any full description of these it may be pointed out that both the true body-cavity and its continuations into the protovertebræ appear later in Torpedo than in Pristiurus and Scyllium. In some cases even the muscle-plates become definitely separated and independent before the true body-cavity has appeared. As [Pg 330] a result of this the primitive continuity of the body-cavity and cavity of the muscle-plates becomes to a certain extent masked, though its presence may easily be detected by the obvious continuity which at first exists between the somatic and splanchnic layers of mesoblast and the two layers of the muscle-plate. In the muscle-plate itself the chief point to be noticed is the fact that the earlier formed bands of muscles (mp´) arise very much later, and are less conspicuous, in Torpedo than in the genera first described. They are however present and functional.
The anatomical relations of the body-cavity itself are precisely the same in Torpedo as in Pristiurus and Scyllium, and the pericardial cavity becomes separated from the peritoneal in the same way in all the genera; the two lateral canals connecting the two cavities being also present in all the three genera. The two independent parietal plates of mesoblast of the posterior parts of the body have ventrally a swollen edge, as in Pristiurus, and in this a cavity appears which forms a posterior continuation of the true body-cavity.
Resumé. The primitive independent mesoblast plates of the two sides of the body become divided into two layers, a somatic and a splanchnic (Hautfaserblatt and Darmfaserblatt). At the same time in the dorsal part of the mesoblast plate a series of transverse splits appear which mark out the limits of the protovertebræ and serve to distinguish a dorsal or vertebral part of the plate from a ventral or parietal part.
Between the somatic and splanchnic layers of the mesoblast plate a cavity arises which is continued quite to the summit of the vertebral part of the plate. This is the primitive body-cavity; and at first the cavity is divided into two lateral and independent halves.
The next change which takes place is the complete separation of the vertebral portion of the plate from the parietal; thereby the upper segmented part of the body-cavity becomes isolated and separated from the lower and unsegmented part. In connection with this change in the constitution of the body-cavity there are formed a series of rectangular plates, each composed of two layers, a somatic and a splanchnic, between which is the cavity originally continuous with the body-cavity. The splanchnic layer of the plates buds off cells to form the rudiments [Pg 331] of the vertebral bodies which are originally segmented in the same planes as the protovertebræ. The plates themselves remain as the muscle-plates and develop a special layer of muscle (mp´) in their splanchnic layer.
In the meantime the parietal plates of the two sides unite ventrally throughout the intestinal and cardiac regions of the body, and the two primitively isolated cavities contained in them coalesce. Posteriorly however the plates do not unite ventrally, and their contained cavities remain distinct.
At first the pericardial cavity is quite continuous with the body-cavity; but by the close of the period included in the present chapter it becomes separated from the body-cavity by a septum in front of the liver, which is however pierced dorsally by two narrow channels.
The parts derived from the two layers of the mesoblast (not including special organs or the vascular system) are as follow:—
From the somatic layer are formed
(1) A considerable part of the voluntary muscular system of the body.
(2) The dermis.
(3) A large part of the intermuscular connective tissue.
(4) Part of the peritoneal epithelium.
From the splanchnic layer are formed
(1) A great part of the voluntary muscular system.
(2) Part of the intermuscular connective tissue (?).
(3) The axial skeleton.
(4) The muscular and connective-tissue wall of the alimentary tract.
(5) A great part of the peritoneal epithelium.
General Considerations. In the history which has just been given of the development of the mesoblast, there are several points which appear to me to throw light upon the primitive origin of that layer. Before entering into these it is however necessary to institute a comparison between the history of the mesoblast in Elasmobranchii and in other Vertebrates, in order to distinguish as far as possible the primitive and the secondary characters present in the various groups.
[Pg 332] Though the Mammals are to be looked on as the most differentiated group amongst the Vertebrates, yet in their embryonic history they retain many very primitive features, and, as has been recently shewn by Hensen[204], present numerous remarkable approximations to the Elasmobranchii. We find accordingly[205] that the primitive lateral plates of mesoblast undergo nearly the same changes in these two groups. In Mammals there is at first a continuous cavity extending through both the parietal and vertebral portions of each plate, and dividing the plates into a somatic and a splanchnic layer: this cavity is the primitive body-cavity. The vertebral portion of each plate with its contained cavity then becomes divided off from the parietal. The later development of these parts is not accurately known, but it seems that the outer portion of each vertebral plate, composed of two layers (somatic and splanchnic) enclosing between them a remnant of the primitive body-cavity, becomes separated off as a muscle-plate. The remainder forms a vertebral rudiment, &c. Thus the extension of the body-cavity into the vertebral portion of the mesoblast, and the constriction of the vertebral portion of the cavity from the remainder, are as distinctive features of Mammals as they are of the Elasmobranchii.
In Birds[206] the horizontal splitting of the mesoblast into somatic and splanchnic layers appears, as in Mammals, to extend at first to the summit of the protovertebræ, but these bodies become so early separated from the parietal plates that this fact has usually been overlooked or denied; but even on the second day of incubation the outer layer of the protovertebræ is continuous with the somatic layer of the lateral plates, and the inner layer and kernel of the protovertebræ with the splanchnic layer of the lateral plates[207]. After the isolation of the protovertebræ the primitive position of the split which separated their somatic and splanchnic layers becomes obscured, but when [Pg 333] on the third day the muscle-plates are formed they are found to be constituted of two layers, an inner and an outer, which enclose between them a central cavity. This remarkable fact, which has not received much attention, though noticeable in most figures, receives a simple explanation as a surviving rudiment on Darwinian principles. The central cavity of the muscle-plate is, in fact, a remnant of the vertebral extension of the body-cavity, and is the same cavity as that found in the muscle-plates of Elasmobranchii. The two layers of the muscle-plate also correspond with the two layers present in Elasmobranchii, the one belonging to the somatic, the other to the splanchnic layer of mesoblast. The remainder of the protovertebræ internal to the muscle-plates is very large in Birds, and is the equivalent of that portion of the protovertebræ which in Elasmobranchii is split off to form the vertebral bodies[208] (Pl. 11, figs. 6, 7, 8, Vr). Thus, though the history of the development of the mesoblast is not precisely the same for Birds as for Elasmobranchii, yet the differences between the two groups are of such a character as to prove in a striking manner that the Avian development is a derivation from a more primary form, like that of the Elasmobranchii.
According to the statements of Bambeke and Götte, the Amphibians present rather remarkable peculiarities in the development of their muscular system. Each side-plate of mesoblast is divided into a somatic and a splanchnic layer, continuous throughout the vertebral and parietal portions of the plate. The vertebral portions (protovertebræ) of the plates soon become separated from the parietal, and form an independent mass of cells constituted of two layers, which were originally continuous with the somatic and splanchnic layers of the parietal plates. The outer or somatic layer of the vertebral plates is formed of a single row of cells, but the inner or splanchnic layer is made up of a central kernel of cells and an inner single layer. This central kernel is the first portion of the vertebral body to undergo [Pg 334] any change, and it becomes converted into the main dorso-lateral muscles of the body, which apparently correspond with the muscles derived from the whole muscle-plate of the Elasmobranchii. From the inner layer of the splanchnic division there are next formed the main internal ventral muscles, rectus abdominis, &c., as well as the chief connective-tissue elements of the parts surrounding the spinal cord. The outer layer of the vertebral plates forms the dermis and subcutaneous connective tissue, as well as some of the superficial muscles of the trunk and the muscles of the limbs.
Dr Götte appears to think that the vertebral plates in Amphibians present a perfectly normal development very similar to that of other Vertebrates. The divergences between Amphibians and other Vertebrates appear, however, to myself, to be very great, and although the very careful account given by Dr Götte is probably to be relied on, yet some further explanation than he has offered of the development of these parts amongst the Amphibians would seem to be required.
A primary stage in which the two layers of the vertebral plates are continuous with the somatic and splanchnic layers of the body-wall is equally characteristic of Amphibians, Elasmobranchii and Mammals. In the subsequent development, however, a great difference between the types becomes apparent, for whereas in Elasmobranchii both layers of the vertebral plates combine to form the muscle-plates, out of which the great dorso-lateral muscles are formed, in Amphibians what appear to be the equivalent muscles are derived from a few of the cells (the kernel) of the inner layer of the vertebral plates only. The cells which form the lateral muscles in Amphibians might be thought to correspond in position with the cells which become, in Elasmobranchii, converted into the special early formed band of muscles (m.p´.), rather than, as their development seems to indicate, with the whole Elasmobranch muscle-plates[209].
[Pg 335] Osseous Fishes are stated to agree with Amphibians in the development of their protovertebræ and muscular system[210], but further observations on this point are required.
Though the development of the general muscular system and muscle-plates does not, according to existing statements, take place on quite the same type throughout the Vertebrate subkingdom, yet the comparison which has been instituted between Elasmobranchii and other Vertebrates appears to prove that there are one or two common features in their development, which may be regarded as primitive, and as having been inherited from the ancestors of Vertebrates. These features are (1) The extension of the body-cavity into the vertebral plates, and subsequent enclosure of this cavity between the two layers of the muscle-plates; (2) The primitive division of the vertebral plate into a somatic and a splanchnic layer, and the formation of a large part of the voluntary muscular system out of the splanchnic layer.
* * * * *
The ultimate derivation of the mesoblast forms one of the numerous burning questions of modern embryology, and there are advocates to be found for almost every one of the possible views the question admits of.
All who accept the doctrine of descent are agreed that primitively only two embryonic layers were present—the epiblast and the hypoblast—and that the mesoblast subsequently appeared as a distinct layer, after a certain complexity of organization had been attained.
The general agreement stops, however, at this point, and the greatest divergence of opinion exists with reference to all further questions which bear on the development of the mesoblast. There appear to be four possibilities as to the origin of this layer.
It may be derived:
(1) entirely from the epiblast,
(2) partly from the epiblast, and partly from the hypoblast,
[Pg 336] (3) entirely from the hypoblast,
(4) or may have no fixed origin.
The fourth of these possibilities may for the present be dismissed, since it can be only maintained should it turn out that all the other views are erroneous. The first possibility is supported by the case of the Cœlenterata, and we might almost say by that of this group only[211].
Amongst the Cœlenterata the mesoblast, when present, is unquestionably a derivative of the epiblast, and when, as is frequently the case, a distinct mesoblast is not present, the muscle-cells form a specialized part of the epidermic cells.
The condition of the mesoblast in these lowly organized animals is exactly what might à priori have been anticipated, but the absence throughout the group of a true body-cavity, or specially developed muscular system of the alimentary tract, prevents the possibility of generalizing for other groups, from the condition of the mesoblast in this one.
In those animals in which a body-cavity and muscular alimentary tract are present, it would certainly appear reasonable to expect the mesoblast to be derived from both the primitive layers: the voluntary muscular system from epiblast, and the splanchnic system from the hypoblast. This view has been taken and strongly advocated by so distinguished an embryologist as Professor Haeckel, and it must be admitted, that on à priori grounds there is much to recommend it; there are, however, so far as I am aware of, comparatively few observed facts in its favour.
Professor Haeckel's own objective arguments in support of his view are as follows:
[Pg 337] (1) From the fact that some investigators derive the mesoblast with absolute confidence from the hypoblast, while others do so with equal confidence from the epiblast, he concludes that it is really derived from both these layers.
(2) A second argument is founded on the supposed derivation of the mesoblast in Amphioxus from both epiblast and hypoblast. Kowalevsky's account (on which apparently Prof. Haeckel's[212] statements are based) appears to me, however, too vague, and his observations too imperfect, for much confidence to be placed in his statements on this head. It does not indeed appear to me that the formation of the layers in Amphioxus, till better known, can be used as an argument for any special view about this question.
(3) Professor Haeckel's own observations on the development of Osseous fish form a third argument in support of his views. These observations do not, however, accord with those of the majority of investigators, and not having been made by means of sections, require further confirmation before they can be definitely accepted.
(4) A fourth argument rests on the fact that the various embryonic layers fuse together to form the primitive streak or axis-cord in higher vertebrates. This he thinks proves that the mesoblast is derived from both the primitive layers. The primitive streak has, however, according to my views, quite another significance to that attributed to it by Professor Haeckel[213]; but in any case Professor Kölliker's researches, and on this point my own observations accord with his, appear to me to prove that the fusion which there takes place is only capable of being used as an argument in favour of an epiblastic origin of the mesoblast, and not of its derivation from both epiblast and hypoblast.
The objective arguments in favour of Professor Haeckel's views are not very conclusive, and he himself does not deny that the mesoblast as a rule apparently arises as a single and undivided mass from one of the two primary layers, and only [Pg 338] subsequently becomes split into somatic and splanchnic strata. This original fusion and subsequent splitting of the mesoblast is explained by him as a secondary condition, a possibility which cannot by any means be thrown on one side. It seems therefore worth while examining how far the history of the somatic and splanchnic layers of the mesoblast in Elasmobranchii and other Vertebrates accords with the supposition that they were primitively split off from the epiblast and the hypoblast respectively.
It is well to consider first of all what parts of the mesoblast of the body might be expected to be derived from the somatic and splanchnic layers on this view of their origin[214].
From the somatic layer of the mesoblast there would no doubt be formed the whole of the voluntary muscular system of the body, the dermis, the subcutaneous connective tissue, and the connective tissue between the muscles. It is probable also, though this point is less certain, that the skeleton would be derived from the somatic layer. From the splanchnic layer would be formed the connective tissue and muscular layers of the alimentary tract, and possibly also the vascular system.
Turning to the actual development of these parts, the discrepancy between theory and fact becomes very remarkable. From the somatic layer of the mesoblast, part of the voluntary muscular system and the dermis is no doubt derived, but the splanchnic layer supplies the material, not only for the muscular wall of the digestive canal and the vascular system, but also for the whole of the axial skeleton and a great part of the voluntary muscular system of the body, including the first-formed muscles. Though remarkable, it is nevertheless not inconceivable, that the skeleton might be derived from the splanchnic mesoblast, but [Pg 339] it is very difficult to understand how there could be formed from it a part of the voluntary muscular system of the body indistinguishably fused with part of the muscular system derived from the somatopleure. No fact in my investigations comes out more clearly than that a great part of the voluntary muscular system is formed from the splanchnic layer of the mesoblast, yet this fact presents a most serious difficulty to the view that the somatic and splanchnic layers of the mesoblast in Vertebrates are respectively derived from the epiblast and hypoblast.
In spite, therefore, of general à priori considerations of a very convincing kind which tell in favour of the double origin of the mesoblast, this view is supported by so few objective facts, and there exists so powerful an array of facts against it, that at present, at least, it seems impossible to maintain it. The full strength of the facts against it will appear more fully in a review of the present state of our knowledge as to the development of the mesoblast in the different groups.
To this I now pass.
In a paper on the Early stages of Development in Vertebrates[215]
a short resumé was given of the
development of the mesoblast throughout the animal kingdom, which it may be
worth while repeating here with a few additions. So far as we know at
present, the mesoblast is derived from the hypoblast in the following
groups:
Echinoderms (Hensen, Agassiz, Metschnikoff, Selenka, Götte), Nematodes (Bütschli), Sagitta (Kowalevsky, Bütschli), Lumbricus and probably other Annelids (Kowalevsky), Brachiopoda (Kowalevsky), Crustaceans (Bobretzky), Insects (Kowalevsky, Ulianin, Dohrn), Myriapods (Metschnikoff), Tunicates (Kowalevsky, Kuppfer), Petromyzon (Owsjanikoff), Osseous fishes (Oellacher, Götte, Kowalevsky), Elasmobranchii (Self), Amphibians (Remak, Stricker, Götte).
The list includes members from the greater number of the groups of the animal kingdom; the most striking omissions being the Cœlenterates, Mollusks, and the Amniotic Vertebrates. The absence of the Cœlenterates has been already explained and my grounds for regarding the Amniotic Vertebrates as [Pg 340] apparent rather than real exceptions have also been pointed out. The Mollusks, however, remain as a large group, in which we as yet know very little as to the formation of the mesoblast.
Dr Rabl[216], who seems recently to have studied the development of Lymnæus by means of sections, gives some figures shewing the origin of the mesoblast; they are, however, too diagrammatic to be of much service in settling the present question, and the memoirs of Professor Lankester[217] and Dr Fol[218] are equally inconclusive for this purpose, for, though they contain figures of elongated and branched mesoblast cells passing from the epiblast to the hypoblast, no satisfactory representations are given of the origin of these cells. I have myself observed in embryos of Turbo or Trochus similar elongated cells to those figured by Lankester and Fol, but was unable clearly to determine whence they arose. The most accurate observations which we have on this question are those of Professor Bobretzky[219]. In Nassa he finds that the three embryonic layers are all established during segmentation. The outermost and smallest cells form the epiblast, somewhat larger cells adjoining these the mesoblast, and the large yolk-cells the hypoblast. These observations do not, however, demonstrate from which of the primary layers the mesoblast is derived.
The evidence at present existing is clearly in favour of the mesoblast being, in almost all groups of animals, developed from the hypoblast, but strong as this evidence is, it has not its full weight unless the actual manner in which the mesoblast is in many groups derived from the hypoblast, is taken into consideration. The most important of these are the Echinoderms, Brachiopods and Sagitta.
In the Echinoderms the mesoblast is in part formed by cells budded off from the hypoblast, the remainder, however, arises as one or more diverticula of the alimentary tract. From the separate cells first budded off there are formed the cutis, part of the connective tissue and the calcareous skeleton[220]. The diverticula [Pg 341] from the alimentary cavity form the water-vascular system and the somatic and splanchnic layers of mesoblast. The cavity of the diverticula after the separation of the water-vascular system, forms the body-cavity. The outer lining layer of the cavity forms the somatic layer of mesoblast and the voluntary muscles; the inner lining layer the splanchnic mesoblast which unites with the epithelium of the alimentary tract. Though this fundamental arrangement would seem to be universal amongst Echinoderms, considerable variations of it are exhibited in different groups.
There is one outgrowth from the alimentary tract in Synapta; two in Echinoids, Asteroids and Ophiura; three in Comatula, and four (?) in Amphiura. The cavity of the outgrowth usually forms the body-cavity, but sometimes in Ophiura and Amphiura (Metschnikoff) the outgrowths are from the first or soon become solid, and only secondarily acquire a cavity, which is however homologous with the body-cavity of the other groups.
In Sagitta[221] the formation of the mesoblast and the alimentary tract takes place in nearly the same fashion as in the Echinoderms. The simple invaginate alimentary cavity becomes divided into three lobes, a central and two lateral. The two lateral lobes are gradually more and more constricted off from the central one, and become eventually quite separated from it; their cavities remain independent, and form in the adult the body-cavity, divided by a mesentery into two distinct lateral sections. The inner layer of each of the two lateral lobes forms the mesoblast of the splanchnopleure, the outer layer the mesoblast of the somatopleure. The central division of the primitive gastræa cavity remains as the alimentary tract of the adult.
The remarkable observations of Kowalevsky[222] on the development of the Brachiopoda have brought to light the unexpected fact that in two genera at least (Argiope and Terebratula) the mesoblast and body-cavity develop as paired constrictions from [Pg 342] the alimentary tract in a manner almost identically the same as in Sagitta.
It thus appears that, so far as can be determined from the facts at our disposal, the mesoblast in almost all cases is derived from the hypoblast, and in three widely separated groups it arises as a pair of diverticula from the alimentary tract, each diverticulum containing a cavity which eventually becomes the body-cavity. I have elsewhere suggested[223] that the origin of the mesoblast from alimentary diverticula is to be regarded as primitive for all higher animals, and that the more general cases in which the mesoblast becomes split off, as an undivided layer, from the hypoblast, are in reality derivates from this. The chief obstacle in the way of this view arises from the difficulty of understanding how the whole voluntary muscular system can have been derived at first from the alimentary tract. That part of a voluntary system of muscles might be derived from the contractile diverticula of the alimentary canal attached to the body-wall is not difficult to understand, but it is not easy to believe that the secondary system so formed could completely replace the primitive muscular system, derived, as it must have been, from the epiblast. In my paper above quoted will be found various speculative suggestions for removing this difficulty, which I do not repeat here. If it be granted, however, that in Sagitta, Brachiopods, and Echinoderms we have genuine examples of the formation of the whole mesoblast from alimentary diverticula, it is easy to see how the formation of the mesoblast in Vertebrates may be a secondary derivate from an origin of this nature.
An attempt has been already made to shew that the mesoblast in Elasmobranchii is formed in a very primitive fashion, and for this reason the Elasmobranchii appear to be especially adapted for determining whether any signs are exhibited of a derivation of the mesoblast as paired diverticula of the alimentary tract. There are, it appears to me, several such features. In the first place, the mesoblast is split off from the hypoblast not as a single mass but as a pair of distinct masses, comparable with the paired diverticula already alluded to. [Pg 343] Secondly, the body-cavity when it appears in the mesoblast plates, does not arise as a single cavity, but as a pair of cavities, one for each plate of mesoblast, and these cavities remain permanently distinct in some parts of the body, and nowhere unite till a comparatively late period. Thirdly, the primitive body-cavity of the embryo is not confined to the region in which a body-cavity exists in the adult, but extends to the summit of the muscle-plates, at first separating parts which become completely fused in the adult to form the great lateral muscles of the body. It is difficult to understand how the body-cavity could have such an extension as this, on the supposition that it represents a primitive split in the mesoblast between the wall of the gut and the body-wall; but its extension to this part is quite intelligible, on the supposition that it represents the cavities of two diverticula of the alimentary tract, from whose muscular walls the voluntary muscular system has been derived. Lastly, I would point out that the derivation of part of the muscular system from what appears as the splanchnopleure is quite intelligible on the assumed hypothesis, but, as far as I see, on no other.
Such are the main features presented by the mesoblast in Elasmobranchii, which favour the view of its having originally formed the walls of the alimentary diverticula. Against this view of its nature are the facts (1) of the mesoblast plates being at first solid, and (2), as a consequence of this, of the body-cavity never communicating with the alimentary canal. These points, in view of our knowledge of embryological modifications, cannot be regarded as great difficulties to my view. We have many examples of organs, which, though in most cases arising as involutions, yet appear in other cases as solid ingrowths. Such examples are afforded by the optic vesicle, auditory vesicle, and probably also by the central nervous system, of Osseous Fish. In most Vertebrates these organs are formed as hollow involutions from the exterior; in Osseous Fish, however, as solid involutions, in which a cavity secondarily appears.
The segmental duct of Elasmobranchii or the Wolffian duct (segmental duct) of Birds are cases of a similar kind, being organs which must originally have been formed as hollow involutions, but which now arise as solid bodies.
[Pg 344] Only one more instance of this kind need be cited, taken from the Echinoderms.
The body-cavity and the mesoblast investing it arise in the case of most Echinoderms as hollow involutions of the alimentary tract, but in some exceptional groups, Ophiura and Amphiura, are stated to be solid at first and only subsequently to become hollow. Should the accuracy of Metschnikoff's account of this point be confirmed, an almost exact parallel to what has been supposed by me to have occurred with the mesoblast in Elasmobranchii, and other groups, will be supplied.
The tendency of our present knowledge appears to be in favour of regarding the body-cavity in Vertebrates as having been primitively the cavity of alimentary diverticula, and the mesoblast as having formed the walls of the diverticula.
This view, to say the least of it, suits the facts which we know far better than any other theory which has been proposed, and though no doubt the à priori difficulties in its way are very great, yet it appears to me to be sufficiently strongly supported to deserve the attention of investigators. In the meantime, however, our knowledge of invertebrate embryology is so new and imperfect that no certainty on a question like that which has just been discussed can be obtained; and any generalizations made at present are not unlikely to be upset by the discovery of fresh facts.
The only other point in connection with the mesoblast which I would call attention to is the formation of the vertebral bodies.
My observations confirm those of Remak and Gegenbaur, shewing that there is a primary segmentation of the vertebral bodies corresponding to that of the muscle-plates, followed by a secondary segmentation in which the central lines of the vertebral bodies are opposite the partitions between the muscle-plates.
The explanation of these changes is not difficult to find. The primary segmentation of the body is that of the muscle-plates, which must have been present at a time when the vertebral bodies had no existence. As soon however as the notochordal sheath was required to be strong as well as flexible, it necessarily became divided into a series of segments.
The conditions under which the lateral muscles can cause the [Pg 345] flexure of the vertebral column are clearly that each muscle-segment shall be capable of acting on two vertebræ; and this condition can only be fulfilled when the muscle-segments are opposite the intervals between the vertebræ. Owing to this necessity, when the vertebral segments became formed, their centres corresponded, not with the centres of the muscle-plates, but with the inter-muscular septa.
These considerations fully explain the secondary segmentation of the vertebræ by which they become opposite the inter-muscular septa. On the other hand, the primary segmentation is clearly a remnant of the time when no vertebral bodies were present, and has no greater morphological significance than the fact that the cells to form the unsegmented investment of the notochord were derived from the segmented muscle-plates, and only secondarily became fused into a continuous tube.
The Urinogenital System.
The first traces of the urinary system become visible at about the time of the appearance of the third visceral cleft. At about this period the somatopleure and splanchnopleure become more or less fused together at the level of the dorsal aorta, and thus, as has been already mentioned, each of the original plates of mesoblast becomes divided into a vertebral plate and lateral plate (Pl. 11, fig. 6). The mass of cells resulting from this fusion corresponds with Waldeyer's intermediate cell-mass in the Fowl.
At about the level of the fifth protovertebra the first trace of the urinary system appears.
From the intermediate cell-mass a solid knob grows outwards towards the epiblast (woodcut, fig. 4, pd). This knob consists at first of 20-30 cells, which agree in character with the neighbouring cells of the intermediate cell-mass, and are at this period rounded. It is mainly, if not entirely, derived from the somatic layer of the mesoblast.
From this knob there grows backwards a solid rod of cells which keeps in very close contact with the epiblast, and rapidly diminishes in size towards its posterior extremity. Its hindermost part consists in section of at most one or two cells. It keeps so close to the epiblast that it might be supposed to be [Pg 346] derived from that layer were it not for the sections shewing its origin from the knob above mentioned. We have in this rod the commencement of what I have elsewhere[224] called the segmental duct.
Fig. 4. Two sections of a Pristiurus Embryo with three visceral clefts.
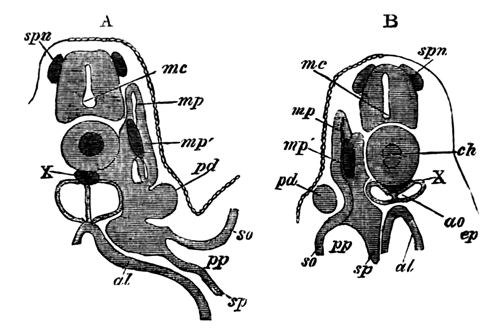
The sections are to shew the development of the segmental duct (pd) or primitive duct of the kidneys. In A (the anterior of the two sections) this appears as a solid knob projecting towards the epiblast. In B is seen a section of the column which has grown backwards from the knob in A.
spn. rudiment of a spinal nerve; mc. medullary canal; ch. notochord; X. string of cells below the notochord; mp. muscle-plate; mp´. specially developed portion of muscle-plate; ao. dorsal aorta; pd. segmental duct; so. somatopleura; sp. splanchnopleura; pp. pleuro-peritoneal or body-cavity; ep. epiblast; al. alimentary canal.
My observations shew that the segmental duct is developed in the way just described in both Pristiurus and Torpedo. Its origin in Pristiurus is shewn in the adjoining woodcut, and in Torpedo in Pl. 11, fig. 7, sd.
At a stage somewhat older than I, the condition of the segmental duct has not very materially altered. It has increased considerably in length, and the knob at its front end is both absolutely smaller, and also consists of fewer cells than before (Pl. 11, fig. 7, sd). These cells have become more columnar, and have begun to arrange themselves radially; thus indicating the early appearance of the lumen of the duct. The cells forming the front part of the rod, as well as those of the knob, commence to exhibit a columnar character, but in the hinder part of the [Pg 347] rod the cells are still rounded. In no part of it has a lumen appeared.
At this period also the knob, partly owing to the commencing separation of the muscle-plate from the remainder of the mesoblast, begins to pass inwards and approach the pleuro-peritoneal cavity.
At the same stage the first not very distinct traces of the remainder of the urinary system become developed. These appear in the form of solid outgrowths from the intermediate cell-mass just at the most dorsal part of the body-cavity.
The outgrowths correspond in numbers with the vertebral segments, and are at first quite disconnected with the segmental duct. At this stage they are only distinctly visible in the first few segments behind the front end of the segmental duct. A full description of them will come more conveniently in the next stage.
By a stage somewhat earlier than K important changes have taken place in the urinary system.
The segmental duct has acquired a lumen in its anterior portion, which opens at its front end into the body-cavity. (Pl. 11, fig. 9, sd.) The lumen is formed by the columnar cells spoken of in the last stage, acquiring a radiating arrangement round a central point, at which a small hole appears. After the lumen has once become formed, it rapidly increases in size.
The duct has also grown considerably in length, but its hind extremity is still as thin, and lies as close to the epiblast, as at first. The segmental involutions which commenced to be formed in the last stage, have now appeared for every vertebral segment along the whole length of the segmental duct, and even for two or three segments behind this.
They are simple independent outgrowths arising from the outer and uppermost angle of the body-cavity, and are at first almost without a trace of a lumen; though their cells are arranged as two layers. They grow in such a way as to encircle the oviduct on its inner and upper side (Pl. 11, fig. 8 and Pl. 12, fig. 14b, st). When the hindermost ones are formed, a slight trace of a lumen is perhaps visible in the front ones. At a stage slightly subsequent to this, in Scyllium canicula, I noticed 29 [Pg 348] of them; the first of them arising in the segment immediately behind the front end of the oviduct (Pl. 12, fig. 17, st), and two of them being formed in segments just posterior to the hinder extremity of the oviduct.
Pl. 12, figs. 16 and 18 represent two longitudinal sections shewing the segmental nature of the involutions and their relation to the segmental duct.
Many of the points which have been mentioned can be seen by referring to Pl. 11 and 12. Anteriorly the segmental duct opens into the pleuro-peritoneal cavity. In the sections behind this there may be seen the segmental duct with a distinct lumen, and also a pair of segmental involutions (Pl. 12, fig. 14a). In the still posterior sections the segmental duct would be quite without a lumen, and would closely adjoin the epiblast.
It seems not out of place to point out that the modes of the development of the segmental duct and of the segmental involutions are strikingly similar. Both arise as solid involutions, from homologous parts of the mesoblast. The segmental duct arises in the vertebral segment immediately in front of that in which the first segmental involution appears; so that the segmental duct appears to be equivalent to a single segmental involution.
The next stage corresponds with the first appearance of the external gills. The segmental duct now communicates by a wide opening with the body-cavity (Pl. 11, fig. 9, sd). It possesses a lumen along its whole length up to the extreme hind end (Pl. 11, fig. 9a). It is, however, at this hinder extremity that the most important change has taken place. This end has grown downwards towards that part of the alimentary canal which still lies behind the anus. This downgrowth is beginning to shew distinct traces of a lumen, and will appear in the next stage as one of the horns by which the segmental ducts communicate with the cloaca (Pl. 11, fig. 9b). All the anterior segmental involutions have now acquired a lumen. But this is still absent in the posterior ones (Pl. 11, fig. 9a).
Owing to the disappearance of the body-cavity in the region behind the anus, the primitive involutions there remain as simple masses of cells still disconnected with the segmental duct (Pl. 11, figs. 9b, 9c and 9d).
[Pg 349] Primitive Ova. The true generative products make their first appearance as the primitive ova between stages I and K.
In the sections of one of my embryos of this stage they are especially well shewn, and the following description is taken from those displayed in that embryo.
They are confined to the region which extends posteriorly nearly to the end of the small intestine and anteriorly to the abdominal opening of the segmental duct.
Their situation in this region is peculiar. There is no trace of a distinct genital ridge, but the ova mainly lie in the dorsal portion of the mesentery, and therefore in a part of the mesoblast which distinctly belongs to the splanchnopleure (Pl. 12, fig. 14a). Some are situated external to the segmental involutions; and others again, though this is not common, in a part of the mesoblast which distinctly belongs to the body-wall (Pl. 12, fig. 14b).
The portion of mesentery, in which the primitive ova are most densely aggregated, corresponds to the future position of the genital ridge, but the other positions occupied by ova are quite outside this. Some ova are in fact situated on the outside of the segmental duct and segmented tubes, and must therefore effect a considerable migration before reaching their final positions in the genital ridge on the inner side of the segmental duct (Pl. 12, fig. 14b).
The condition of the tissue in which the ova appear may at once be gathered from an examination of the figures given. It consists of an irregular epithelium of cells partly belonging to the somatopleure and partly to the splanchnopleure, but passing uninterruptedly from one layer to the other. The cells which compose it are irregular in shape, but frequently columnar (Pl. 12, figs. 14a and 14b).
They are formed of a nucleus which stains deeply, invested by a very delicate layer of protoplasm. At the junction of somatopleure and splanchnopleure they are more rounded than elsewhere. Very few loose connective-tissue cells are present. The cells just described vary from .008 Mm. to .01 Mm. in diameter.
The primitive ova are situated amongst them and stand out with extraordinary clearness, to which justice is hardly done in my figures.
[Pg 350] The normal full-sized ova exhibit the following structure. They consist of a mass of somewhat granular protoplasm of irregular, but more or less rounded, form. Their size varies from .016 - .036 Mm. In their interior a nucleus is present, which varies from .012 - .016 Mm., but its size as a rule bears no relation to the size of the containing cell.
This is illustrated by the subjoined list of measurements.
| Size of Primitive ova in degrees of micrometer scale with F. ocul 2. |
Size of nucleus of Primitive ova in degrees of micrometer scale with F. ocul 2. |
| 10 | 8 |
| 13 | 8 |
| 13 | 8 |
| 14 | 7 |
| 15 | 7 |
| 13 | 7½ |
| 11 | 8 |
| 16 | 5½ |
| 12 | 7 |
| 10 | 7 |
| 15 | 6 |
| 13 | 6 |
| 12 | 7 |
The numbers given refer to degrees on my micrometer scale.
Since it is the ratio alone which it is necessary to call attention to, the numbers are not reduced to decimals of a millimeter. Each degree of my scale is equal, however, with the object glass employed, to .002 Mm.
This series brings out the result I have just mentioned with great clearness.
In one case we find a cell has three times the diameter of the nucleus 16 : 5½; in another case 10 : 8, the nucleus has only a slightly smaller diameter than the cell. The irrationality of the ratio is fairly shewn in some of my figures, though none of the largest cells with very small nuclei have been represented.
The nuclei are granular, and stain fairly well with hæmatoxylin. They usually contain a single deeply stained nucleolus, but in many cases, especially where large (and this independently [Pg 351] of the size of the cell), they contain two nucleoli (Pl. 12, figs. 14c and 14d), and are at times so lobed as to give an apparent indication of commencing division.
A multi-nucleolar condition of the nuclei, like that figured by Götte[225], does not appear till near the close of embryonic life, and is then found equally in the large ova and in those not larger than the ova which exist at this early date.
As regards the relation of the primitive ova to each other and the neighbouring cells, there are a few points which deserve attention. In the first place, the ova are, as a rule, collected in masses at particular points, and not distributed uniformly (fig. 14a). The masses in some cases appear as if they had resulted from the division of one primitive ovum, but can hardly be adduced as instances of a commencing coalescence; since if the ova thus aggregated were to coalesce, an ovum would be produced of a very much greater size than any which is found during the early stages. Though at this stage no indication is present of such a coalescence of cells to form ova as is believed to take place by Götte, still the origin of the primitive ova is not quite clear. One would naturally expect to find a great number of cells intermediate between primitive ova and ordinary columnar cells. Cells which may be intermediate are no doubt found, but not nearly so frequently as might have been anticipated. One or two cells are shewn in Pl. 12, fig. 14a, x, which are perhaps of an intermediate character; but in most sections it is not possible to satisfy oneself that any such intermediate cells are present.
In one case what appeared to be an intermediate cell was measured, and presented a diameter of .012 Mm. while its nucleus was .008 Mm. Apart from certain features of the nucleus, which at this stage are hardly very marked, the easiest method of distinguishing a primitive ovum from an adjacent cell is the presence of a large quantity of protoplasm around the nucleus. The nucleus of one of the smallest primitive ova is not larger than the nucleus of an ordinary cell (being about .008 Mm. in both). It is perhaps the similarity in the size of the nuclei which renders it difficult at first to distinguish developing primitive ova from ordinary cells. Except with the [Pg 352] very thinnest sections a small extra quantity of protoplasm around a nucleus might easily escape detection, and the developing cell might only become visible when it had attained to the size of a small typical primitive ovum.
It deserves to be noticed that the nuclei even of some of the largest primitive ova scarcely exceed the surrounding nuclei in size. This appears to me to be an argument of some weight in shewing that the great size of primitive ova is not due to the fact of their having been formed by a coalescence of different cells (in which case the nucleus would have increased in the same proportion as the cell); but to an increase by a normal method of growth in the protoplasm around the nucleus.
It appears to me to be a point of great importance certainly to determine whether the primitive ova arise by a metamorphosis of adjoining cells, or may not be introduced from elsewhere. In some of the lower animals, e.g. Hydrozoa, there is no question that the ova are derived from the epiblast; we might therefore expect to find that they had the same origin in Vertebrates. Further than this, ova are frequently capable in a young state of executing amœboid movements, and accordingly of migrating from one layer to another. In the Elasmobranchii the primitive ova exhibit in a hardened state an irregular form which might appear to indicate that they possess a power of altering their shape, a view which is further supported by some of them being at the present stage situated in a position very different from that which they eventually occupy, and which they can only reach by migration. If it could be shewn that there were no intermediate stages between the primitive ova and the adjoining cells (their migratory powers being admitted) a strong presumption would be offered in favour of their having migrated from elsewhere to their present position. In view of this possibility I have made some special investigations, which have however led to no very satisfactory results. There are to be seen in the stages immediately preceding the present one, numerous cells in a corresponding position to that of the primitive ova, which might very well be intermediate between the primitive ova and ordinary cells, but which offer no sufficiently well marked features for a certain determination of their true nature.
[Pg 353] In the particular embryo whose primitive ova have been described these bodies were more conspicuous than in the majority of cases, but at the same time they presented no special or peculiar characters.
In a somewhat older embryo of Scyllium the cells amongst which the primitive ova lay had become very distinctly differentiated as an epithelium (the germinal epithelium of Waldeyer) well separated by what might almost be called a basement membrane from the adjoining connective-tissue cells. Hardly any indication of a germinal ridge had appeared, but the ova were more definitely confined than in previous embryos to the restricted area which eventually forms this. The ova on the average were somewhat smaller than in the previous cases.
In several embryos intermediate in age between the embryo whose primitive ova were described at the commencement of this section and the embryo last described, the primitive ova presented some peculiarities, about the meaning of which I am not quite clear, but which may perhaps throw some light on the origin of these bodies.
Instead of the protoplasm around the nucleus being clear or slightly granular, as in the cases just described, it was filled in the most typical instances with numerous highly refracting bodies resembling yolk-spherules. In osmic acid specimens (Pl. 12, fig. 15) these stain very darkly, and it is then as a rule very difficult to see the nucleus; in specimens hardened in picric acid and stained with hæmatoxylin these bodies are stained of a deep purple colour, but the nucleus can in most cases be distinctly seen. In addition to the instances in which the protoplasm of the ova is quite filled with these bodies, there are others in which they only occupy a small area adjoining the nucleus (Pl. 12, fig. 15a), and finally some in which only one or two of these bodies are present. The protoplasm of the primitive ova appears in fact to present a series of gradations between a state in which it is completely filled with highly refracting spherules and one in which these are completely absent.
This state of things naturally leads to the view that the primitive ova, when they are first formed, are filled with these spherules, which are probably yolk-spherules, but that they [Pg 354] gradually lose them in the course of development. Against this interpretation is the fact that the primitive ova in the younger embryo first described are completely without these bodies; this embryo however unquestionably presented an abnormally early development of the ova; and I am satisfied that embryos present considerable variations in this respect.
If the primitive ova are in reality in the first instance filled with yolk-spherules, the question arises as to whether, considering that they are the only mesoblast cells filled at this period with yolk-spherules, we must not suppose that they have migrated from some peripheral part of the blastoderm into their present position. To this question I can give no satisfactory answer. Against a view which would regard the spherules in the protoplasm as bodies which appear subsequently to the first formation of the ova, is the fact that hitherto no instances in which these spherules were present have been met with in the late stages of development; and they seem therefore to be confined to the first stages.
Notochord.
The changes undergone by the notochord during this period present considerable differences according to the genus examined. One type of development is characteristic of Scyllium and Pristiurus; a second type, of Torpedo.
My observations being far more complete for Scyllium and Pristiurus than for Torpedo, it is to the two former genera only that the following account applies, unless the contrary is expressly stated. Only the development of the parts of the notochord in the trunk are here dealt with; the cephalic section of the notochord is treated of in a subsequent section.
During stage G the notochord is composed of flattened cells arranged vertically, rendering the histological characters of the notochord difficult to determine in transverse sections. In longitudinal sections, however, the form and arrangement of the cells can be recognised with great ease. At the beginning of stage G each cell is composed of a nucleus invested by granular protoplasm frequently vacuolated and containing in suspension numerous yolk-spherules. It is difficult to determine whether [Pg 355] there is only one vacuole for each cell, or whether in some cases there may not be more than one.
Round the exterior of the notochord there is present a distinct though delicate cuticular sheath.
The vacuoles are at first small, but during stage G rapidly increase in size, while at the same time the yolk-spherules completely vanish from the notochord.
As a result of the rapid growth of the vacuoles, the nuclei, surrounded in each case by a small amount of protoplasm, become pushed to the centre of the notochord, the remainder of the protoplasm being carried to the edge. The notochord thus becomes composed during stages H and I (Pl. 11, fig. 4-6) of a central area mainly formed of nuclei with a small quantity of protoplasm around them, and of a thin peripheral layer of protoplasm without nuclei, the widish space between the two being filled with clear fluid. The exterior of the cells is indurated, so that they may be said to be invested by a membrane[226]; the cells themselves have a flattened form, and each extends from the edge to the centre of the notochord, the long axis of each being rather greater than half the diameter of the cord.
The nuclei of the notochord are elliptical vesicles, consisting of a membrane filled with granular contents, amongst which is situated a distinct nucleolus. They stain deeply with hæmatoxylin. Their long diameter in Scyllium is about 0.02 Mm.
The diameter of the whole notochord in Pristiurus during stage I is about 0.1 Mm. in the region of the back, and about 0.08 Mm. near the posterior end of the body.
Owing to the form of its constituent cells, the notochord presents in transverse sections a dark central area surrounded by a lighter peripheral one, but its true structure cannot be unravelled without the assistance of longitudinal sections. In these (Pl. 12, fig. 10) the nuclei form an irregular double row in the centre of the cord. Their outlines are very clear, but those of the individual cells cannot for certain be made out. It is, however, easy to see that the cells have a flattened and wedge-shaped form, with the narrow ends overlapping and interlocking at the centre of the notochord.
[Pg 356] By the close of stage I the cuticular sheath of the notochord has greatly increased in thickness.
During the period intermediate between stages I and K the notochord undergoes considerable transformations. Its cells cease to be flattened, and become irregularly polygonal, and appear but slightly more compressed in longitudinal sections than in transverse ones. The vacuolation of the cells proceeds rapidly, and there is left in each cell only a very thin layer of protoplasm around the nucleus. Each cell, as in the earlier stages, is bounded by a membrane-like wall.
Accompanying these general changes special alterations take place in the distribution of the nuclei and the protoplasm. The nuclei, accompanied by protoplasm, gradually leave the centre and migrate towards the periphery of the notochord. At the same time the protoplasm of the cells forms a special layer in contact with the investing sheath.
The changes by which this takes place can easily be followed in longitudinal sections. In Pl. 12, fig. 11 the migration of the nuclei has commenced. They are still, however, more or less aggregated at the centre, and very little protoplasm is present at the edges of the notochord. The cells, though more or less irregularly polygonal, are still somewhat flattened. In Pl. 12, fig. 12 the notochord has made a further progress. The nuclei now mainly lie at the side of the notochord, where they exist in a somewhat shrivelled state, though still invested by a layer of protoplasm.
A large portion of the protoplasm of the cord forms an almost continuous layer in close contact with the sheath, which is more distinctly visible in some cases than in others.
While the changes above described are taking place the notochord increases in size. At the age of fig. 11 it is in the anterior part of the body of Pristiurus about 0.11 Mm. At the age of fig. 12 it is in the same species 0.12 Mm., while in Scyllium stellare it reaches about 0.17 Mm.
During stage K (Pl. 11, fig. 8) the vacuolation of the cells of the notochord becomes even more complete than during the earlier stages, and in the central cells hardly any protoplasm is present, though a starved nucleus surrounded by a little protoplasm may be found in an occasional corner.
[Pg 357] The whole notochord becomes very delicate, and can with great difficulty be conserved whole in transverse sections.
The layer of protoplasm which appeared during the last stage on the inner side of the cuticular membrane of the notochord becomes during the present stage a far thicker and more definite structure. It forms a continuous layer with irregular prominences on its inner surface; and contains numerous nuclei. The layer sometimes presents in transverse sections hardly any indication of a division into a number of separate cells, but in longitudinal sections this is generally very obvious. The cells are directed very obliquely forwards, and consist of an oblong nucleus invested by protoplasm. The layer formed by them is very delicate and very easily destroyed. In one example its thickness varied from .004 to .006 Mm., in another it reached .012 Mm. The thickness of the cuticular membrane is about .002 Mm. or rather less.
The diameter of a notochord in the anterior part of the body of a Pristiurus embryo of this stage is about 0.21 Mm. Round the exterior of the notochord the mesoblast cells are commencing to arrange themselves as a special sheath.
In Torpedo the notochord at first presents the same structure as in Pristiurus, i.e. it forms a cylindrical rod of flattened cells.
The vacuolation of these cells does not however commence till a relatively very much later period than in Pristiurus, and also presents a very different character (Pl. 11, fig. 7).
The vacuoles are smaller, more numerous, and more rounded than in the other genera, and there can be no question that in many cases there is more than one vacuole in a cell. The most striking point in which the notochord of Torpedo differs from that of Pristiurus consists in the fact that in Torpedo there is never any aggregation of the nuclei at the centre of the cord, but the nuclei are always distributed uniformly through it. As the vacuolation proceeds the differences between Torpedo and the other genera become less and less marked. The vacuoles become angular in form, and the cells of the cord cease to be flattened, and become polygonal.
At my final stage for Torpedo (slightly younger than K) the only important feature distinguishing the notochord from that [Pg 358] of Pristiurus, is the absence of any signs of nuclei or protoplasm passing to the periphery. Around the exterior of the cord there is early found in Torpedo a special investment of mesoblastic cells.
EXPLANATION OF PLATES 11 AND 12.
Complete List of Reference Letters.
al. Alimentary tract. an. Point where anus will be formed. ao. Dorsal aorta. ar. Rudiment of anterior root of spinal nerve. b. Anterior fin. c. Connective-tissue cells. cav. Cardinal vein. ch. Notochord. df. Dorsal fin. ep. Epiblast. ge. Germinal epithelium. ht. Heart. l. Liver. mp. Muscle-plate. mp´. Early formed band of muscles from the splanchnic layer of the muscle-plates. nc. Neural canal. p. Protoplasm from yolk in the alimentary tract. pc. Pericardial cavity. po. Primitive ovum. pp. body-cavity. pr. Rudiment of posterior root of spinal nerve. sd. Segmental duct. sh. Cuticular sheath of notochord. so. Somatic layer of mesoblast. sp. Splanchnic layer of mesoblast. spc. Spinal cord. sp.v. Spiral valve. sr. Interrenal body. st. Segmental tube. sv. Sinus venosus. ua. Umbilical artery. um. Umbilical cord. uv. Umbilical vein. V. Splanchnic vein. v. Blood-vessel. vc. Visceral cleft. vr. Vertebral rudiment. W. White matter of spinal cord. x. Subnotochordal rod (except in fig. 14a). y. Passage connecting the neural and alimentary canals.
Plate 11.
Fig. 1. Section from the caudal region of a Pristiurus embryo belonging to stage H. Zeiss C, ocul. 1. Osmic acid specimen.
It shews (1) the constriction of the subnotochordal rod (x) from the summit of the alimentary canal. (2) The formation of the body-cavity in the muscle-plate and the ventral thickening of the parietal plate.
Fig. 1a. Portion of alimentary wall of the same embryo, shewing the formation of the subnotochord rod (x).
Fig. 2. Section through the caudal vesicle of a Pristiurus embryo belonging to stage H. Zeiss C, ocul. 1.
It shews the bilobed condition of the alimentary vesicle and the fusion of the mesoblast and hypoblast at the caudal vesicle.
Fig. 3a. Sections from the caudal region of a Pristiurus embryo belonging to stage H. Zeiss C, ocul. 1. Picric acid specimen.
It shews the communication which exists posteriorly between the neural and alimentary canals, and also by comparison with 3b it exhibits the dilatation undergone by the alimentary canal in the caudal vesicle.
Fig. 3b. Section from the caudal region of an embryo slightly younger than 3a. Zeiss C, ocul. 1. Osmic acid specimen.
[Pg 359] Fig. 4. Section from the cardiac region of a Pristiurus embryo belonging to stage H. Zeiss C, ocul. 1. Osmic acid specimen.
It shews the formation of the heart (ht) as a cavity between the splanchnopleure and the wall of the throat.
Fig. 5. Section from the posterior dorsal region of a Scyllium embryo, belonging to stage H. Zeiss C, ocul. 1. Osmic acid specimen.
It shews the general features of an embryo of stage H, more especially the relations of the body-cavity in the parietal and vertebral portions of the lateral plate, and the early-formed band of muscle (mp´) in the splanchnic layer of the vertebral plate.
Fig. 6. Section from the œsophageal region of Scyllium embryo belonging to stage I. Zeiss C, ocul. 1. Chromic acid specimen.
It shews the formation of the rudiments of the posterior nerve-roots (pr) and of the vertebral rudiments (Vr).
Fig. 7. Section of a Torpedo embryo belonging to stage slightly later than I. Zeiss C, ocul. 1, reduced 1/3. Osmic acid specimen.
It shews (1) the formation of the anterior and posterior nerve-roots. (2) The solid knob from which the segmental duct (sd) originates.
Fig. 8. Section from the dorsal region of a Scyllium embryo belonging to a stage intermediate between I and K. Zeiss C, ocul. 1. Chromic acid specimen.
It illustrates the structure of the primitive ova, segmental tubes, notochord, etc.
Fig. 8a. Section from the caudal region of an embryo of the same age as 8. Zeiss A, ocul. 1.
It shews (1) the solid œsophagus. (2) The narrow passage connecting the pericardial (pc) and body cavities (pp).
Fig. 9. Section of a Pristiurus embryo belonging to stage K. Zeiss A, ocul. 1. Osmic acid specimen.
It shews the formation of the liver (l), the structure of the anterior fins (b), and the anterior opening of the segmental duct into the body-cavity (sd).
Figs. 9a, 9b, 9c, 9d. Four sections through the anterior region of the same embryo as 9. Osmic acid specimens.
The sections shew (1) the atrophy of the post-anal section of the alimentary tract (9b, 9c, 9d). (2) The existence of the segmental tubes behind the anus (9b, 9c, 9d). With reference to these it deserves to be noted that the segmental tubes behind the anus are quite disconnected, as is proved by the fact that a tube is absent on one side in 9c but reappears in 9d. (3) The downward prolongation of the segmental duct to join the posterior or cloacal extremity of the alimentary tract (9b).
Plate 12.
Fig. 10. Longitudinal and horizontal section of a Scyllium embryo of stage H. Zeiss C, ocul. 1. Reduced by 1/3. Picric acid specimen.
It shews (1) the structure of the notochord; (2) the appearance of the early formed band of muscles (mp´) in the splanchnic layer of the protovertebra.
Fig. 11. Longitudinal and horizontal sections of an embryo belonging to stage I. Zeiss C, ocul. 1. Chromic acid specimen. It illustrates the same points as the previous section, but in addition shews the formation of the rudiments of the vertebral bodies (Vr) which are seen to have the same segmentation as the muscle-plates.
[Pg 360] Fig. 12.[227] Longitudinal and horizontal section of an embryo belonging to the stage intermediate between I and K. Zeiss C, ocul. 1. Osmic acid specimen illustrating the same points as the previous section.
Fig. 13. Longitudinal and horizontal section of an embryo belonging to stage K. Zeiss C, ocul. 1, and illustrating same points as previous section.
Figs. 14a, 14b, 14c, 14d. Figures taken from preparations of an embryo of an age intermediate between I and K, and illustrating the structure of the primitive ova. Figs. 14a and 14b are portions of transverse sections. Zeiss C, ocul. 3 reduced 1/3. Figs. 14c and 14d are individual ova, shewing the lobate form of nucleus. Zeiss F, ocul. 2.
Fig. 15. Osmic acid preparation of primitive ova belonging to stage K. Zeiss immersion No. 2, ocul. 1. The protoplasm of the ova is seen to be nearly filled with bodies resembling yolk-spherules: and one ovum is apparently undergoing division.
Fig. 15a. Picric acid preparation shewing a primitive ovum partially filled with bodies resembling yolk-spherules.
Fig. 16. Horizontal and longitudinal section of Scyllium embryo belonging to stage K. Zeiss A, ocul. 1. Picric acid preparation. The connective-tissue cells are omitted.
The section shews that there is one segmental tube to each vertebral segment.
Fig. 17. Portion of a Scyllium embryo belonging to stage K, viewed as a transparent object.
It shews the segmental duct and the segmental involutions—two of which are seen to belong to segments behind the end of the alimentary tract.
Fig. 18. Vertical longitudinal section of a Scyllium embryo belonging to stage K. Zeiss A, ocul. 1. Hardened in a mixture of osmic and chromic acid. It shews
(1) the commissures connecting together the posterior roots of the spinal nerves;
(2) the junction of the anterior and posterior roots;
(3) the relations of the segmental ducts to the segmental involutions and the alternation of calibre in the segmental tube;
(4) the germinal epithelium lining the body-cavity.
[192] Unless the contrary is stated, the facts recorded in this chapter apply only to the genera Scyllium and Pristiurus.
[193] The layers are known as epidermic (horny) and mucous layers by English writers, and as Hornschicht and Schleimschicht by the Germans. For their existence in all Vertebrates, vide Leydig Ueber allgemeine Bedeckungen der Amphibien, p. 20. Bonn, 1876.
[194] Vide Leydig, loc. cit.
[195] Vide Götte, Entwicklungsgeschichte der Unke.
[196]
Vide Self, Development of Spinal Nerves in
Elasmobranchii.
Phil.
Trans. 1876. [This Edition, No.
VIII.]
[197] For Birds, vide Elements of Embryology, Foster and Balfour, pp. 144, 145, and for Mammals, Kölliker, Entwicklungsgeschichte, p. 283.
[198] For the nervous supply in fishes, vide Stannius, Peripher. Nerv. System d. Fische. In Osseous Fishes he states that the thoracic fin is supplied by branches from the first three though sometimes from the first four spinal nerves. In Acipenser there are branches from the first six nerves. In Spinax the limb is supplied by the rami anteriores of the fourth and succeeding ten spinal nerves. In the Rays not only do the sixteen anterior spinal nerves unite to supply the fin, but in all there are rami anteriores from thirty spinal nerves which pass to the thoracic limb.
[199] Philosophical Transactions, 1871.
[200] Ursprung d. Wirbelthiere and Functionswechsels.
[201] Grundriss d. Vergleichenden Anat. p. 494.
[202] Loc. cit.
[203] No attempt has been made to describe in detail the different appearances presented by the protovertebræ in the various parts of the body, but in each stage a protovertebra from the dorsal region is taken as typical.
[204] Zeitschrift f. Anat. Entwicklungsgeschichte, Vol. 1.
[205] Hensen loc. cit.
[206] For the history of protovertebræ and muscle-plates in Birds, vide Elements of Embryology, Foster and Balfour. The statement there made that the horizontal splitting of the mesoblast does not extend to the summit of the vertebral plate, must however be regarded as doubtful.
[207] Vide Elements of Embryology, p. 56.
[208] Dr Götte, Entwicklungsgeschichte der Unke, p. 534, gives a different account of the development of the protovertebræ from that in the text. He states that the muscle-plates do not give rise to the main dorso-lateral muscles, but only to some superficial ventral muscles, while the dorso-lateral muscles are according to him formed from part of the kernel of the protovertebræ internal to the muscle-plates. The account given in the text is the result of my own investigations, and accords precisely with the recent statements of Professor Kölliker, Entwicklungsgeschichte, 1876.
[209] The type of development of the muscle-plates of Amphibians would become identical with that of Elasmobranchii if their first-formed mass of muscle corresponded with the early-formed muscles of Elasmobranchii, and the remaining cells of both layers of the protovertebræ became in the course of development converted into muscle-cells indistinguishable from those formed at first. Is it possible that, owing to the distinctness of the first-formed mass of muscle, Dr Götte can have overlooked the fact that its subsequent growth is carried on at the expense of the adjacent cells of the somatic layer?
[210]
Ehrlich, Ueber den peripher. Theil d. Urwirbel.
Archiv f. Mic. Anat. Vol. XI.
[211] The most important other instances in addition to that of the Cœlenterata which can be adduced in favour of the epiblastic origin of the mesoblast are the Bird and Mammal, in which according to the recent observations of Hensen for the Mammal, and Kölliker for the Mammal and Bird, the mesoblast is split off from the epiblast. If the views I have elsewhere put forward about the meaning of the primitive groove be accepted, the derivation of the mesoblast from the epiblast in these instances would be apparent rather than real, and have no deep morphological significance for the present question.
Other instances may be brought forward from various groups, but none of these are sufficiently well confirmed to be of any value in the determination of the present question.
[212] Vide Anthropogenie, p. 197.
[213]
Vide Self, Development of Elasmobranch
Fishes,
Journal of Anat.
and Phys. Vol. X. note on
p. 682, and also Review of Professor Kölliker's
Entwicklungsgeschichte des Menschen u. d. höheren Thiere,
Journal of Anat. And
Phys. Vol. X.
[214] Professor Haeckel speaks of the splitting of the mesoblast in Vertebrates into a somatic and splanchnic layer as a secondary process (Gastrula u. Eifurchung d. Thiere), but does not make it clear whether he regards this secondary splitting as taking place along the old lines. It appears to me to be fairly certain that even if the original unsplit condition of the mesoblast is to be regarded as a secondary condition, yet that the splitting of this must take place along the old lines, otherwise a change in the position of the body-cavity in the adult would have to be supposed—an unlikely change producing unnecessary complication. The succeeding argument is based on the assumption that the unsplit condition is a secondary condition, but that the split which eventually appears in this occurs along the old lines, separating the primitive splanchnopleure from the primitive somatopleure.
[215] Quart. Jl. of Micros. Science, July, 1875. [This Edition, No. VI.]
[216] Jenaische Zeitschrift, Vol. IX.
[217] Quart. Jl. of Micros. Science, Vol. XXV. 1874, and Phil. Trans. 1875.
[218] Archives de Zoologie, Vol. IV.
[219] Archiv f. Micr. Anat. Vol. XIII.
[220] The recent researches of Selenka, Zeitschrift f. Wiss. Zoologie, Vol. XXVII. 1876, demonstrate that in Echinoderms the muscles are derived from the cells first split off from the hypoblast, and that the diverticula only form the water-vascular system and the epithelial lining of the body-cavity.
[221]
Kowalevsky, Würmer u. Arthropoden,
Mém.
Acad. Pétersbourg, 1871.
[222]
Zur Entwicklungsgeschichte d. Brachiopoden
,
Protokoll d. ersten Session der Versammlung Russischer Naturforscher in
Kasan, 1873. Published in Kaiserliche Gesellschaft
Moskau, 1874 (Russian). Abstracted in Hoffmann and Schwalbe, Jahresbericht f. 1873.
[223] Comparison of Early Stages, Quart. Jl. Micros. Science, July, 1875. [This Edition, No. VI.]
[224]
Urinogenital Organs of Vertebrates,
Journ. of Anat. and Phys. Vol. X. [This Edition,
No. VII.]
[225] Entwicklungsgeschichte der Unke, Pl. 1, fig. 8.
[226] This membrane is better looked upon, as is done by Gegenbaur and Götte, as intercellular matter.
[227] The apparent structure in the sheath of the notochord in this and the succeeding figure is merely the result of an attempt on the part of the engraver to represent the dark colour of the sheath in the original figure.
External Epiblast.
The change already alluded to in the previous chapter (p. 317) by which the external epiblast or epidermis becomes divided into two layers, is completed before the close of stage L.
In the tail region at this stage three distinct strata may be recognized in the epidermis. (1) An outer stratum of flattened horny cells, which fuse together to form an almost continuous membrane. (2) A middle stratum of irregular partly rounded and partly flattened cells. (3) An internal stratum of columnar cells, bounded towards the mesoblast by a distinct basement membrane (Pl. 13, fig. 8), unquestionably pertaining to the epiblast. This layer is especially thickened in the terminal parts of the paired fins (Pl. 13, fig. 1). The two former of these strata together constitute the epidermic layer of the skin, and the latter the mucous layer.
In the anterior parts of the body during stage L the skin only presents two distinct strata, viz. an inner somewhat irregular layer of rounded cells, the mucous layer, and an outer layer of flattened cells (Pl. 13, fig. 8).
The remaining history of the external epiblast, consisting as it does of a record of the gradual increase in thickness of the epidermic strata, and a topographical description of its variations in structure and thickness in different parts, is of no special interest and need not detain us here.
In the late embryonic periods subsequent to stage Q the layers of the skin cease to be so distinct as at an earlier period, [Pg 362] partly owing to the innermost layer becoming less columnar, and partly to the presence of a large number of mucous cells, which have by that stage made their appearance.
I have followed with some care the development of the placoid scales, but my observations so completely accord with those of Dr O. Hertwig[228], that it is not necessary to record them. The so-called enamel layer is a simple product of the thickening and calcification of the basement membrane, and since this membrane is derived from the mucous layer of the epidermis, the enamel is clearly to be viewed as an epidermic product. There is no indication of a gradual conversion of the bases of the columnar cells forming the mucous layer of the epidermis into enamel prisms, as is frequently stated to occur in the formation of the enamel of the teeth in higher Vertebrates.
Lateral line.
The lateral line and the nervous structures appended to it have been recently studied from an embryological point of view by Götte[229] in Amphibians and by Semper[230] in Elasmobranchii.
The most important morphological result which these two distinguished investigators believe themselves to have arrived at is the direct derivation of the lateral nerve from the ectoderm. On this point there is a complete accord between them, and Semper especially explains that it is extremely easy to establish the fact.
As will appear from the sequel, I have not been so fortunate as Semper in elucidating the origin of the lateral nerve, and my observations bear an interpretation not in the least in accordance with the views of my predecessors, though not perhaps quite conclusive against them.
It must be premised that two distinct structures have to be dealt with, viz. the lateral line formed of modified epidermis, and the lateral nerve whose origin is in question.
The lateral line is the first of the two to make its appearance, at a stage slightly subsequent to K, in the form of a [Pg 363] linear thickening of the inner row of cells of the external epiblast, on each side, at the level of the notochord.
This thickening, in my youngest embryo in which it is found, has but a very small longitudinal extension, being present through about 10 thin sections in the last part of the head and first part of the trunk. The thickening, though short, is very broad, measuring about 0.28 Mm. in transverse section, and presents no signs of a commencing differentiation of nervous structures. The large intestinal branch of the vagus can be seen in all the anterior sections in close proximity to this line, and appears to me to give off to it posteriorly a small special branch which can be traced through a few sections, vide Pl. 13, fig. 2, n.l. But this branch is not sufficiently well marked to enable me to be certain of its real character. In any case the posterior part of the lateral line is absolutely without any adjoining nervous structures or traces of such.
The rudiment of the epidermic part of the lateral line is formed of specially elongated cells of the mucous layer of the epiblast, but around the bases of these certain rounder cells of a somewhat curious appearance are intercalated.
There is between this and my next youngest embryo an unfortunately large gap with reference to the lateral line, although in almost every other respect the two embryos might be regarded as belonging to the same stage. The lateral line in the older embryo extends from the hind part of the head to a point well behind the anus, and is accompanied by a nerve for at least two-thirds of its length.
In the foremost section in which it appears the intestinal branch of the vagus is situated not far from it, and may be seen at intervals giving off branches to it. There is no sign that these are otherwise than perfectly normal branches of the vagus. Near the level of the last visceral cleft the intestinal branch of the vagus gives off a fair-sized branch, which from the first occupies a position close to the lateral line though well within the mesoblast (Pl. 13, fig. 3a, n.l). This branch is the lateral nerve, and though somewhat larger, is otherwise much like the nerve I fancied I could see originating from the intestinal branch of the vagus during the previous stage.
It rapidly thins out posteriorly and also approaches closer [Pg 364] and closer to the lateral line. At the front end of the trunk it is quite in contact with it, and a short way behind this region the cells of the lateral line arrange themselves in a gable-like form, in the angle of which the nerve is situated (Pl. 13, figs. 3b, and 3c). In this position the nerve though small is still very distinct in all good sections, and is formed of a rod of protoplasm, with scattered nuclei, in which I could not detect a distinct indication of cell-areas. The hinder part of the nerve becomes continually smaller and smaller, without however presenting any indication of becoming fused with the epiblast, and eventually ceases to be visible some considerable distance in front of the posterior end of the lateral line.
The lateral line itself presents some points of not inconsiderable interest. In the first place, it is very narrow anteriorly and throughout the greater part of its length, but widens out at its hinder end, and is widest of all at its termination, which is perfectly abrupt. The following measurements of it were taken from an embryo belonging to stage L, which though not quite my second youngest embryo is only slightly older. At its hinder end it was 0.17 Mm. broad. At a point not far from this it was 0.09 Mm. broad, and anteriorly it was 0.05 Mm. broad. These measurements clearly shew that the lateral line is broadest at what may be called its growing-point, a fact which explains its extraordinary breadth in the anterior part of the body at my first stage, viz. 0.28 Mm., a breadth which strangely contrasts with the breadth, viz. 0.05 Mm., which it has in the same part of the body at the present stage.
It still continues to form a linear area of modified epidermis, and has no segmental characters. Anteriorly it is formed by the cells of the mucous layer becoming more columnar (Pl. 13 fig. 3a). In its middle region the cells of the mucous layer in it are still simply elongated, but, as has been said above, have a gable-like arrangement, so as partially to enclose the nerve (Pl. 13, fig. 3b). Nearer the hind end of the trunk a space appears in it between its columnar cells and the flattened cells of the outermost layer of the skin (Pl. 13, fig. 3c), and this space becomes posteriorly invested by a very definite layer of cells. The space (Pl. 13, fig. 3d) or lumen has a slit-like section, and is not formed by the closing in of an originally open groove, but by [Pg 365] the formation of a cavity in the midst of the cells of the lateral line. Its walls are formed by a layer of columnar cells on the inner side, and flattened cells on the outer side, both layers however appearing to be derived from the mucous layer of the epidermis. The outer layer of cells attains its greatest thickness dorsally.
During stages M, N, O, the lateral nerve gradually passes inwards into the connective tissue between the dorso-lateral and the ventro-lateral muscles, and becomes even before the close of stage N completely isolated from the lateral line.
The growth of the lateral line itself remains for some time almost stationary; anteriorly the cells retain the gable-like arrangement which characterised them at an earlier period, but cease to enclose the nerve; posteriorly the line retains its original more complicated constitution as a closed canal. In stage O the cells of the anterior part of the line, as well as those of the posterior, commence to assume a tubular arrangement, and the lateral line takes the form of a canal. The tubular form is due to a hollowing out of the lateral line itself and a rearrangement of its cells. As the lateral line becomes converted into a canal it recedes from the surface.
In stage P the first indication of segmental apertures to the exterior make their appearance, vide Pl. 13, fig. 4. The lateral line forms a canal situated completely below the skin, but at intervals (corresponding with segments) sends upwards and outwards prolongations towards the exterior. These prolongations do not during stage P acquire external openings. As is shewn in my figure, a special area of the inner border of the canal of the lateral line becomes distinguished by its structure from the remainder.
No account of the lateral line would be complete without some allusion to the similar sensory structures which have such a wide distribution on the heads of Elasmobranchii; and this is especially important in the present instance, owing to the light thrown by a study of their development on the origin of the nerves which supply the sense-organs of this class. The so-called mucous canals of the head originate in the same way as does the lateral line; they are products of the mucous layer of the epidermis. They eventually form either canals with numerous [Pg 366] openings to the exterior, or isolated tubes with terminal ampulliform dilatations.
I have not definitely determined whether the canal-system of the head arises in connection with the lateral line, or only eventually becomes so connected. The important point to be noticed is, that at first no nervous structures are to be seen in connection with it. In stage O nerves for the mucous canals make their appearance as delicate branches of the main stems. These nerve-stems are very much ramified, and their branches have, in a large number of instances, an obvious tendency towards a particular sense-organ (Pl. 13, figs. 5 and 6).
I have not during stage O been able to detect a case of direct continuity between the two. This is, however, established in the succeeding stage P, in the case of the canals, and the facility with which it may be observed would probably render the embryo Elasmobranch a very favourable object for studying the connection between nerves and terminal sense-organs. The nerve (Pl. 13, fig. 7) dilates somewhat before uniting with the sense-organ, and the protoplasm of the nerve and the sense-organ become completely fused. The basement membrane of the skin is not continuous across their point of junction, and appears to unite with a delicate membrane-like structure, which invests the termination of the nerve. The ampullæ would seem to receive their nervous supply somewhat later than the canals, and the terminal swellings of the nerves supplying them are larger than in the case of the canals, and the connection between the ampullæ and the nerves not so clear. In the case of the head, there can for Elasmobranchii be hardly a question that the nerves which supply the mucous canals grow centrifugally from the original cranial nerve-stems, and do not originate in a peripheral manner from the integument.
This is an important point to make certain of in settling any doubtful
features in the nervous supply of the lateral line. Professor Semper[231], with whom as dealing with Elasmobranchii we are more
directly concerned, makes the following statement: At the time when at
the front end the lateral nerve has already completely separated itself
from the ectoderm, and is situated amongst the muscles, it still lies in
the middle of the body close [Pg 367] to the ectoderm, and at the hind end of the
body is not yet completely segmented off (abgegliedert) from the ectoderm.
Although the last sentence
of this quotation may seem to be opposed to my statements, yet it appears
to me probable that Professor Semper has merely seen the lateral nerve
partially enclosed in the ectoderm. This position of the nerve no doubt
affords a presumption, but only a presumption, in favour of a
direct origin of the lateral nerve from the ectoderm; but against this
interpretation of it are the following facts:
(1) That the front part of the lateral line is undoubtedly supplied by branches which arise in the ordinary way from the intestinal branch of the vagus; and we should not expect to find part of the lateral line supplied by nerves which originate in one way, and the remainder supplied by a nerve having a completely different and abnormal mode of origin.
(2) The growth of the lateral line is quite independent of that of the lateral nerve: the latter arises subsequently to the lateral line, and, so far as is shewn by the inconclusive observation of my earliest stage, as an offshoot from the intestinal branch of the vagus; and though it grows along at first in close contact with the lateral line, yet it never presents, so far as I have seen, any indubitable indication of becoming split off from this, or of fusing with it.
(3) The fact that the cranial representatives of the lateral line are supplied with nerves which originate in the normal way[232], affords a strong argument in favour of the lateral line receiving an ordinary nerve-supply.
Considering all these facts, I am led to the conclusion that the lateral nerve in Elasmobranchii arises as a branch of the vagus, and not as a direct product of the external epiblast.
An interesting feature about the lateral line and the similar cephalic structures, is the fact of these being the only sense-organs in Elasmobranchii which originate entirely from the mucous layer of the epiblast. This, coupled with the well-known facts about the Amphibian epiblast, and the fact that the [Pg 368] mucous canals are the only sense-organs which originate subsequently to the distinct differentiation of the epiblast into mucous and horny layers, goes far to prove[233] that the mucous layer is to be regarded as the active layer of the epiblast, and that after this has become differentiated, an organ formed from the epiblast is always a product of it.
Muscle-plates.
The muscle-plates at the close of stage K were flattened angular bodies with the apex directed forwards, their ventral edge being opposite the segmental duct, and their dorsal edge on a level with the middle of the spinal cord. They were composed of two layers, formed for the most part of columnar cells, but a small part of their splanchnic layer opposite the notochord had already become differentiated into longitudinal muscles.
During stage L the growth of these plates is very rapid, and their upper ends extend to the summit of the neural canal, and their lower ones nearly meet in the median ventral line. The original band of muscles (Pl. 11, fig. 8, m.p´), whose growth was so slow during stages I and K, now increases with great rapidity, and forms the nucleus of the whole voluntary muscular system. It extends upwards and downwards by the continuous conversion of fresh cells of the splanchnic layer into muscle-cells. At the same time it grows rapidly in thickness, but it requires some little patience and care to unravel the details of this growth; and it will be necessary to enter on a slight digression as to the relations of the muscle-plates to the surrounding connective tissue.
As the muscle-plates grow dorsalwards and ventralwards their ends dive into the general connective tissue, whose origin has already been described (Pl. 13, fig. 1). At the same time the connective-tissue cells, which by this process become situated between the ends of the muscle-plates and the skin, grow upwards and downwards, and gradually form a complete layer separating the muscle-plates from the skin. The cells forming [Pg 369] the ends of the muscle-plates retain unaltered their primitive undifferentiated character, and the separation between them and the surrounding connective-tissue cells is very marked. This however ceases to be the case in the parts of the muscle-plates on a level with the notochord and lower part of the medullary canal; the thinnest sections and most careful examination are needed to elucidate the changes taking place in this region. The cells which form the somatic layer of the muscle-plates then begin to elongate and become converted into muscle-cells, at the same time that they are increasing in number to meet the rapid demands upon them. One result of these changes is the loss of the original clearness in the external boundary between the muscle-plates and the adjoining connective-tissue cells, which is only in exceptional cases to be seen so distinctly as it may be in Pl. 13, figs. 1 and 8. Longitudinal horizontal sections are the most instructive for studying the growth of the muscles, but transverse sections are also needed. The interpretation of the transverse ones is however rendered difficult, both by rapid alterations in the thickness of the connective-tissue layer between the skin and the muscle-plates (shewn in Pl. 13, fig. 8), and by the angular shape of the muscle-plates themselves.
A careful study of both longitudinal and transverse sections has enabled me to satisfy myself of the fact that the cells of the somatic layer of the protovertebræ, equally with the cells of the splanchnic layer, are converted into muscle-cells, and some of these are represented in the act of undergoing this conversion in Pl. 13, fig. 8; but the difficulty of distinguishing the outline of the somatic layer of the muscle-plates, at the time its cells become converted into muscle-cells, renders it very difficult to determine whether any cells of this layer join the surrounding connective tissue. General considerations certainly lead me to think that they do not; but my observations do not definitely settle the point.
From these facts it is clear, as was briefly stated in the last chapter, that both layers of the muscle-plate are concerned in forming the great lateral muscle, though the splanchnic layer is converted into muscles very much sooner than the somatic[234].
[Pg 370] The remainder of the history of the muscle-plates presents no points of special interest.
Till the close of stage L, the muscle-plates are not distinctly divided into dorsal and ventral segments, but this division, which is so characteristic of the adult, commences to manifest itself during stage M, and is quite completed in the succeeding stage. It is effected by the appearance, nearly opposite the lateral line, of a layer of connective tissue which divides the muscles on each side into a dorso-lateral and ventro-lateral section. Even during stage O the ends of the muscle-plates are formed of undifferentiated columnar cells. The peculiar outlines of the intermuscular septa gradually appear during the later stages of development, causing the well-known appearances of the muscles in transverse sections, but require no special notice here.
With reference to the histological features of the development of the muscle-fibres, I have not pushed my investigations very far. The primitive cells present the ordinary division, well known since Remak, into a striated portion and a non-striated portion, and in the latter a nucleus is to be seen which soon undergoes division and gives rise to several nuclei in the non-striated part, while the striated part of each cell becomes divided up into a number of fibrillæ. I have not however determined what exact relation the original cells hold to the eventual primitive bundles, or anything with reference to the development of the sarcolemma.
The Muscles of the Limbs.—These are formed during stage O coincidently with the cartilaginous skeleton, in the form of two bands of longitudinal fibres on the dorsal and ventral surfaces of the limbs. Dr Kleinenberg first called my attention to the fact that he had proved the limb-muscles in Lacerta to be derived from the muscle-plates. This I at first believed did not hold good for Elasmobranchii, but have since determined that it does so. Between stages K and L the muscle-plates grow downwards as far as the limbs and then turn outwards and grow into them [Pg 371] (Pl. 18, fig. 1). Small portions of several muscle-plates come in this way to be situated in the limbs, and are very soon segmented off from the remainder of the muscle-plates. The portions of muscle-plates thus introduced into the limbs soon lose their original distinctness, and can no longer be recognized in stage L. There can however be but little doubt that they supply the tissue for the muscles of the limbs. The muscle-plates themselves after giving off these buds to the limbs grow downwards, and by stage L cease to shew any trace of what has occurred (Pl. 13, fig. 1). This fact, coupled with the late development of the muscles of the limbs (stage O), caused me to fall into my original error.
The Vertebral Column and Notochord.
In the previous chapter (p. 325) an account was given of the origin of the tissue destined to form the vertebral bodies; it merely remains to describe the changes undergone by this in becoming converted into the permanent vertebræ.
This subject has already been dealt with by a considerable number of anatomists, and my investigations coincide in the main with the results of my predecessors. Especially the researches of Gegenbaur[235] may be singled out as containing the pith of the whole subject, and my results, while agreeing in all but minor points with his, do not supplement them to any very great extent. I cannot do more than confirm Götte's[236] account of the development of the hæmal arches, and may add that Cartier[237] has given a good account of the later development of the centra. Under the circumstances it has not appeared to me to be worth while recording with great detail my investigations; but I hope to be able to give a somewhat more complete history of the whole subject than has appeared in any single previous memoir.
At their first appearance the cells destined to form the permanent vertebræ present the same segmentation as the muscle-plates. [Pg 372] This segmentation soon disappears, and between stages K and L the tissue of the vertebral column forms a continuous investment of the notochord which cannot be distinguished from the adjoining connective tissue. Immediately surrounding the notochord a layer formed of a single row of cells may be observed, which is not however very distinctly marked[238].
During the stage L there appear four special concentrations of mesoblastic tissue adjoining the notochord, two of them dorsal and two of them ventral. They are not segmented, and form four ridges seated on the sides of the notochord. They are united with each other by a delicate layer of tissue, and constitute the rudiments of the neural and hæmal arches. In longitudinal sections of stage L special concentrated wedge-shaped masses of tissue are to be seen between the muscle-plates, which must not be confused with these rudiments. Immediately around the notochord the delicate investment of cells previously mentioned, is still present.
The rudiments of the arches increase in size and distinctness in the succeeding stages, and by stage N have unquestionably assumed the constitution of embryonic cartilage. In the meantime there has appeared surrounding the sheath of the notochord a well-marked layer of tissue which stains deeply with hæmatoxylin, and with the highest power may be observed to contain flattened nuclei. It is barely thicker than the adjoining sheath, but is nevertheless the rudiment of the vertebral bodies. Pl. 13, fig. 9, vb. Whence does this layer arise? To this question I cannot give a quite satisfactory answer. It is natural to conclude that it is derived from the previously existing mesoblastic investment of the notochord, but in the case of the vertebral column I have not been able to prove this. Observations on the base of the brain afford fairly conclusive evidence that the homologous tissue present there has this origin. Gegenbaur apparently answers the question of the origin of this layer in the way suggested above, and gives a figure in support of his conclusion (Pl. XXII. fig. 3)[239].
[Pg 373] The layer of tissue which forms the vertebral bodies rapidly increases in thickness, and very soon, at a somewhat earlier period than represented in Gegenbaur's Pl. XXII. fig. 4, a distinct membrane (Kölliker's Membrana Elastica Externa) may easily be recognized surrounding it and separating it from the adjoining tissue of the arches. Gegenbaur's figure gives an excellent representation of the appearance of this layer at the period under consideration. It is formed of a homogeneous basis containing elongated concentrically arranged nuclei, and constitutes a uniform unsegmented investment for the notochord (vide Pl. 13, fig. 10).
The neural and hæmal arches now either cease altogether to be united with each other by a layer of embryonic cartilage, or else the layer uniting them is so delicate that it cannot be recognized as true cartilage. They have moreover by stage P undergone a series of important changes. The tissue of the neural arches does not any longer form a continuous sheet, but is divided into (1) a series of arches encircling the spinal cord, and (2) a basal portion resting on the cartilaginous sheath of the notochord. There are two arches to each muscle-plate, one continuous with the basal portion of the arch-tissue and forming the true arch, which springs opposite the centre of a vertebral body, and the second not so continuous, which forms what is usually known as the intercalated piece. Between every pair of true arches the two roots of a single spinal nerve pass out. The anterior root passes out in front of an intercalated piece and the posterior behind it[240].
The basal portion of the arch-tissue likewise undergoes differentiation into a vertebral part continuous with the true arch and formed of hyaline cartilage, and an intervertebral segment formed of a more fibrous tissue.
The hæmal arches, like the neural arches, become divided into a layer of tissue adjoining the cartilaginous sheath of the notochord, and processes springing out from this opposite the [Pg 374] centres of the vertebræ. These processes throughout the region of the trunk in front of the anus pass into the space between the dorsal and ventral muscles, and are to be regarded as rudiments of ribs. The tissue with which they are continuous, which is exactly equivalent to the tissue from which the neural arches originate, is not truly a part of the rib. In the tail, behind the anus and kidneys, the cardinal veins fuse to form an unpaired caudal vein below the aorta, and in this part a fresh series of processes originates on each side from the hæmal tissue adjoining the cartilaginous sheath of the notochord, and eventually, by the junction of the processes of the two sides, a canal which contains the aorta and caudal vein is formed below the notochord. These processes for a few segments coexist with small ribs (vide Pl. 13, fig. 10), a fact which shews (1) that they cannot be regarded as modified ribs, and (2) that the tissue from which they spring is to be viewed as a kind of general basis for all the hæmal processes which may arise, and is not specially connected with any one set of processes.
While these changes (all of which are effected during stage P) are taking place in the arches, the tissue of the vertebral bodies or cartilaginous investment of the notochord, though much thicker than before, still remains as a continuous tube whose wall exhibits no segmental differentiations.
It is in stage Q that these differentiations first appear in the
vertebral regions opposite the origin of the neural arches. The outermost
part of the cartilage at these points becomes hyaline and almost
undistinguishable in structure from the tissue of the arches[241]. These patches of hyaline cartilage grow larger and
cause the vertebral parts of the column to constrict the notochord, whilst
the intervertebral parts remain more passive, but become composed of cells
with very little intercellular substance. Coincidently also with these
changes, part of the layer internal to the hyaline cartilage becomes
modified to form a somewhat peculiar tissue, the intercellular substance of
which does not stain, and in which calcification eventually arises (Pl. 13, fig. 11). The innermost layer adjoining the
notochord retains its primitive [Pg 375] fibrous character, and is distinguishable
as a separate layer through both the vertebral and the intervertebral
regions. As a result of these changes a transverse section through the
centre of the vertebral regions now exhibits three successive rings (vide Pl. 13, fig. 11), an
external ring of hyaline cartilage invested by the membrana elastica
externa
(m.el), followed by a ring
of calcifying cartilage, and internal to this a ring of fibrous cartilage,
which adjoins the now slightly constricted notochord. A transverse section
of an intervertebral region shews only a thick outer and thin inner ring of
fibrous cartilage, the latter in contact with the sheath of the
unconstricted notochord.
The constriction of the notochord proceeds till in the centre of the vertebræ it merely forms a fibrous band. The tissue internal to the calcifying cartilage then becomes hyaline, so that there is formed in the centre of each vertebral body a ring of hyaline cartilage immediately surrounding the fibrous band which connects the two unconstricted segments of the notochord. The intervertebral tissue becomes more and more fibrous. In Cartier's paper before quoted there is a figure (fig. 3) which represents the appearance presented by a longitudinal section of the vertebral column at this stage.
The relation of the vertebral bodies to the arches requires a short notice. The vertebral hyaline cartilage becomes almost precisely similar to the tissue of the arches, and the result is, that were it not for the "membrana elastica externa" it would be hardly possible to distinguish the limits of the two tissues. This membrane however persists till the hyaline cartilage has become a very thick layer (Pl. 13, fig. 11), but I have failed to detect it in the adult, so that I cannot there clearly distinguish the arches from the body of the vertebræ. From a comparison however of the adult with the embryo, it is clear that the arches at most form but a small part of what is usually spoken of as the body of the vertebræ.
The changes in the notochord itself during the stages subsequent to K are not of great importance. The central part retains for some time its previous structure, being formed of large vacuolated cells with an occasional triangular patch of protoplasm containing the starved nucleus and invested by indurated layers of protoplasm. These indurated layers are all [Pg 376] fused, and are probably rightly regarded by Gegenbaur and Götte as representing a sparse intercellular matter. The external protoplasmic layer of the notochord ceases shortly after stage K to exhibit any traces of a division into separate cells, but forms a continuous layer with irregular prominences and numerous nuclei (Pl. 13, fig. 9). In the stages subsequent to P further changes take place in the notochord: the remains of the cells become more scanty and the intercellular tissue assumes a radiating arrangement, giving to sections of the notochord the appearance of a number of lines radiating from the centre to the periphery (Pl. 13, fig. 11).
The sheath of the notochord at first grows in thickness, and during
stage L there is no difficulty in seeing in it the fine radial markings
already noticed by Müller[242] and Gegenbaur[243], and regarded by them as indicating pores. Closely
investing the sheath of the notochord there is to be seen a distinct
membrane, which, though as a rule closely adherent to the sheath, in some
examples separates itself from it. It is perhaps the membrane identified by
W. Müller[244] (though not by Gegenbaur) as
Kölliker's membrana elastica interna
. After the formation of the
cartilaginous investment of the notochord, this membrane becomes more
difficult to see than in the earlier stage, though I still fancy that I
have been able to detect it. The sheath of notochord also appears to me to
become thinner, and its radial striation is certainly less easy to detect[245].
EXPLANATION OF PLATE 13.
Complete List of Reference Letters.
al. Alimentary tract. ao. Aorta. c. Connective tissue. cav. Cardinal vein. ch. Notochord. ep. Epiblast. ha. Hæmal arch. l. Liver. ll. Lateral line. mc. Mucous canal of the head. mel. Membrana elastica externa. mp. Muscle-plate. mp´. Muscles of muscle-plate. na. Neural arch. nl. Nervus lateralis. rp. Rib process. sd. Segmental duct. sh. Sheath of notochord. spc. Spinal cord. spg. Spinal ganglion. syg. Sympathetic ganglion. um. Ductus choledochus. v. Blood-vessel. [Pg 377] var. Vertebral arch. vb. Vertebral body. vcau. Caudal vein. vin. Intestinal branch of the vagus. vop. Ramus ophthalmicus of the fifth nerve. x. Subnotochordal rod.
Fig. 1. Section through the anterior part of an embryo of Scyllium canicula during stage L.
c. Peculiar large cells which are found at the dorsal part of the spinal cord. Sympathetic ganglion shewn at syg. Zeiss A, ocul. 1.
Fig. 2. Section through the lateral line at the time of its first formation.
The cells marked nl were not sufficiently distinct to make it quite certain that they really formed part of the lateral nerve. Zeiss B, ocul. 2.
Figs. 3a, 3b, 3c, 3d. Four sections of the lateral line from an embryo belonging to stage L. 3a is the most anterior. In 3a the lateral nerve (nl) is seen to lie in the mesoblast at some little distance from the lateral line. In 3b and 3c it lies in immediate contact with and partly enclosed by the modified epiblast cells of the lateral line. In 3d, the hindermost section, the lateral line is much larger than in the other sections, but no trace is present of the lateral nerve. The sections were taken from the following slides of my series of the embryo (the series commencing at the tail end) 3d (46), 3c (64), 3b (84), 3a (93). The figures all drawn on the same scale, but 3 a is not from the same side of the body as the other sections.
Fig. 4. Section through lateral line of an embryo of stage P at the point where it is acquiring an opening to the exterior. The peculiar modified cells of its innermost part deserve to be noticed. Zeiss D, ocul. 2.
Fig. 5. Mucous canals of the head with branches of the ramus ophthalmicus growing towards them. Stage O. Zeiss A, ocul. 2.
Fig. 6. Mucous canals of head with branches of the ramus ophthalmicus growing towards them. Stage between O and P. Zeiss a a, ocul. 2.
Fig. 7. Junction of a nerve and mucous canal. Stage P. Zeiss D, ocul. 2.
Fig. 8. Longitudinal and horizontal section through the muscle-plates and adjoining structures at a stage intermediate between L and M. The section is intended to shew the gradual conversion of the cells of the somatic layer of muscle-plates into muscles.
Fig. 9. Longitudinal section through the notochord and adjoining parts to shew the first appearance of the cartilaginous notochordal sheath which forms the vertebral centra. Stage N.
Fig. 10. Transverse section through the tail of an embryo of stage P to shew the coexistence of the rib-process and hæmal arches in the first few sections behind the point where the latter appear. Zeiss C, ocul. 1.
Fig. 11. Transverse section through the centre of a caudal vertebra of an embryo somewhat older than Q. It shews (1) the similarity between the arch-tissue and the hyaline tissue of the outer layer of the vertebral centrum, and (2) the separation of the two by the membrana elastica externa[246] (mel). It shews also the differentiation of three layers in the vertebral centrum: vide p. 374.
[228] Jenaische Zeitschrift, Vol. VIII.
[229] Entwicklungsgeschichte d. Unke.
[230] Urogenitalsystem d. Selachier. Semper's Arbeiten, Bd. II.
[231] Loc. cit. p. 398.
[232] Götte extends his statements about the lateral nerve to the nerves supplying the mucous canals in the head; but my observations appear to me, as far as Elasmobranchii are concerned, nearly conclusive against such a derivation of the nerves in the head.
[233] I believe that Götte, amongst his very numerous valuable remarks in the Entwicklungsgeschichte der Unke, has put forward a view similar to this, though I cannot put my hand on the reference.
[234] The difference between Dr Götte's account of the development of the muscles and my own consists mainly in my attributing to the somatic layer of the muscle-plates a share in the formation of the great lateral muscles, which he denies to it. In an earlier section of this Monograph, pp. 333, 334, too much stress was unintentionally laid on the divergence of our views; a divergence which appears to have, in part at least, arisen, not from our observations being opposed, but from Dr Götte's having taken the highly differentiated Bombinator as his type instead of the less differentiated Elasmobranch.
[235] Das Kopfskelet d. Selachier, p. 123.
[236] Entwicklungsgeschichte d. Unke, pp. 433-4.
[237] Zeitschrift f. Wiss. Anat. Bd.XXV., Supplement.
[239] None of my specimens resembles this figure, and the layer when first formed is in my embryos much thinner than represented by Gegenbaur, and the histological structure of the embryonic cartilage is very different from that of the cartilage in the figures alluded to. Götte's very valuable researches with reference to the origin of this layer in Amphibians tend to confirm the view advocated in the text.
[240] In the adult Scyllium it is well known that the posterior root pierces the intercalated cartilage and the anterior root the true neural arch. This however does not seem to be the case in the embryo at stage P.
[241] A good representation of a longitudinal section at this stage is given by Cartier (Zeitschrift f. Wiss. Zoologie, Bd. XXV., Supplement Pl. IV. fig. 1), who also gives a fair description of the succeeding changes of the vertebral column.
[242] Jenaische Zeitschrift, Vol. VI.
[243] Loc. cit.
[244] Loc. cit.
[245] Gegenbaur makes the reserve statement with reference to the sheath of the notochord. For my own sections the statement in the text certainly holds good. Fortunately the point is one of no importance.
[246] The slight difference observable between these two tissues in the arrangement of their nuclei has been much exaggerated by the engraver.
The spinal nerves.
The development of the spinal nerves has been already treated by me at considerable length in a paper read before the Royal Society in December, 1875[247], and I have but little fresh matter to add to the facts narrated in that paper. The succeeding account, though fairly complete, is much less full than the previous one in the Philosophical Transactions, but a number of morphological considerations bearing on this subject are discussed.
The rudiments of the posterior roots make their appearance considerably before those of the anterior roots. They arise during stage I, as outgrowths from the spinal cord, at a time when the muscle-plates do not extend beyond a third of the way up the sides of the spinal cord, and in a part where no scattered mesoblast-cells are present. They are formed first in the anterior part of the body and successively in the posterior parts, in the following way. At a point where a spinal nerve is about to arise, the cells of the dorsal part of the cord begin to proliferate, and the uniform outline of the cord becomes broken (Pl. 14, fig. 3). There is formed in this way a small prominence of cells springing from the summit of the spinal cord, and constituting a rudiment of a pair of posterior roots. In sections anterior to the point where a nerve is about to appear, the nerve-rudiments are always very distinctly formed. Such a section is shewn in Pl. 14, fig. 2, and the rudiments may there be seen [Pg 379] as two club-shaped masses of cells, which have grown outwards and downwards from the extreme dorsal summit of the neural canal and in contact with its walls. The rudiments of the two sides meet at their point of origin at the dorsal median line, and are dorsally perfectly continuous with the walls of the canal.
It is a remarkable fact that rudiments of posterior roots are to be seen in every section. This may be interpreted as meaning that the rudiments are in very close contact with each other, but more probably means, as I hope to shew in the sequel, that there arises from the spinal cord a continuous outgrowth from which discontinuous processes (the rudiments of posterior roots) grow out.
After their first formation these rudiments grow rapidly ventralwards in close contact with the spinal cord (vide Pl. 14, fig. 1, and Pl. 11, figs. 6 and 7), but soon meet with and become partially enclosed in the mesoblastic tissue (Pl. 11, fig. 7). The similarity of the mesoblast and nerve-tissue in Scyllium and Pristiurus embryos hardened in picric or chromic acid, render the nerves in these genera, at the stage when they first become enveloped in mesoblast, difficult objects to observe; but no similar difficulty is encountered in the case of Torpedo embryos.
While the rudiments of the posterior roots are still quite short, those of the anterior roots make their first appearance. Each of these (Pl. 14, fig. 4, a.r.) arises as a very small but distinct conical outgrowth from a ventral corner of the spinal cord. From the very first the rudiments of the anterior roots have an indistinct form of peripheral termination and somewhat fibrous appearance, while the protoplasm of which they are composed becomes attenuated towards its end. The points of origin of the anterior roots from the spinal cord are separated by considerable intervals. In this fact, and also in the fact of the nerves of the two sides never being united with each other in the median line, the anterior roots exhibit a marked contrast to the posterior. There are thus constituted, before the close of stage I, the rudiments of both the anterior and posterior roots of the spinal nerves. The rudiments of both of these take their origin from the involuted epiblast of the neural canal, and the two roots of each spinal nerve are at first quite unconnected [Pg 380] with each other. It is scarcely necessary to state that the pairs of roots correspond in number with the muscle-plates.
It is not my intention to enter with any detail into the subsequent changes of the rudiments whose origin has been described, but a few points especially connected with their early development are sufficiently important to call for attention.
One feature of the posterior roots at their first formation is the fact that they appear as processes of a continuous outgrowth of the spinal cord. This state of affairs is not of long continuance, and before the close of stage I each posterior root has a separate junction with the spinal cord. What then becomes of the originally continuous outgrowth? It has not been possible for me to trace the fate of this step by step; but the discovery that at a slightly later period (stage K) there is present a continuous commissure independent of the spinal cord connecting the dorsal and central extremities of all the spinal nerves, renders it very probable that the original continuous outgrowth becomes converted into this commissure. Like all the other nervous structures, this commissure is far more easily seen in embryos hardened in a mixture of osmic and chromic acids or osmic acid, than in those hardened in picric acid. Its existence must be regarded as one of the most remarkable results of my researches upon the Elasmobranch nervous system. At stage K it is fairly thick, though it becomes much thinner at a slightly later period. Its condition during stage K is shewn in Pl. 12, fig. 18, com. What it has been possible for me to make out of its eventual fate is mentioned subsequently[248].
A second feature of the earliest condition of the posterior roots is their attachment to the extreme dorsal summit of the spinal cord—a point of attachment very different from that which they eventually acquire. Before the commencement of stage K this state of things has become altered; and the posterior roots spring from the spinal cord in the position normal for Vertebrates.
This apparent migration caused me at first great perplexity, [Pg 381] and I do not feel quite satisfied that I have yet got completely to the bottom of its meaning. The explanation which appears to me most probable has suggested itself in the course of some observations on the development of the thin roof of the fourth ventricle. A growth of cells appears to take place in the median dorsal line of the roof of the spinal cord. This growth tends to divaricate the two lateral parts of the cord, which are originally contiguous in the dorsal line, and causes therefore the posterior roots, which at first spring from the dorsal summit, to assume an apparent attachment to the side of the cord at some little distance from the summit. If this is the true explanation of the change of position which takes place, it must be regarded as due rather to peculiar growths in the spinal cord, than to any alteration in the absolute attachment of the nerves.
By stage K the rudiment of the posterior root has become greatly elongated, and exhibits a division into three distinct portions (Pl. 14, fig. 6):
(1) A proximal portion, in which is situated the pedicle of attachment to the wall of the neural canal.
(2) An enlarged portion, which may conveniently from its future fate be called the spinal ganglion.
(3) A distal portion beyond this.
The proximal portion presents a fairly uniform diameter, and ends dorsally in a rounded expansion; it is attached, remarkably enough, not by its extremity, but by its side, to the spinal cord. The dorsal extremities of the posterior roots are therefore free. It seems almost certain that the free dorsal extremities of these roots serve as the starting points for the dorsal commissure before mentioned, which connects the roots together. The attachment of the posterior nerve-root to the spinal cord is, on account of its small size, very difficult to observe. In favourable specimens there may however be seen a distinct cellular prominence from the spinal cord, which becomes continuous with a small prominence on the lateral border of the nerve-root near its distal extremity. The proximal extremity of the rudiment is composed of cells, which, by their small size and circular form, are easily distinguished from those which form the succeeding or ganglionic portion of the nerve. This succeeding part has a swollen configuration, and is composed [Pg 382] of large elongated cells with oval nuclei. The remainder of the rudiment forms the commencement of the true nerve.
The anterior root, which, at the close of stage I, formed a small and inconspicuous prominence from the spinal cord, grows rapidly during the succeeding stages, and soon forms an elongated cellular structure with a wide attachment to the spinal cord (Pl. 14, fig. 5). At first it passes obliquely and nearly horizontally outwards, but, before reaching the muscle-plate of its side, takes a bend downwards (Pl. 14, fig. 7).
I have not definitely made out when the anterior and posterior roots unite, but this may easily be seen to take place before the close of stage K (Pl. 12, fig. 18).
One feature of some interest with reference to the anterior roots, is the fact that they arise not vertically below, but alternately with the dorsal roots, a condition which persists in the adult.
Although I have made some efforts to determine the eventual fate of the commissure uniting the dorsal roots, these have not hitherto been crowned with success. It grows thinner and thinner, becoming at the same time composed of fibrous protoplasm with imbedded nuclei (Pl. 14, figs. 8 and 9). By stage M it is so small as to be quite indistinguishable in transverse sections; and I have failed in stage P to recognize it at all. I can only conclude that it gradually atrophies, and finally vanishes without leaving a trace. Both its appearance and history are very remarkable, and deserve the careful attention of future investigators.
There can be little doubt that it is some sort of remnant of an ancestral structure in the nervous system; and it would appear to indicate that the central nervous system must originally have been formed of a median and two lateral strands. At the same time I very much doubt whether it can be brought into relation with the three rows of ganglion-cells (a median and two lateral) which are so frequently present on the ventral side of annelidan nerve-cords.
My results may be summarised as follows:—Along the extreme dorsal summit of the spinal cord there arises on each side a continuous outgrowth. From each outgrowth processes corresponding in number to the muscle-plates grow downwards. [Pg 383] These are the rudiments of the posterior nerve-roots. The outgrowths, though at first attached to the spinal cord throughout their whole length, soon cease to be so, and remain in connection with it at certain points only, which form the primitive junctions of the posterior roots with the spinal cord. The original outgrowth on each side remains as a bridge, uniting together the dorsal extremities of all the posterior roots. The posterior roots, though primitively attached to the dorsal summit of the spinal cord, eventually come to arise from its sides. The original homogeneous rudiments before the close of stage K become differentiated into a root, a ganglion, and a nerve.
The anterior roots, like the posterior, are outgrowths from the spinal cord, but are united independently with it, and the points from which they spring originally, remain as those by which they are permanently attached. The anterior roots arise, not vertically below, but in the intervals between the posterior roots. They are at first quite separate from the posterior roots; but before the close of stage K a junction is effected between each posterior root and the corresponding anterior root. The anterior root joins the posterior at some little distance below its ganglion.
* * * * *
The results here arrived at are nearly in direct opposition to those of the majority of investigators, though in accordance, at least so far as the posterior roots are concerned, with the beautiful observations of Hensen 'on the Development of Mammalia[249].'
Mr Marshall[250] has more recently published a paper on the development of the nerves in Birds, in which he shews in a most striking manner that the observations recorded here for Elasmobranchii hold good for the posterior roots of Birds. The similarity between his figures and my own is very noticeable. A further discussion of the literature would be quite unprofitable, and I proceed at once to certain considerations suggested by the above observations.
[Pg 384]General considerations. One point of general anatomy upon which my observations throw considerable light, is the primitive origin of nerves. So long as it was admitted that the spinal and cerebral nerves developed in the embryo independently of the central nervous system, their mode of origin always presented to my mind considerable difficulties. It never appeared clear how it was possible for a state of things to have arisen in which the central nervous system as well as the peripheral terminations of nerves, whether motor or sensory, were formed independently of each other; while between them a third structure was developed, which, growing out either towards the centre or towards the periphery, ultimately brought the two into connection. That such a condition could be a primitive one seemed scarcely possible.
Still more remarkable did it appear, on the supposition that the primitive mode of formation of these parts was represented in the developmental history of Vertebrates, that we should find similar structural elements in the central and in the peripheral nervous systems. The central nervous system arises from the epiblast, and yet contains precisely similar nerve-cells and nerve-fibres to the peripheral nervous system, which, when derived from the mesoblast, was necessarily supposed to have an origin completely different from that of the central nervous system. Both of these difficulties are to a great extent removed by the facts of the development of these parts in Elasmobranchii.
It is possible to suppose that in their primitive differentiation contractile and sensory systems may, as in Hydra[251], have been developed from the protoplasm of even the same cell. As the sensory and motor systems became more complicated, the sensory portion of a cell would become separated by an increasing interval from the muscular part of a cell, and the two parts of a cell would only be connected by a long protoplasmic process. When such a condition as that was reached, the sensory portion of the cell would be called a ganglion-cell or terminal sensory organ, the connecting process a nerve, and the contractile portion of the cell a muscle-cell. When these organs were in this condition, it might not impossibly happen for the general developmental growth which tended to separate the [Pg 385] ganglion-cell and the muscle-cell to be so rapid as to render it impossible for the growth of the connecting nerve to keep pace with it, and that thus the process connecting the ganglion-cell and the muscle-cell might become ruptured. Nevertheless the tendency of the process to grow from the ganglion-cell to the muscle-cell, would remain, and when the rapid developmental growth had ceased, the two would become united again by the growth of the process which had previously been ruptured. It will be seen that this hypothesis, which I have considered only with reference to a single nerve and muscle-cell, might be extended so as to apply to a complicated central nervous system and peripheral nerves and muscles, and also could apply equally as well to the sensory as to the motor terminations of a nerve. In the case of the sensory termination, we should only have to suppose that the centre nervous cell became more and more separated by the general growth from the recipient terminal sensory cell, and that during the general growth the connection between the two was mechanically ruptured but restored again on the termination of the more rapid growth.
As the descendants of the animal in which the rupture occurred became progressively more complicated, the two terminal cells must have become widely separated at a continually earlier period, till finally they may have been separated at a period of development when they were indistinguishable from the surrounding embryonic cells; and since the rupture would also occur at this period, the primitive junction between the nerve-centre and termination would escape detection. The object of this hypothesis is to explain the facts, so far as they are known, of the development of the nervous system in Vertebrates.
In Vertebrates we certainly appear to have an outgrowth from the nervous system, which eventually becomes united with the muscle or sensory terminal organs. The ingenious hypothetical scheme of development of the nerves given by Hensen[252] would be far preferable to the one suggested if it could be brought into conformity with the facts. There is, however, at present no evidence for Hensen's view, as he himself admits, but considering how little we know of the finer details of the development of nerves, it seems not impossible that such [Pg 386] evidence may be eventually forthcoming. The evidence from my own observation is, so far as it goes, against it. At a time anterior to the outgrowth of the spinal nerves, I have shewn[253] that the spinal cord is completely invested by a delicate hyaline membrane. It is difficult to believe that this is pierced by a number of fine processes, which completely escape detection, but which must, nevertheless, be present on the hypothesis of Hensen.
The facts of the development of nerves in Vertebrates are unquestionably still involved in considerable doubt. It may, I think, be considered as certain, that in Elasmobranchii the roots of the spinal and cranial nerves are outgrowths of the central nervous system. How the final terminations of the nerves are formed is, however, far from being settled. Götte[254], whose account of the development of the spinal ganglia is completely in accordance with the ordinary views, yet states[255] that the growth of the nerve fibres themselves is a centrifugal one from the ganglia. My own investigations prove that the ganglia have a centrifugal development, and also appear to demonstrate that the nerves themselves near the ganglion have a similar manner of growth. Moreover, the account given in the preceding chapter of the manner in which the nerves become connected with the mucous canals of the head, goes far to prove that the whole growth of the nerves is a centrifugal one. The combination of all these converging observations tells strongly in favour of this view.
On the other hand, Calberla[256] believes that in the tails of larval Amphibians he has seen connective-tissue cells unite with nerve-processes, and become converted into nerves, but he admits that he cannot definitely prove that the axis-cylinder has not a centrifugal growth, while the connective-tissue cells merely become converted into the sheath of the nerve. If Calberla's view be adopted, that the nerves are developed directly out of a chain of originally indifferent cells, each cell of the chain being converted in turn into a section of the nerve, an altogether different origin of nerves from that I have just suggested would seem to be indicated.
[Pg 387] The obvious difficulty, already alluded to, of understanding how it is, according to the generally accepted mode of development of the spinal nerves, that precisely similar nerve-cells and nerves should arise in structures which have such different origins as the central nervous system and the spinal nerves, is completely removed if my statements on the development of the nerves in Elasmobranch represent the truth.
One point brought out in my investigations appears to me to have bearings upon the origin of the central canal of the vertebrate nervous system, and in consequence upon the origin of the vertebrate nervous system itself. This point is, that the posterior nerve-rudiments make their first appearance at the extreme dorsal summit of the spinal cord. The transverse section of the ventral nervous cord of an ordinary segmented Annelid consists of two symmetrical halves placed side by side. If by a mechanical folding the two lateral halves of the nervous cord became bent towards each other, while into the groove between the two the external skin became pushed, we should have an approximation to the vertebrate nervous system. Such a folding as this might take place to give extra rigidity to the body in the absence of a vertebral column.
If this folding were then completed in such a way that the groove, lined by external skin and situated between the two lateral columns of the nervous system, became converted into a canal, above and below which the two columns of the nervous system united, we should have in the transformed nervous cord an organ strongly resembling the spinal cord of Vertebrates.
It is well known that the nerve-cells are always situated on the ventral side of the abdominal nerve-cord of Annelids, either as a continuous layer, or in the form of two, or more usually, three bands. The dorsal side of the cord is composed of nerve-fibres or white matter. If the folding I have supposed were to take place in the Annelid nervous cord, the grey and white matters would have very nearly the same relative situations as they have in the Vertebrate spinal cord. The grey matter would be situated in the interior and line the central canal, and the white matter would nearly surround the grey. The nerves would then arise, not from the sides of the nervous cord as in existing Annelids, but from its extreme ventral summit. One [Pg 388] of the most striking features which I have brought to light with reference to the development of the posterior roots, is the fact of their growing out from the extreme dorsal summit of the neural canal, a position analogous to the ventral summit of the Annelidan nervous cord. Thus the posterior roots of the nerves in Elasmobranchii[257] arise, in the exact manner which might have been anticipated, were the spinal canal due to such a folding as I have suggested.
The argument from the position of the outgrowth of nerves becomes the more striking from its great peculiarity, and forms a feature which would be most perplexing without some such explanation as I have proposed. The central epithelium of the neural canal, according to this view, represents the external skin, and its ciliation in certain cases may, perhaps, be explained as a remnant of the ciliation of the external skin still found amongst many of the lower Annelids.
I have employed the comparison of the Vertebrate and Annelidan nervous cords, not so much to prove a genetic relation between the two, as to shew the à priori possibility of the formation of a spinal cord, and the à posteriori evidence we have of the vertebrate canal having been formed in the way indicated. I have not made use of what is really my strongest argument, viz. that the embryological mode of formation of the spinal canal by a folding in of the external epiblast is the very method by which I supposed the spinal canal to have been formed in the ancestors of Vertebrates. My object has been to suggest a meaning for the peculiar primitive position of the posterior roots, rather than to attempt to explain in full the origin of the spinal canal.
Although the homologies between the Vertebrate and the Annelidan nervous systems are not necessarily involved in the questions which arise with reference to the formation of the spinal canal, they have nevertheless considerable bearings on it.
Two views have recently been put forward on this subject. [Pg 389] Professor Gegenbaur[258] looks upon the central nervous system of Vertebrates as equivalent to the superior œsophageal ganglia of Annelids and Arthropods only, while Professors Leydig[259] and Semper[260] and Dr Dohrn[261] compare it with the whole Annelidan nervous system.
The first of these two views is only possible on the supposition that Vertebrates are descended from unsegmented ancestors, and even then presents considerable difficulties. If the ancestors of Vertebrates were segmented animals, and several of the recent researches tend to shew that they were, they must almost certainly have possessed a nervous cord like that of existing Annelids. If such were the case, it is almost inconceivable that the greater portion of the nervous system which forms the ventral cord can have become lost, and the system reduced to the superior œsophageal ganglia. Dr Dohrn[262], who has speculated very profoundly on this matter, has attempted to explain and remove some of the difficulties which arise in comparing the nervous systems of Vertebrates and Annelids. He supposes that the segmented Annelids, from which Vertebrates are descended, were swimming animals. He further supposes that their alimentary canal was pierced by a number of gill-slits, and that the anterior amongst these served for the introduction of nutriment into the alimentary canal, in fact as supplementary mouths as well as for respiration. Eventually the old mouth and throat atrophied, and one pair of coalesced gill-slits came to serve as the sole mouth. Thus it came about that on the disappearance of that portion of the alimentary canal, which penetrated the œsophageal nervous ring, the latter structure ceased to be visible as such, and no part of the alimentary [Pg 390] canal was any longer enclosed by a commissure of the central nervous system. With the change of mouth Dr Dohrn also supposes that there took place a change, which would for a swimming animal be one of no great difficulty, of the ventral for the dorsal surface. This general explanation of Dr Dohrn's, apart from the considerable difficulty of the fresh mouth, appears to me to be fairly satisfactory. Dr Dohrn has not however in my opinion satisfactorily dealt with the questions of detail which arise in connection with this comparison. One of the most important points for his theory is to settle the position where the nervous system was formerly pierced by the œsophagus. This position he fixes in the fourth ventricle, and supports his hypothesis by the thinness of the roof of the spinal canal in this place, and the absence (?) of nervous structures in it.
It appears to me that this thinness cannot be used as an argument. In the first place, if the hypothesis I have suggested as to the formation of the spinal canal be accepted, the formation of the canal must be supposed to have occurred in point of time either after or before the loss of the primitive mouth. If, on the one hand, the spinal canal made its appearance before the atrophy of the primitive mouth, the folding to form it must necessarily have ceased behind the mouth; and, on the supposition of the œsophageal ring having been situated in the region of the fourth ventricle, a continuation of the spinal canal could not be present in front of this part. If, on the other hand, the cerebro-spinal canal appeared after the disappearance of the primitive mouth, its roof must necessarily also be a formation subsequent to the atrophy of the mouth, and varieties of structure in it can have no bearing upon the previous position of the mouth.
But apart from speculations upon the origin of the spinal cord, there are strong arguments against Dr Dohrn's view about the fourth ventricle. In the first place, were the fourth ventricle to be the part of the nervous system which previously formed the œsophageal commissures, we should expect to find the opening in the nervous system at this point to be visible at an early period of development, and at a later period to cease to be so. The reverse is however the case. In early embryonic life the roof of the fourth ventricle is indistinguishable from other [Pg 391] parts of the nervous system, and only thins out at a later period. Further than this, any explanation of the thin roof of the fourth ventricle ought also to elucidate the nearly similar structure in the sinus rhomboidalis, and cannot be considered satisfactory unless it does so.
The peculiarities of the cerebro-spinal canal in the region of the brain appear to me to present considerable difficulties in the way of comparing the central nervous system of Vertebrates and segmented Annelids. The manner in which the cerebro-spinal canal is prolonged into the optic vesicles, the cerebral and the optic lobes is certainly opposed both to an intelligible explanation of the spinal canal itself, and also to a comparison of the two nervous systems under consideration.
Its continuation into the cerebral hemispheres and into the optic lobes (mid-brain) may perhaps be looked upon as due to peculiar secondary growths of those two ganglia, but it is very difficult to understand its continuation into the optic vesicles.
If it be granted that the spinal canal has arisen from a folding in of the external skin, then the present inner surface of the optic vesicle must also have been its original outer surface, and it follows as a necessary consequence that the present position of the rods and cones behind and not in front of the nervous structures of the retina was not the primitive one. The rods and cones arise, as is well known, from the inner surface of the outer portion of the optic vesicle, and must, according to the above view, be supposed originally to have been situated on the external surface, and have only come to occupy their present position during the folding in, which resulted in the spinal canal. On à priori grounds we should certainly expect the rods and cones to have resulted from the differentiation of a layer of cells external to the conducting nervous structures. The position of the rods and cones posterior to these suggests therefore that some peculiar infolding has occurred, and may be used as an argument to prove that the medullary groove is no mere embryonic structure, but the embryonic repetition of an ancestral change. The supposition of such a change of position in the rods and cones necessarily implies that the folding in to form the spinal canal must have been a very slow one. It must have given time to the refracting media of the eye [Pg 392] gradually to travel round, so as still to maintain their primitive position, while in successive generations a rudimentary spinal furrow carrying with it the retina became gradually converted into a canal[263].
If Dr Dohrn's comparison of the vertebrate nervous system with that of segmented Annelids be accepted, the following two points must in my opinion be admitted:—
(1) That the formation of the cerebro-spinal canal was subsequent to the loss of the old mouth.
(2) That the position of the old mouth is still unknown.
The well-known view of looking at the pituitary and pineal growths as the remnants of the primitive œsophagus, has no doubt some features to recommend it. Nearly conclusive against it is the fact that the pituitary involution is not, as used to be supposed, a growth towards the infundibulum of the hypoblast of the œsophagus, but of the epiblast of the mouth. It is almost inconceivable that an involution from the present mouth can have assisted in forming part of the old œsophagus.
There is a view not involving the difficulty of the œsophageal ring, fresh mouth[264], and of the change of the ventral to the dorsal [Pg 393] surface, which, though so far unsupported by any firm basis of observed facts, nevertheless appears to me worth suggesting. It assumes that Vertebrates are descended not through the present line of segmented Vermes, but through some other line which has now, so far as is known, completely vanished. This line must be supposed to have originated from the same unsegmented Vermes as the present segmented Annelids. They therefore acquired fundamentally similar segmental and other Annelidan organs.
The difference between the two branches of the Vermes lay in the nervous system. The unsegmented ancestors of the present Annelids seem to have had a pair of super-œsophageal ganglia, from which two main nervous stems extended backwards, one on each side of the body. Such a nervous system in fact as is possessed by existing Nemertines or Turbellarians[265]. As the Vermes became segmented and formed the Annelids, these side nerves seem to have developed ganglia, corresponding in number with the segments, and finally, approximating on the ventral surface, to have formed the ventral cord[266].
The other branch of Vermes which I suppose to have been the ancestors of Vertebrates started from the same stock as existing Annelids, but I conceive the lateral nerve-cords, instead of approximating ventrally, to have done so dorsally, and thus a dorsal cord to have become formed analogous to the ventral cord of living Annelids, only without an œsophageal nerve-ring[267].
[Pg 394] It appears to me, (if the difficulties of comparing the Annelidan ventral cord with the spinal cord of Vertebrates are found to be insurmountable), that this hypothesis would involve far fewer improbabilities than one which supposes the whole central nervous system of Vertebrates to be homologous with the super-œsophageal ganglia. The mode of formation of a nervous system presupposed in my hypothesis, well accords with what we know of the formation of the ventral cord in existing Annelids.
The supposition of the existence of another branch of segmented Vermes is not a very great difficulty. Even at the present day we have possibly more than one branch of Vermes which have independently acquired segmentation. viz.: the Chætopodous Annelids and the Hirudinea. If the latter is an isolated branch, it is especially interesting from having independently developed a series of segmental organs like those of Chætopodous Annelids, which we must suppose the ancestors of Vertebrates also to have done if they too form an independent branch.
In addition to the difficulty of imagining a fresh line of segmented Vermes, there is another difficulty to my view, viz.: the fact that in almost all Vermes, the blood flows forwards in the dorsal vessel, and backwards in the ventral vessel. This condition of the circulation very well suits the view of a change of the dorsal for the ventral surfaces, but is opposed to these surfaces being the same for Vertebrates and Vermes. I cannot however regard this point as a very serious difficulty to my view, considering how undefined is the circulation in the unsegmented groups of the Vermes.
Sympathetic nervous system.
Between stages K and L there may be seen short branches from the spinal nerves, which take a course towards the median line of the body, and terminate in small irregular cellular masses immediately dorsal to the cardinal veins (Pl. 18, fig. 1, sy.g.). These form the first traces that have come under my notice of the sympathetic nervous system. In the youngest of my embryos in which I have detected these it has not been possible for me [Pg 395] either definitely to determine the antero-posterior limits of the system, or to make certain whether the terminal masses of cells which form the ganglia are connected by a longitudinal commissure. In a stage slightly younger than L the ganglia are much more definite, the anterior one is situated in the cardiac region close to the end of the intestinal branch of the vagus, and the last of them quite at the posterior end of the abdominal cavity. The anterior ganglia are the largest; the commissural cord, if developed, is still very indistinct. In stage L the commissural cord becomes definite, though not very easy to see even in longitudinal sections, and the ganglia become so considerable as not to be easily overlooked. They are represented in Pl. 13, fig. 1, sy.g. and in Pl. 18, fig. 2, in the normal position immediately above the cardinal veins. The branches connecting them with the trunks of the spinal nerves may still be seen without difficulty. In later stages these branches cannot so easily be made out in sections, but the ganglia themselves continue as fairly conspicuous objects. The segmental arrangement of the ganglia is shewn in Pl. 18, fig. 3, a longitudinal and vertical section of an embryo between stages L and M with the junctions of the sympathetic ganglia and spinal nerves. The ganglia occupy the intervals between the successive segments of the kidneys.
The sympathetic system only came under my notice at a comparatively late period in my investigations, and the above facts do not in all points clear up its development[268]. My observations seem to point to the sympathetic system arising as an off-shoot from the cerebrospinal system. Intestinal branches would seem to be developed on the main nerve stems of this in the thoracic and abdominal regions, each of these then develops a ganglion, and the ganglia become connected by a longitudinal commissure. On this view a typical spinal nerve has the following parts: two roots, a dorsal and ventral, the dorsal one ganglionated, and three main branches, (1) a ramus dorsalis, (2) a ramus ventralis, and (3) a ramus intestinalis. This scheme may be advantageously compared with that of a typical cranial nerve according to Gegenbaur. It may be noted that it brings [Pg 396] the sympathetic nervous system into accord with the other parts of the nervous system as a product of the epiblast, and derived from outgrowths from the neural axis. It is clear, however, that my investigations, though they may naturally be interpreted in this way, do not definitely exclude a completely different method of development for the sympathetic system.
EXPLANATION OF PLATE 14.
This Plate illustrates the Formation of the Spinal Nerves.
Complete List of Reference Letters.
ar. Anterior root of a spinal nerve. ch. Notochord. com. Commissure connecting the posterior roots of the spinal nerves. i. Mesoblastic investment of spinal cord. mp. Muscle-plate. n. Spinal nerve. nc. Neural canal. pr. Posterior root of a spinal nerve. spg. Ganglion on posterior root of spinal nerve. v.r. Vertebral rudiment. w. White matter of spinal cord. y. Point where the spinal cord became segmented off from the superjacent epiblast.
Figs. 1, 2, and 3. Three sections of a Pristiurus embryo belonging to stage I. Fig. 1 passes through the heart, fig. 2 through the anterior part of the dorsal region, fig. 3 through a point slightly behind this. (Zeiss CC, ocul. 2.) In fig. 3 there is visible a slight proliferation of cells from the dorsal summit of the neural canal. In fig. 2 this proliferation definitely constitutes two club-shaped masses of cells (pr)—the rudiments of the posterior nerve-roots,—both attached to the dorsal summit of the spinal cord. In fig. 1 the rudiments of the posterior roots are of considerable length.
Fig. 4. Section through the dorsal region of a Torpedo embryo slightly older than stage I, with three visceral clefts. (Zeiss CC, ocul. 2.) The section shews the formation of a pair of dorsal nerve-rudiments (pr) and a ventral nerve-rudiment (ar). The latter is shewn in its youngest condition, and is not distinctly cellular.
Fig. 5. Section through the dorsal region of a Torpedo embryo slightly younger than stage K. (Zeiss CC, ocul. 2.) The connective-tissue cells are omitted. The rudiment of the ganglion (spg) on the posterior root has appeared, and the junction of posterior root with the cord is difficult to detect. The anterior root forms an elongated cellular structure.
Fig. 6. Section through the dorsal region of a Pristiurus embryo of stage K. (Zeiss CC, ocul. 2.) The section especially illustrates the attachment of the posterior root to the spinal cord.
Fig. 7. Section through the same embryo as fig. 6. (Zeiss CC, ocul. 1.) The section contains an anterior root, which takes its origin at a point opposite the interval between two posterior roots.
Fig. 8. A series of posterior roots with their central ends united by a dorsal commissure, from a longitudinal and vertical section of a Scyllium embryo belonging to a stage intermediate between L and M. The embryo was hardened in a mixture of osmic and chromic acids.
Fig. 9. The central end of a posterior nerve-root from the same embryo, with the commissure springing out from it on either side.
[247] Phil. Trans. Vol. 166, p. 175. [This Edition, No. VIII.]
[248] It is not by any means always possible to detect this commissure in transverse sections. As I have suggested, in connection with a similar commissure connecting the vagus branches, it perhaps easily falls out of the section, and is always so small that the hole left would certainly be invisible.
[249] Zeit. f. Anat. u. Entwicklungsgeschichte, Vol. I.
[250] Journal of Anatomy and Physiology, Vol. XI. April, 1877.
[251] Kleinenberg Hydra.
[252] Virchow's Archiv, Vol. XXXI. 1864.
[253] Phil. Trans., 1876. [This Edition, No. VIII.]
[254] Entwicklungsgeschichte der Unke.
[255] Loc. cit. p. 516.
[256] Archiv für Micros. Anat., Vol. XI. 1875.
[257] There are strong reasons for regarding the posterior roots as the primitive ones. These are spoken of later, but I may state that they depend:
(1) On the fact that only posterior roots exist in the brain.
(2) That only posterior roots exist in Amphioxus.
(3) That the posterior roots develop at an earlier period than the anterior.
[258] Grundriss d. vergleichenden Anat. p. 264.
[259] Bau des thierischen Körpers.
[260] Stammesverwandtschaft d. Wirbelthiere u. Wirbellosen and Die Verwandtschaftsbeziehungen d. gegliederten Thiere. This latter work, for a copy of which I return my best thanks to the author, came into my hands after what follows was written, and I much regret only to have been able to make one or two passing allusions to it. The work is a most important contribution to the questions about to be discussed, and contains a great deal that is very suggestive; some of the conclusions with reference to the Nervous System appear to me however to be directly opposed to the observations on Spinal Nerves above recorded.
[261] Ursprung d. Wirbelthiere u. Princip des Functionswechsels.
[262] Loc. cit.
[263] Professor Huxley informs me that he has for many years entertained somewhat similar views to those in the text about the position of the rods and cones, and has been accustomed to teach them in his lectures.
[264]
Professor Semper (Die Verwandtschaftsbeziehungen d.
gegliederten Thiere,
Arbeiten aus d. Zool.-zoot.
Institut, Würzburg, 1876) has some interesting speculations on the
difficult question of the vertebrate mouth, which have unfortunately come
to my knowledge too late to be either fully discussed or incorporated in
the text. These speculations are founded on a comparison of the condition
of the mouth in Turbellarians and Nemertines. He comes to the conclusion
that there was a primitive mouth on the cardiac side of the
supra-œsophageal ganglion, which is the existing mouth of
Turbellarians and Vertebrates and the opening of the proboscis of
Nemertines, but which has been replaced by a fresh mouth on the neural
side in Annelids and Nemertines. In Nemertines however the two mouths
co-exist—the vertebrate mouth as the opening of the proboscis, and
the Annelid mouth as the opening for the alimentary tract. This ingenious
hypothesis is supported by certain anatomical facts, which do not appear
to me of great weight, but for which the reader must refer to the original
paper. It no doubt avoids the difficulty of the present position of the
vertebrate mouth, but unfortunately at the same time substitutes an equal
difficulty in the origin of the Annelidan mouth. This Professor Semper
attempts to get over by an hypothesis which to my mind is not very
satisfactory (p. 378), which, however, and
this Professor Semper does not appear to have noticed, could equally
well be employed to explain the origin of a Vertebrate mouth as a
secondary formation subsequent to the Annelidan mouth. Under these
circumstances this fresh hypothesis does not bring us very much nearer to
a solution of the vertebrate-annelid mouth question, but merely
substitutes one difficulty for another; and does not appear to me so
satisfactory as the hypothesis suggested in the text.
At the same time Professor Semper's hypothesis suggests an explanation of that curious organ the Nemertine proboscis. If the order of changes suggested by him were altered it might be possible to suppose that there never was more than one mouth for all Vermes, but that the proboscis in Nemertines gradually split itself off from the œsophagus to which it originally belonged, and became quite free and provided with a separate opening and perhaps carried with it the so-called vagus of Professors Semper and Leydig.
[265] It is not of course to be supposed that the primitive nervous system was pierced by a proboscis like that of the Nemertines.
[266] This is Gegenbaur's view of the development of the ventral cord, and I regard it in the meantime as the most probable view which has been suggested.
[267] A dorsal instead of a ventral approximation of the lateral nerve-cords would be possible in the descendants of such living segmented Vermes as Saccocirrus and Polygordius.
[268] The formation out of the sympathetic ganglia of the so-called paired suprarenal bodies is dealt with in connection with the vascular system. The original views of Leydig on these bodies are fully borne out by the facts of their development.
The Development of the Brain.
General History. In stage G the brain presents a very simple constitution (Pl. 8, fig. G), and is in fact little more than a dilated termination to the cerebro-spinal axis. Its length is nearly one-third that of the whole body, being proportionately very much greater than in the adult.
It is divided by very slight constrictions into three lobes, the posterior of which is considerably the largest. These are known as the fore-brain, the mid-brain, and the hind-brain. The anterior part of the brain is bent slightly downwards about an axis passing through the mid-brain. The walls of the brain, composed of several rows of elongated columnar cells, have a fairly uniform thickness, and even the roof of the hind-brain is as thick as any other part. Towards the end of stage G the section of the hind-brain becomes somewhat triangular with the apex of the triangle directed downwards.
In Pristiurus during stage H no very important changes take place in the constitution of the brain. In Scyllium, however, indications appear in the hind-brain of its future division into a cerebellum and medulla oblongata. The cavity of the anterior part dilates and becomes rounded, while that of the posterior part assumes in section an hour-glass shape, owing to an increase in the thickness of the lateral parts of the walls. At the same time the place of the original thick roof is taken by a very thin layer, which is formed not so much through a change in the character and arrangements of the cells composing the roof, as [Pg 398] by a divarication of the two sides of the hind-brain, and the simultaneous introduction of a fresh structure in the form of a thin sheet of cells connecting dorsally the diverging lateral halves of this part of the brain. By stage I, the hind-brain in Pristiurus also acquires an hour-glass shaped section, but the roof has hardly begun to thin out (Pl. 15, figs. 4a and 4b).
During stages I and K the cranial flexure becomes more and more pronounced, and causes the mid-brain definitely to form the termination of the long axis of the embryo (Pl. 15, figs. 1, 2, etc.), and before the close of stage K a thin coating of white matter has appeared on the exterior of the whole brain, but no other histological changes of interest have occurred.
During stage L an apparent rectification of the cranial flexure commences, and is completed by stage Q. The changes involved in this process may be advantageously studied by comparing the longitudinal sections of the brain during stages L, P, and Q, represented in Pl. 16, figs. 1a, 5 and 7a.
It will be seen, first of all, that so far from the flexure of the brain itself being diminished, it is increased, and in P (fig. 5) the angle in the floor of the mid-brain becomes very acute indeed; in other words, the anterior part of the brain has been bent upon the posterior through nearly two right angles, and the infundibulum, or primitive front end of the brain, now points nearly directly backwards. At the same time the cerebral hemispheres have grown directly forwards, and if figures 1a and 5 in Pl. 16 be compared it will be seen that in the older brain of the two the cerebral hemispheres have assumed a position which might be looked on as the result of their having been pushed dorsalwards and forwards against the mid-brain, and having in the process pressed in and nearly obliterated the original thalamencephalon. The thalamencephalon in fig. 1a, belonging to stage L, is relatively large, but in fig. 5, belonging to stage P, it only occupies a very small space between the front wall of the mid-brain and the hind wall of the cerebral hemispheres. It is therefore in part by the change in position of the cerebral hemispheres that the angle between the trabeculæ and parachordals becomes increased, i.e. their flexure diminished, while at the same time the flexure of the brain itself is increased. More important perhaps in the apparent rectification of the cranial [Pg 399] flexure than any of the previously mentioned points, is the appearance of a bend in the hind-brain which tends to correct the original cranial flexure. The gradual growth of this fresh flexure can be studied in the longitudinal sections which have been represented. It is at its maximum in stage Q. This short preliminary sketch of the development of the brain as a whole will serve as an introduction to the history of the individual divisions of the brain.
Fore-brain. In its earliest condition the fore-brain forms a single vesicle without a trace of separate divisions, but buds off very early the optic vesicles, whose history is described with that of the eye (Pl. 15, fig. 3, op.v). Between stages I and K the posterior part of the fore-brain sends outwards a papilliform process towards the exterior, which forms the rudiment of the pineal gland (Pl. 15, fig. 1, pn). Immediately in front of the rudiment a constriction appears, causing a division of the fore-brain into a large anterior and a small posterior portion. This constriction is shallow at first, but towards the close of stage K becomes much deeper (Pl. 15, fig. 2 and fig. 16a), leaving however the two cavities of the two divisions of the fore-brain united ventrally by a somewhat wide canal.
The posterior of the two divisions of the fore-brain forms the thalamencephalon. Its anterior wall adjoining the cerebral rudiment becomes excessively thin (Pl. 15, fig. 11); and its base till the close of stage K is in close contact with the mouth involution, and presents but a very inconspicuous prominence which marks the eventual position of the infundibulum (Pl. 15, figs. 9a, 12, 16a, in). The anterior and larger division of the fore-brain forms the rudiment of the cerebral hemispheres and olfactory lobes. Up to stage K this rudiment remains perfectly simple, and exhibits no signs, either externally or internally, of a longitudinal constriction into two lobes. From the canal uniting the two divisions of the fore-brain (which eventually forms part of the thalamencephalon) there spring the hollow optic nerves. A slight ventral constriction separating the cerebral rudiment from that part of the brain where these are attached appears even before the close of stage K (Pl. 15, fig. 11, op.n).
During stage L the infundibulum becomes much produced, and forms a wide sack in contact with the pituitary body, and [Pg 400] its cavity communicates with that of the third ventricle by an elongated slit-like aperture. This may be seen by comparing Pl. 16, figs. 1a and 1c. In fig. 1c taken along the middle line, there is present a long opening into the infundibulum (in), which is shewn to be very narrow by being no longer present in fig. 1a representing a section slightly to one side of the middle line. During the same stage the pineal gland grows into a sack-like body, springing from the roof of the thalamencephalon, fig. 1b, pn. This latter (the thalamencephalon) is now dorsally separated from the cerebral rudiment by a deep constriction, and also ventrally by a less well marked constriction. At its side also a deep constriction is being formed in it, immediately behind the pineal gland. The cerebral rudiment is still quite unpaired and exhibits no sign of becoming constricted into two lobes.
During the next two stages the changes in the fore-brain are of no great importance, and I pass at once to stage O. The infundibulum is now nearly in the same condition as during stage L, though (as is well shewn in the figure of a longitudinal section of the next stage) it points more directly backwards than before. The remaining parts of the thalamencephalon have however undergone considerable changes. The more important of these are illustrated by a section of stage O, Pl. 16, fig. 3, transverse to the long axis of the embryo, and therefore, owing to the cranial flexure, cutting the thalamencephalon longitudinally and horizontally; and for stage P in a longitudinal and vertical section through the brain (Pl. 16, fig. 5). In the first place the roof of the thalamencephalon has become very much shortened by the approximation of the cerebral rudiment to the mid-brain. The pineal sack has also become greatly elongated, and its somewhat dilated extremity is situated between the cerebral rudiment and the external skin. It opens into the hind end of the third ventricle, and its posterior wall is continuous with the front wall of the mid-brain. The sides of the thalamencephalon have become much thickened, and form distinct optic thalami (op.) united by a very well marked posterior commissure (pc.). The anterior wall of the thalamencephalon as well as its roof are very thin. The optic nerves have become by stage O quite solid except at their roots, into which the ventricles of the fore-brain are for a short [Pg 401] distance prolonged. This solidification is arrived at, so far as I have determined, without the intervention of a fold. The nerves are fibrous, and a commencement of the chiasma is certainly present. From the chiasma there appears to pass out on each side a band of fibres, which runs near the outer surface of the brain to the base of the optic lobes (mid-brain), and here the fibres of the two sides again cross.
By stage O important changes are perceptible in the cerebral rudiment. In the first place there has appeared a slight fold at its anterior extremity (Pl. 16, fig. 3, x), destined to form a vertical septum dividing it into two hemispheres, and secondly, lateral outgrowths (vide Pl. 16, fig. 2, ol.l), to form the olfactory lobes. Its thin posterior wall presents on each side a fold which projects into the central cavity. From the peripheral end of each olfactory lobe a nerve similar in its histological constitution to any other cranial nerve makes its appearance (Pl. 16, fig. 2); this divides into a number of branches, one of which passes into the connective tissue between the two layers of epithelium in each Schneiderian fold. On the root of this nerve there is a large development of ganglionic cells. I have not definitely observed its origin, but have no reason to doubt that it is a direct outgrowth from the olfactory lobe, exactly similar in its mode of development to any other nerve of the body.
The cerebral rudiment undergoes great changes during stage P. In addition to a great increase in the thickness of its walls, the fold which appeared in the last stage has grown backwards, and now divides it in front into two lobes, the rudiments of the cerebral hemispheres. The greater and posterior section is still however quite undivided, and the cavities of the lobes (lateral ventricles) though separated in front are still quite continuous behind. At the same time, the olfactory lobes, each containing a prolongation of the ventricle, have become much more pronounced (vide Pl. 16, figs. 4a and 4c, ol.l). The root of the olfactory nerve is now very thick, and the ganglion cells it contains are directly prolonged into the ganglionic portion of the olfactory bulb; in consequence of which it becomes rather difficult to fix on the exact line of demarcation between the bulb and the nerve.
Stage Q is the latest period in which I have investigated the [Pg 402]
development of the brain. Its structure is represented for this stage in
general view in Pl. 16, figs. 6a,
6b, 6c, in longitudinal section in Pl. 16, figs. 7a, 7b, and in transverse
section Pl. 16, figs. 8a-d. The
transverse sections are taken from a somewhat older embryo than the
longitudinal. In the thalamencephalon there is no fresh point of great
importance to be noticed. The pineal gland remains as before, and has
become, if anything, longer than it was, and extends further forwards over
the summit of the cerebrum. It is situated, as might be expected, in the
connective tissue within the cranial cavity (fig. 8a, pn), and does not extend outside the skull, as it
appears to do, according to Götte's investigations, in Amphibians. Götte[269] compares the pineal gland with the long persisting
pore which leads into the cavity of the brain in the embryo of Amphioxus,
and we might add the Ascidians, and calls it ein
Umbildungsprodukt einer letzten Verbindung des Hirns mit der Oberhaut.
This suggestion appears to me a very good one, though no facts have come
under my notice which confirm it. The sacci vasculosi are perhaps indicated
at this stage in the two lateral divisions of the trilobed ventricle of the
infundibulum (fig. 8c).
The lateral ventricles (fig. 8a) are now quite separated by a median partition, and a slight external constriction marks the lobes of the two hemispheres; these, however, are still united by nervous structures for the greater part of their extent. The olfactory lobes are formed of a distinct bulb and stalk (fig. 8a, ol.l), and contain, as before, prolongations of the lateral ventricles. The so-called optic chiasma is very distinct (fig. 8b, op.n), but the fibres from the optic nerves appear to me simply to cross and not to intermingle.
The mid-brain. The mid-brain is at first fairly marked off from both the fore and hind brains, but less conspicuously from the latter than from the former. Its roof becomes progressively thinner and its sides thicker up to stage P, its cavity remaining quite simple. The thinness of the roof gives it, in isolated brains of stage P, a bilobed appearance (vide Pl. 16, fig. 4b, mb, in which the distinctness of this character is by no means exaggerated): During stage Q it becomes really bilobed through [Pg 403] the formation in its roof of a shallow median furrow (Pl. 16, fig. 8b). Its cavity exhibits at the same time the indication of a division into a central and two lateral parts.
The hind-brain. The hind-brain has at first a fairly uniform structure, but by the close of stage I, the anterior part becomes distinguished from the remainder by the fact, that its roof does not become thin as does that of the posterior part. This anterior, and at first very insignificant portion, forms the rudiment of the cerebellum. Its cavity is quite simple and is continued uninterruptedly into that of the remainder of the hind-brain. The cerebellum assumes in the course of development a greater and greater prominence, and eventually at the close of stage Q overlaps both the optic lobes in front and the medulla behind (Pl. 16, fig. 7a). It exhibits in surface-views of the hardened brain of stages P and Q the appearance of a median constriction, and the portion of the ventricle contained in it is prolonged into two lateral outgrowths (Pl. 16, figs. 8c and 8d, cb).
The posterior section of the hind-brain which forms the medulla undergoes changes of a somewhat complicated character. In the first place its roof becomes in front very much extended and thinned out. At the raphe, where the two lateral halves of the brain originally united, a separation, as it were, takes place, and the two sides of the brain become pushed apart, remaining united by only a very thin layer of nervous matter (Pl. 15, fig. 6, iv.v.). As a result of this peculiar growth in the brain, the roots of the nerves of the two sides which were originally in contact at the dorsal summit of the brain become carried away from one another, and appear to rise at the sides of the brain (Pl. 15, figs. 6 and 7). Other changes also take place in the walls of the brain. Each lateral wall presents two projections towards the interior (Pl. 15, fig. 5a). The ventral of these vanish, and the dorsal approximate so as nearly to divide the cavity of the hind-brain, or fourth ventricle, into a large dorsal and a small ventral channel (Pl. 15, fig. 6), and this latter becomes completely obliterated in the later stages. The dorsal pair, while approximating, also become more prominent, and stretch into the dorsal moiety of the fourth ventricle (Pl. 15, fig. 6). They are still very prominent at stage Q (Pl. 16, [Pg 404] fig. 8d, ft), and correspond in position with the fasciculi teretes of human anatomy. Part of the root of the seventh nerve originates from them. They project freely in front into the cavity of the fourth ventricle (Pl. 16, fig. 7a, ft).
By stage Q restiform tracts are indistinctly marked off from the remainder of the brain, and are anteriorly continued into the cerebellum, of which they form the peduncles. Near their junction with the cerebellum they form prominent bodies (Pl. 16, fig. 7a, rt), which are regarded by Miklucho-Maclay[270] as representing the true cerebellum.
By stage O the medulla presents posteriorly, projecting into its cavity, a series of lobes which correspond with the main roots (not the branches) of the vagus and glosso-pharyngeal nerves (Pl. 17, fig. 5). There appear to me to be present seven or eight projections: their number cannot however be quite certainly determined. The first of them belongs to the root of the glosso-pharyngeal, the next one is interposed between the glosso-pharyngeal and the first root of the vagus, and is without any corresponding nerve-root. The next five correspond to the five main roots of the vagus. For each projection to which a nerve pertains there is a special nucleus of nervous matter, from which the root springs. These nuclei do not stain like the remainder of the walls of the medulla, and stand out accordingly very conspicuously in stained sections.
The coating of white matter which appeared at the end of stage K, on the exterior of each lateral half of the hind-brain, extends from a point just dorsal to the attachment of the nerve-roots to the ventral edge of the medulla, and is specially connected with the tissue of the upper of the two already described projections into the fourth ventricle.
A rudiment of the tela vasculosa makes its appearance during stage Q, and is represented by the folds in the wall of the fourth ventricle in my figure of that stage (Pl. 16, fig. 7a, tv).
* * * * *
The development of the brain in Elasmobranchii has already been worked out by Professor Huxley, and a brief but in many respects very complete account of it is given in his recent paper [Pg 405] on Ceratodus[271]. He says, pp. 30 and 31, "The development of the cerebral hemispheres in Plagiostome Fishes differs from the process by which they arise in the higher Vertebrata. In a very early stage, when the first and second visceral clefts of the embryo Scyllium are provided with only a few short branchial filaments, the anterior cerebral vesicle is already distinctly divided into the thalamencephalon (from which the large infundibulum proceeds below, and the small tubular peduncle of the pineal gland above, while the optic nerve leaves its sides) and a large single oval vesicle of the hemispheres. On the ventral face of the integument covering these are two oval depressions, the rudimentary olfactory sacs.
As development proceeds the vesicle of the
hemispheres becomes divided by the ingrowth of a median longitudinal
septum, and the olfactory lobes grow out from the posterior lateral regions
of each ventricle thus formed, and eventually rise on to the dorsal faces
of the hemispheres, instead of, as in most Vertebrata, remaining on their
ventral sides. I may remark, that I cannot accept the views of
Miklucho-Maclay, whose proposal to alter the nomenclature of the parts of
the Elasmobranch's brain, appears to me to be based upon a
misinterpretation of the facts of development.
The last sentence of the paragraph brings me to the one part on which it
is necessary to say a few words, viz. the
views of Miklucho-Maclay. His views have not received any general
acceptance, but the facts narrated in the preceding pages shew, beyond a
doubt, that he has 'misinterpreted' the facts of development, and that the
ordinary view of the homology of the parts is the correct one. A comparison
of the figures I have given of the embryo brain with similar figures of the
brain of higher Vertebrates shews this point conclusively. Miklucho-Maclay
has been misled by the large size of the cerebellum, but, as we have seen,
this body does not begin to be conspicuous till late in embryonic life.
Amongst the features of the embryonic brain of Elasmobranchii, the long
persisting unpaired condition of the cerebral hemisphere, upon which so
much stress has already been laid by Professor Huxley, appears to me to be
one of great [Pg 406] importance, and may not improbably be
regarded as a real ancestral feature. Some observations have recently been
published by Professor B. G. Wilder[272]
upon this point, and upon the homologies and development of the olfactory
lobes. Fairly good figures are given to illustrate the development of the
cerebral hemispheres, but the conclusions arrived at are in part opposed to
my own results. Professor Wilder says: The true hemispheres are the
lateral masses, more or less completely fused in the middle line, and
sometimes developing at the plane of union a bundle of longitudinal
commissural fibres. The hemispheres retain their typical condition as
anterior protrusions of the anterior vesicle; but they lie mesiad of the
olfactory lobes, and in Mustelus at least seem to be formed after
them.
The italics are my own. From what has been said above, it is
clear that the statement italicised, for Scyllium at least, completely
reverses the order of development. Still more divergent from my conclusions
are Professor Wilder's statements on the olfactory lobes. He says: The
true olfactory lobe, or rhinencephalon, seems, therefore, to embrace only
the hollow base of the crus, more or less thickened, and more or less
distinguishable from the main mass as a hollow process. The olfactory bulb,
with the more or less elongated crus of many Plagiostomes, seems to be
developed independently, or in connection with the olfactory sack, as are
the general nerves;
and again, But the young and adult brains since
examined shew that the ventricle (i.e. the ventricle of the
olfactory lobe) ends as a rounded cul-de-sac before reaching the
lobe.
The majority of the statements contained in the above quotations are not borne out by my observations. Even the few preparations of which I have given figures, appear to me to prove that (1) the olfactory lobes (crura and bulbs) are direct outgrowths from the cerebral rudiment, and develop quite independently of the olfactory sack; (2) that the ventricle of the cerebral rudiment does not stop short at the base of the crus; (3) that from the bulb a nerve grows out which has a centrifugal growth like other nerves of the body, and places the central olfactory lobe in communication with the peripheral olfactory [Pg 407] sack. In some other Vertebrates this nerve seems hardly to be developed, but it is easily intelligible, that if in the ordinary course of growth the olfactory sack became approximated to the olfactory lobe, the nerve which grew out from the latter to the sack might become so short as to escape detection.
Organs of Sense.
The olfactory organ. The olfactory pit is the latest formed of the three organs of special sense. It appears during a stage intermediate between I and K, as a pair of slight thickenings of the external epiblast, in the normal vertebrate position on the under side of the fore-brain immediately in front of the mouth (Pl. 15, figs. 1 and 2, ol).
The epiblast cells which form this thickening are very columnar, but present no special peculiarities. Each thickened patch of skin soon becomes involuted as a shallow pit, which remains in this condition till the close of the stage K. The epithelium very early becomes raised into a series of folds (Schneiderian folds). These are bilaterally symmetrical, and diverge like the barbs of a feather from a median line (Pl. 15, fig. 14). The nasal pits at the close of stage K are still separated by a considerable interval from the walls of the brain, and no rudiment of an olfactory lobe arises till a later period; but a description of the development of this as an integral part of the brain has already been given, p. 401.
Eye. The eye does not present in its early development any very special features of interest. The optic vesicles arise as hollow outgrowths from the base of the fore-brain (Pl. 15, fig. 3, op.v), from which they soon become partially constricted, and form vesicles united to the base of the brain by comparatively narrow hollow stalks, the rudiments of the optic nerves. The constriction to which the stalk or optic nerve is due takes place from above and backwards, so that the optic nerves open into the base of the front part of the thalamencephalon (Pl. 15, fig. 13a, op.n). After the establishment of the optic nerves, there take place the formation of the lens and the pushing in of the anterior wall of the optic vesicle towards the posterior.
[Pg 408] The lens arises in the usual vertebrate fashion. The epiblast in front of the optic vesicle becomes very much thickened, and then involuted as a shallow pit, which eventually deepens and narrows. The walls of the pit are soon constricted off as a nearly spherical mass of cells enclosing a very small central cavity, in some cases indeed so small as to be barely recognizable (Pl. 15, fig. 7, l). The pushing in of the anterior wall of the optic vesicle towards the posterior takes place in quite the normal manner; but, as has been already noticed by Götte[273] and others, is not a simple mechanical result of the formation of the lens, as is shewn by the fact that the vesicle assumes a flattened form even before the appearance of the lens. The whole exterior of the optic cup becomes invested by mesoblast, but no mesoblastic cells grow in between the lens and the adjoining wall of the optic cup.
Round the exterior of the lens, and around the exterior and interior of the optic cup, there appear membrane-like structures, similar to those already described round the spinal cord and other organs. These membrane-like structures appear with a varying distinctness, but at the close of stage K stand out with such remarkable clearness as to leave no doubt that they are not artificial products (Pl. 15, fig. 13a)[274]. They form the rudiments of the hyaloid membrane and lens capsule. Similar, though less well marked membranes, may often be seen lining the central cavity of the lens and the space between the two walls of the optic cup. The optic cup is at first very shallow, but owing to the rapid growth of the free edge of its walls soon becomes fairly deep. The growth extends to the whole circumference of the walls except the point of entrance of the optic nerve (Pl. 15, fig. 13a), where no growth takes place; here accordingly a gap is left in the walls which forms the well-known choroid slit. While this double walled cup is increasing in size, the wall lining the cavity of the cup becomes thick, and the outer wall very thin (fig. 13a). No further differentiations arise before the close of stage K.
The lens is carried outwards with the growth of the optic cup, leaving the cavity of the cup quite empty. It also grows in size, and its central cavity becomes larger. Still later its anterior [Pg 409] wall becomes very thin, and its posterior wall thick, and doubly convex (fig. 13a). Its changes, however, so exactly correspond to those already known in other Vertebrates, that a detailed description of them would be superfluous.
No mesoblast passes into the optic cup round its edge, but a process of mesoblast, accompanied by a blood-vessel, passes into the space between the lens and the wall of the optic cup through the choroid slit (fig. 13a, ch). This process of tissue is very easily seen, and swells out on entering the optic cup into a mushroom-like expansion. It forms the processus falciformis, and from it is derived the vitreous humour.
About the development of the parts of the eye, subsequently to stage K, I shall not say much. The iris appears during stage O, as an ingrowing fold of both layers of the optic cup with a layer of mesoblast on its outer surface, which tends to close over the front of the lens. Both the epiblast layers comprising the iris are somewhat atrophied, and the outer one is strongly pigmented. At stage O the mesoblast first also grows in between the external skin and the lens to form the rudiment of the mesoblastic structures of the eye in front of the lens. The layer, when first formed, is of a great tenuity.
The points in my observations, to which I attach the greatest importance, are the formation of the lens capsule and the hyaloid membrane; with the development of these may be treated also that of the vitreous humour and rudimentary processus falciformis. The development of these parts in Elasmobranchii has recently been dealt with by Dr Bergmeister[275], and his observations with reference to the vitreous humour and processus falciformis, the discovery of which in embryo Elasmobranchii is due to him, are very complete. I cannot, however, accept his view that the hyaloid membrane is a mesoblastic product. Through the choroid slit there grows, as has been said, a process of mesoblast, the processus falciformis, which on entering the optic cup dilates, and therefore appears mushroom-shaped in section. At the earliest stage (K) a blood-vessel appeared in connection with it, but no vascular structure came under my notice in the later stages. The structure of this process during stage P is shewn in Pl. 17, fig. 6, p.fal.; it [Pg 410] is there seen to be composed of mesoblast-cells with fibrous prolongations. The cells, as has been noticed by Bergmeister, form a special border round its dilated extremity. This process is formed much earlier than the vitreous humour, which is first seen in stage O. In hardened specimens this latter appears either as a gelatinous mass with a meshwork of fibres or (as shewn in Pl. 17, fig. 6) with elongated fibres proceeding from the end of the processus falciformis. These fibres are probably a product of the hardening reagent, but perhaps represent some preformed structure in the vitreous humour. I have failed to detect in it any cellular elements. It is more or less firmly attached to the hyaloid membrane.
On each side of the processus falciformis in stage P a slight fold of the optic cup is to be seen, but folds so large as those represented by Bergmeister have never come under my notice, though this may be due to my not having cut sections of such late embryos as he has. The hyaloid membrane appears long before the vitreous humour as a delicate basement membrane round the inner surface of the optic cup (Pl. 15, fig. 13a), which is perfectly continuous with a similar membrane round the outer surface. In the course of development the hyaloid membrane becomes thicker than the membrane outside the optic cup, with which however it remains continuous. This is very clear in my sections of stage M. By stage O the membrane outside the cup has ceased to be distinguishable, but the hyaloid membrane may nevertheless be traced to the very edge of the cup round the developing iris; but does not unite with the lens capsule. It can also be traced quite to the junction of the two layers of the optic cup at the side of the choroid slit (Pl. 17, fig. 6, hy.m). When the vitreous humour becomes artificially separated from the retina, the hyaloid membrane sometimes remains attached to the former, but at other times retains in preference its attachment to the retina. My observations do not throw any light upon the junction of the hyaloid membrane and lens capsule to form the suspensory ligament, nor have I ever seen (as described by Bergmeister) the hyaloid membrane extending across the free end of the processus falciformis and separating the latter from the vitreous humour. This however probably appears at a period subsequent to the latest one investigated by [Pg 411] me. The lens capsule arises at about the same period as the hyaloid membrane, and is a product of the cells of the lens. It can be very distinctly seen in all the stages subsequent to its first formation. The proof of its being a product of the epiblastic lens, and not of the mesoblast, lies mainly in the fact of there being no mesoblast at hand to give rise to it at the time of its formation, vide Pl. 15, fig. 13a. If the above observations are correct, it is clear that the hyaloid membrane and lens capsule are respectively products of the retina and lens; so that it becomes necessary to go back to the older views of Kölliker and others in preference to the more modern ones of Lieberkühn and Arnold. It would take me too far from my subject to discuss the arguments used by the later investigators to maintain their view that the hyaloid membrane and lens capsule are mesoblastic products; but it will suffice to say that the continuity of the hyaloid membrane over the pecten in birds is no conclusive argument against its retinal origin, considering the great amount of apparently independent growth which membranes, when once formed, are capable of exhibiting.
Bergmeister's and my own observations on the vitreous humour clearly prove that this is derived from an ingrowth through the choroid slit. On the other hand, the researches of Lieberkühn and Arnold on the Mammalian Eye appear to demonstrate that a layer of mesoblast becomes in Mammalia involuted with the lens, and from this the vitreous humour (including the membrana capsulo-pupillaris) is said to be in part formed. Lieberkühn states that in Birds the vitreous humour is formed in a similar fashion. I cannot, however, accept his results on this point. It appears, therefore, that, so far as is known, all groups of Vertebrata, with the exception of Mammalia, conform to the Elasmobranch type. The differences between the types of Mammalia and remaining Vertebrata are, however, not so great as might at first sight appear. They are merely dependent on slight differences in the manner in which the mesoblast enters the optic cup. In the one case it grows in round one specialized part of the edge of the cup, i.e. the choroid slit; in the other, round the whole edge, including the choroid slit. Perhaps the mode of formation of the vitreous humour in Mammalia may be correlated with the early closing of the choroid slit.
[Pg 412] Auditory Organ. With reference to the development of the organ of hearing I have very little to say. Opposite the interval between the seventh and the glosso-pharyngeal nerves the external epiblast becomes thickened, and eventually involuted as a vesicle which remains however in communication with the exterior by a narrow duct. Towards the close of stage K the auditory sack presents three protuberances—one pointing forwards, a second backwards, and a third outwards. These are respectively the rudiments of the anterior and posterior vertical and external horizontal semicircular canals. These rudiments are easily visible from the exterior (Pl. 15, fig. 2).
* * * * *
As has been already pointed out, the epiblast of Elasmobranchii during the early periods of development exhibits no division into an epidermic and a nervous layer, and in accordance with its primitive undifferentiated condition, those portions of the organs of sense which are at this time directly derived from the external integument are formed indiscriminately from the whole, and not from an inner or so-called nervous part of it only. In the Amphibians the auditory sack and lens are derived from the nervous division of the epiblast only, while the same division of the layer plays the major part in forming the olfactory organ. It is also stated that in Birds and Mammals the part of the epiblast corresponding to the nervous layer is alone concerned in the formation of the lens, though this does not appear to be the case with the olfactory or auditory organs in these groups of Vertebrates.
Mouth involution and Pituitary body.
The development of the mouth involution and the pituitary body is closely related to that of the brain, and may conveniently be dealt with here. The epiblast in the angle formed by the cranial flexure becomes involuted as a hollow process situated in close proximity to the base of the brain. This hollow process is the mouth involution, and it is bordered on its posterior surface by the front wall of the alimentary tract, and on its anterior by the base of the fore-brain.
[Pg 413] The uppermost end of this does not till near the close of stage K become markedly constricted off from the remainder, but is nevertheless the rudiment of the pituitary body. Pl. 15, figs. 9a and 12, m shew in a most conclusive manner the correctness of the above account, and demonstrate that it is from the mouth involution, and not, as has usually been stated, from the alimentary canal, that the pituitary body is derived.
This fact was mentioned in my preliminary account of Elasmobranch development[276]; and has also been shewn to be the case in Amphibians by Götte[277]; and in Birds by Mihalkowics[278]. The fact is of considerable importance with reference to speculations as to the meaning of this body.
Plate 15, fig. 7 represents a transverse section through the head during a stage between I and K; but, owing to the cranial flexure, it cuts the fore part of the head longitudinally and horizontally, and passes through both the fore-brain (fb) and the hind-brain (iv.v.). Close to the base of the fore-brain are seen the mouth (m), and the pituitary involution from this (pt.). In contact with the pituitary involution is the blind anterior termination of the throat, which a little way back opens to the exterior by the first visceral cleft (I. v.c.). This figure alone suffices to demonstrate the correctness of the above account of the pituitary body; but the truth of this is still further confirmed by other figures on the same plate (figs. 9a and 12, m); in which the mouth involution is in contact with, but still separated from, the front end of the alimentary tract. By the close of stage K, the septum between the mouth and throat becomes pierced, and the two are placed in communication. This condition is shewn in Pl. 15, fig. 16a, and Pl. 16, figs. 1a, 1c, pt. In these figures the pituitary involution has become very partially constricted off from the mouth involution, though still in direct communication with it. In later stages the pituitary involution becomes longer and dilated terminally, while the passage connecting it with the mouth becomes narrower [Pg 414] and narrower, and is finally reduced to a solid cord, which in its turn disappears. The remaining vesicle then becomes divided into lobes, and connects itself closely with the infundibulum (Pl. 16, figs. 5 and 6 pt). The later stages for Elasmobranchii are fully described by W. Müller in his important memoir on the Comparative Anatomy and development of this organ[279].
Development of the Cranial Nerves.
The present section deals with the whole development (so far as I have succeeded in elucidating it) of the cranial nerves (excluding the optic and olfactory nerves and the nerves of the eye-muscles) from their first appearance to their attainment of the adult condition. My description commences with the first development of the nerves, to this succeeds a short description of the nerves in the adult Scyllium, and the section is completed by an account of the gradual steps by which the adult condition is attained.
Early Development of the Cranial Nerves.—Before the close of stage H the more important of the cranial nerves make their appearance. The fifth and the seventh are the first to be formed. The fifth arises by stage G (Pl. 15, fig. 3, V), near the anterior end of the hind-brain, as an outgrowth from the extreme dorsal summit of the brain, in identically the same way as the dorsal root of a spinal nerve.
The roots of the two sides sprout out from the summit of the brain, in contact with each other, and grow ventralwards, one on each side of the brain, in close contact with its walls. I have failed to detect more than one root for the two embryonic branches of the fifth (ophthalmic and mandibular), and no trace of an anterior or ventral root has been met with in any of my sections.
The seventh nerve is formed nearly simultaneously with or shortly after the fifth, and some little distance behind and independently of it, opposite the anterior end of the thickening of the epiblast to form the auditory involution. It arises precisely [Pg 415] like the fifth, from the extreme dorsal summit of the neural axis (Pl. 15, fig. 4a, VII). So far as I have been able to determine, the auditory nerve and the seventh proper possess only a single root common to the two. There is no anterior root for the seventh any more than for the fifth.
Behind the auditory involution, at a stage subsequent to that in which the fifth and seventh nerves appear, there arise a series of roots from the dorsal summit of the hind-brain, which form the rudiments of the glosso-pharyngeal and vagus nerves. These roots are formed towards the close of stage H, but are still quite short at the beginning of stage I. Their manner of development resembles that of the previously described cranial nerves. The central ends of the roots of the opposite sides are at first in contact with each other, and there is nothing to distinguish the roots of the glosso-pharyngeal and of the vagus nerves from the dorsal roots of spinal nerves. Like the dorsal roots of the spinal nerves, they appear as a series of ventral prolongations of a continuous outgrowth from the brain, which outgrowth is moreover continuous with that for the spinal nerves[280]. The outgrowth of the vagus and glosso-pharyngeal nerves is not continuous with that of the seventh nerve. This is shewn by Pl. 15, figs. 4a and 4b. The outgrowth of the seventh nerve though present in 4a is completely absent in 4b which represents a section just behind 4a.
Thus, by the end of stage I, there have appeared the rudiments of the 5th, 7th, 8th, 9th and 10th cranial nerves, all of which spring from the hind-brain. These nerves all develop precisely as do the posterior roots of the spinal nerves, and it is a remarkable fact that hitherto I have failed to find a trace in the brain of a root of any cranial nerve arising from the ventral corner of the brain as do the anterior roots of the spinal nerves[281].
[Pg 416] It is admittedly difficult to prove a negative, and it may still turn out that there are anterior roots of the brain similar to those of the spinal cord; in the mean time, however, the balance of evidence is in favour of there being none such. This at first sight appears a somewhat startling conclusion, but a little consideration shews that it is not seriously opposed to the facts which we know. In the first place it has been shewn by myself[282] that in Amphioxus (whose vertebrate nature I cannot doubt) only dorsal nerve-roots are present. Yet the nerves of Amphioxus are clearly mixed motor and sensory nerves, and it appears to me far more probable that Amphioxus represents a phase of development in which the nerves had not acquired two roots, rather than one in which the anterior root has been lost. In other words, the condition of the nerves in Amphioxus appears to me to point to the conclusion that primitively the cranio-spinal nerves of vertebrates were nerves of mixed function with one root only, and that root a dorsal one; and that the present anterior or ventral root is a secondary acquisition. This conclusion is further supported by the fact that the posterior roots develop in point of time before the anterior roots. If it be admitted that the vertebrate nerves primitively had only a single root, then the retention of that condition in the brain implies that this became differentiated from the remainder of the nervous system at a very early period before the acquirement of anterior nerve-roots, and that these eventually become developed only in the case of spinal nerves, and not in the case of the already highly modified cranial nerves.
* * * * *
Subsequent Changes of the Nerves. To simplify my description of the subsequent growth of the cranial nerves, I have inserted a short description of their distribution in the adult. [Pg 417] This is taken from a dissection of Scyllium stellare, which like other species has some individualities of its own not found in the other Elasmobranchii. For points not touched on in this description I must refer the reader to the more detailed accounts of my predecessors, amongst whom may specially be mentioned Stannius[283] for Carcharias, Spinax, Raja, Chimæra, &c.; Gegenbaur[284] for Hexanchus; Jackson and Clarke[285] for Echinorhinus.
The ordinary nomenclature has been employed for the branches of the fifth and seventh nerves, though embryological data to be adduced in the sequel throw serious doubts upon it. Since I am without observations on the origin of the nerves to the muscles of the eyes, all account of these is omitted.
The fifth nerve arises from the brain by three roots[286]: (1) an anterior more or less ventral root; (2) a root slightly behind, but close to the former[287], formed by the coalescence of two distinct strands, one arising from a dorsal part of the medulla, and a second and larger from the ventral; (3) a dorsal and posterior root, in its origin quite distinct and well separated from the other two, and situated slightly behind the dorsal strand of the second root. This root a little way from its attachment becomes enclosed for a short distance in the same sheath as the dorsal part of the second root, and a slight mixture of fibres seems to occur, but the majority of its fibres have no connection with those of the second root. The first and second roots of the fifth appear to me partially to unite, but before their junction the ramus ophthalmicus profundus is given off from the first of them.
The fifth nerve, according to the usual nomenclature,
has three main divisions. The first of these is the ophthalmic. It is
formed by the coalescence of two entirely independent branches of the
fifth, which unite on leaving the orbit. The dorsalmost of these, or ramus
ophthalmicus superficialis, originates from the third and posterior of the
roots of the fifth, nearly the whole of which appears to enter into its
formation. This root is situated on the dorsal part of the lobi
trigemini,
at a point posterior to that of the other roots of the
fifth or even of the seventh nerve. The branch itself enters the orbit
by a separate foramen, and, keeping on the dorsal side of it, reenters the
cartilage at its anterior wall, and is there joined by the ramus
ophthalmicus profundus. This latter nerve arises from the anterior
root of the fifth, separately pierces the wall of the orbit, and takes a
course slightly ventral to the superior ophthalmic nerve, but does not (as
is usual with Elasmobranchii) [Pg 418] run below the superior rectus and superior
oblique muscles of the eye. The nerve formed by the coalescence of the
superficial and deep ophthalmic branches courses a short way below the
surface, and supplies the mucous canals of the front of the snout. It is a
purely sensory nerve. Strong grounds will be adduced in the sequel for
regarding the ramus ophthalmicus superficialis, though not the
ophthalmicus profundus, as in reality a branch of the seventh, and
not of the fifth nerve.
The second division of the fifth nerve is the superior maxillary, which appears to me to arise from both the first and second roots of the fifth, though mainly from the first. It divides once into two main branches. The first of these—the buccal nerve of Stannius—after passing forwards along the base of the orbit takes its course obliquely across the palatine arch and behind and below the nasal sack, supplying by the way numerous mucous canals, and dividing at last into two branches, one of these passing directly forwards on the ventral surface of the snout, and the second keeping along the front border of the mouth. The second division of the superior maxillary nerve (superior maxillary of Stannius), after giving off a small branch, which passes backwards in company with a branch from the inferior maxillary nerve to the levator maxillæ superioris, itself keeps close to the buccal nerve, and eventually divides into numerous fine twigs to the mucous canals of the skin at the posterior region of the upper jaw. It anastomoses with the buccal nerve. The inferior maxillary nerve arises mainly from the second root of the fifth. After sending a small branch to the levator maxillæ superioris, it passes outwards along the line separating the musculus adductor mandibulæ from the musculus levator labii superioris, and after giving branches to these muscles takes a course forward along the border of the lower jaw. It appears to be a mixed motor and sensory nerve.
The seventh or facial nerve arises by a root close to, but behind and below the second root of the fifth, and is intimately fused with this. It divides almost at once into a small anterior branch and large posterior.
The anterior branch is the palatine nerve. It gives off at first one or two very small twigs, which pursue a course towards the spiracle, and probably represent the spiracular nerves of other Elasmobranchii. Immediately after giving off these branches it divides into two stems, a posterior smaller and an anterior larger one. The former eventually takes a course which tends towards the angle of the jaw, and is distributed to the mucous membrane of the roof of the mouth, while the larger one bends forwards and supplies the mucous membrane at the edge of the upper jaw. The main stem of the seventh, after giving off a branch to the dorsal section of the musculus constrictor superficialis, passes outwards to the junction of the upper and lower jaws, where it divides into two branches, an anterior superficial branch, which runs immediately below the skin on the surface of the lower jaw, and a second branch, which takes a deep course along the posterior border of the lower jaw, between it and the hyoid, and sends a series of branches backwards to the ventral section of the musculus constrictor superficialis. The main stem of the facial is mixed motor and sensory. I have [Pg 419] not noticed a dorsal branch, similar to that described by Jackson and Clarke.
The auditory nerve arises immediately behind the seventh, but requires no special notice here. A short way behind the auditory is situated the root of the glossopharyngeal nerve. This nerve takes an oblique course backwards through the skull, and gives off in its passage a very small dorsal branch, which passes upwards and backwards through the cartilage towards the roof of the skull. At the point where the main stem leaves the cartilage it divides into two branches, an anterior smaller branch to the hinder border of the hyoid arch, and a posterior and larger one to anterior border of the first branchial arch. It forks, in fact, over the first visceral cleft.
The vagus arises by a great number of distinct strands from the sides of the medulla. In the example dissected there were twelve in all. The anterior three of these were the largest; the middle one having the most ventral origin. The next four were very small and in pairs, and were separated by a considerable interval from the next four, also very small, and these again by a marked interval from the hindermost strand.
The common stem formed by the junction of these gives off immediately on leaving the skull a branch which forks on the second branchial cleft; a second for the third cleft is next given off; the main stem then divides into a dorsal branch—the lateral nerve—and a ventral one—the branchio-intestinal nerve—which, after giving off the branches for the two last branchial clefts, supplies the heart and intestinal tract. The lateral nerve passes back towards the posterior end of the body, internal to the lateral line, and between the dorso-lateral and ventro-lateral muscles. It gives off at its origin a fine nerve, which has a course nearly parallel to its own. The main stem of the vagus, at a short distance from its central end, receives a nerve which springs from the ventral side of the medulla, on about a level with the most posterior of the true roots of the vagus. This small nerve corresponds with the ventral or anterior roots of the vagus described by Gegenbaur, Jackson, and Clarke (though in the species investigated by the latter authors these roots did not join the vagus, but the anterior spinal nerves). Similar roots are also mentioned by Stannius, who found two of them in the Elasmobranchii dissected by him; it is possible that a second may be present in Scyllium, but have been overlooked by me, or perhaps may have been exceptionally absent in the example dissected.
The Fifth Nerve. The thinning of the roof of the brain, in the manner already described, produces a great change in the apparent position of the roots of all the nerves. The central ends of the rudiments of the two sides are, as has been mentioned, at first in contact dorsally but, when by the growth of the roof of the brain its two lateral halves become pushed apart, the nerves also shift their position and become widely separated. The roots of the fifth nerve are so influenced by these changes [Pg 420] that they spring from the brain about half way up its sides, and a little ventral to the border of its thin roof. While this change has been taking place in the point of attachment of the fifth nerve, it has not remained in other respects in a stationary condition.
During stage H it already exhibits two distinct branches known as the mandibular and ophthalmic. These branches first lie outside a section of the body-cavity which exists in the front part of the head. The ophthalmic branch of the fifth being situated near the anterior end of this, and the mandibular near the posterior end.
In stage I the body-cavity in this part becomes divided into two parts one behind the other, the posterior being situated in the mandibular arch. The bifurcation of the nerve then takes place over the summit of the posterior of the two divisions of the body-cavity, Pl. 15, figs. 9b, V, and 10, V, &c., and at first both branches keep close to the sides of this.
The anterior or ophthalmic branch of the fifth soon leaves the walls of the cavity just spoken of and tends towards the eye, and there comes in close contact with the most anterior section of the body-cavity which exists in the head. These relations it retains unchanged till the close of stage K. Between stages I and K it may easily be seen from the surface; but, before the close of stage K, the increased density of the tissues renders it invisible in the living embryo.
The posterior branch of the fifth extends downwards into the mandibular arch in close contact with the posterior and outer wall of the body space already alluded to. At first no branches from it can be seen, but I have detected by the close of stage K, by an examination of the living embryo, a branch springing from it a short way from its central extremity, and passing forwards, Pl. 15, fig. 2, V This branch I take to be the rudiment of the superior maxillary division of the fifth nerve. It is shewn in section, Pl. 15, fig. 15a, V.
In the stages after K the anatomy of the nerves becomes increasingly difficult to follow, and accordingly I must plead indulgence for the imperfections in my observations on all the nerves subsequently to this date. In the fifth I find up to stage O a single ophthalmic branch (Pl. 17, fig. 4b, V. op.th.), [Pg 421] which passes forwards slightly dorsal to the eye and parallel and ventral to a branch of the seventh, which will be described when I come to that nerve. I have been unable to observe that this branch divides into a ramus superficialis and ramus profundus, and subsequently to stage O I have no observations on it.
By stage O the fifth may be observed to have two very distinct roots, and a large ganglionic mass is developed close to their junction (Gasserian ganglion), Pl. 17, fig. 4a. But in addition to this ganglionic enlargement, all of the branches have special ganglia of their own, Pl. 17, fig. 4b.
Summary. The fifth nerve has almost from the beginning two branches, the ophthalmic (probably the inferior ophthalmic of the adult) and the inferior maxillary. The superior maxillary nerve arises later than the other two as a branch from the inferior, originating comparatively far from its root. There is at first but a single root for the whole nerve, which subsequently becomes divided into two. Ganglionic swellings are developed on the common stem and main branches of the nerve.
A general view of the nerve is shewn in the diagram in Pl. 17, fig. 1.
* * * * *
Seventh and Auditory Nerves. There appears in my earliest sections a single large rudiment in the position of the seventh and auditory nerves; but in longitudinal sections of an embryo somewhat older than stage I, in which the auditory organ forms a fairly deep pit, still widely open to the exterior, there are to be seen immediately in front of the ear the rudiments of two nerves, which come into contact where they join the brain and have their roots still closely connected at the end of stage K (Pl. 15, figs. 10 and 15a and 15b). The anterior of these pursues a straight course to the hyoid arch (Pl. 15, fig. 10, VII), the second of the two (Pl. 15, fig. 10, au.n.), which is clearly the rudiment of the auditory nerve, develops a ganglionic enlargement and, turning backward, closely hugs the ventral wall of the auditory involution.
The observation just recorded appears to lead to the following conclusions with reference to the development of the auditory nerve. A single rudiment arises from the brain for the auditory and seventh nerves. This rudiment subsequently [Pg 422] becomes split into two parts, an anterior to form the seventh nerve, and a posterior to form the auditory nerve. The ganglionic part of the auditory nerve is derived from the primitive outgrowths from the brain, and not from the auditory involution. I do not feel perfectly confident that an independent origin of the auditory nerve might not have escaped my notice; but, admitting the correctness of the view which attributes to the seventh and auditory a common origin, it follows that the auditory nerve primitively arose in connection with the seventh, of which it may either, as Gegenbaur believes, be a distinct part—the ramus dorsalis—or else may possibly have formed part of a commissure, homologous with that uniting the dorsal roots of the spinal nerves, connecting the seventh with the glossopharyngeal nerve. In either case it must be supposed secondarily to have become separate and independent in consequence of the development of the organ of hearing.
My sections of embryos of stage K and the subsequent stages do not bring to light many new facts with reference to the auditory nerve: they demonstrate however that its ganglionic part increases greatly in size, and in stage O there is a distinct root for the auditory nerve in contact with that for the seventh.
The history of the seventh nerve in its later stages presents points of great interest. Near the close of stage K there may be observed, in the living embryos and in sections, two branches of the seventh in addition to the original trunk to the hyoid arch, both arising from its anterior side; one passes straight forwards close to the external skin, but is at first only traceable a short way in front of the fifth, and a second passes downwards into the mandibular arch in such a fashion, that the seventh nerve forks over the hyomandibular cleft (vide Pl. 15, fig. 2, VII.; 15a, VII.). My sections shew both these branches with great clearness. A third branch has also come under my notice, whose course leads me to suppose that it supplies the roof of the palate.
In the later stages my attention has been specially directed to the very remarkable anterior branch of the seventh. This may, in stages L to O, be traced passing on a level with the root of the fifth nerve above the eye, and apparently terminating [Pg 423] in branches to the skin in front of the eye (Pl. 17, figs. 3, VII.; 4a, VII, a). It courses close beneath the skin (though this does not appear in the sections represented on account of their obliqueness), and runs parallel and dorsal to the ophthalmic branch of the fifth nerve, and may easily be seen in this position in longitudinal sections belonging to stage O; but its changes after this stage have hitherto baffled me, and its final fate is therefore, to a certain extent, a matter of speculation.
The two other branches of the seventh, viz., the hyoid or main branch and mandibular branch, retain their primitive arrangement till the close of stage O.
The fate of the remarkable anterior branch of the seventh nerve is one of the most interesting points which has started up in the course of my investigations on the development of the cranial nerves, and it is a matter of very great regret to me that I have not been able to clear up for certain its later history.
Its primitive distribution leads to the supposition that it becomes the nerve known in the adult as the ramus ophthalmicus[TN10] superficialis of the fifth nerve, and this is the view which I admit myself to be inclined to adopt. There are several points in the anatomy of this nerve in the adult which tell in favour of accepting this view with reference to it. In the first place, the ramus ophthalmicus superficialis rises from the brain (vide description above, p. 417), quite independently of the ramus ophthalmicus profundus, and not in very close connection with the other branches of the fifth, and also considerably behind these, quite as far back indeed as the ventral root of the seventh. There is therefore nothing in the position of its root opposed to its being regarded as a branch of the seventh nerve. Secondly, its distribution, which might at first sight be regarded as peculiar, presents no very strange features if it is looked on as a ramus dorsalis of the seventh, whose apparent anterior instead of dorsal course is due to the cranial flexure. If, however, the distribution of the ramus ophthalmicus superficialis is used as an argument against my view, a satisfactory reply is to be found in the fact that a branch of the seventh nerve certainly has the distribution in question in the embryo, and that there is no reason why it should not retain it in the adult.
[Pg 424] Finally, the junction of the two rami ophthalmici, most remarkable if they are branches of a single nerve, would present nothing astonishing when they are regarded as branches of two separate nerves.
If this view be adopted, certain modifications of the more generally accepted views of the morphology of the cranial nerves will be necessitated; but this subject is treated of at the end of this section.
Some doubt hangs over the fate of the other branches of the seventh nerve, but their destination is not so obscure as that of the anterior branch. The branch to the roof of the mouth can be at once identified as the 'palatine nerve', and it only remains to speak of the mandibular branch.
It may be noticed first of all with reference to this branch, that the seventh behaves precisely like the less modified succeeding cranial nerves. It forks in fact over a visceral cleft (the hyomandibular) the two sides of which it supplies; the branch at the anterior side of the cleft is the later developed and smaller of the two. There cannot be much doubt that the mandibular branch must be identified with the spiracular nerve (præ-spiracular branch Jackson and Clarke) of the adult, and if the chorda tympani of Mammals is correctly regarded as the mandibular branch of the seventh nerve, then the spiracular nerve must represent it. Jackson and Clarke[288] take a different view of the homology of the chorda tympani, and regard it as equivalent to the ramus mandibularis internus (one of the two branches into which the seventh eventually divides), because this nerve takes its course over the ligament connecting the mandible with the hyoid. This view I cannot accept so long as it is admitted that the chorda tympani is the branch of a cranial nerve supplying the anterior side of a cleft. The ramus mandibularis internus, instead of forming with the main branch of the seventh a fork over the spiracle, passes to its destination completely behind and below the spiracle, and therefore fails to fulfil the conditions requisite for regarding it as a branch to the anterior wall of a visceral cleft. It is indeed clear that the ramus mandibularis internus cannot be identified with the embryonic mandibular branch of the seventh (which passes above the spiracle or [Pg 425] hyomandibular cleft) when there is present in the adult another nerve (the spiracular nerve), which exactly corresponds in distribution with the embryonic nerve in question. My view accords precisely with that already expressed by Gegenbaur in his masterly paper on the nerves of Hexanchus, in which he distinctly states that he looks upon the spiracular nerve as the homologue of an anterior branchial branch of a division of the vagus. In the adult the spiracular nerve is sometimes represented by one or two branches of the palatine, e.g. Scyllium, but at other times arises independently from the main stem of the seventh[289]. The only difficulty in my identification of the embryonic mandibular branch with the adult spiracular nerve, is the extremely small size of the latter in the adult, compared with the size of mandibular in the embryo; but it is hardly surprising to find an atrophy of the spiracular nerve accompanying an atrophy of the spiracle itself. The palatine appears to me to have been rightly regarded by Jackson and Clarke as the great superficial petrosal of Mammals.
On the common root of the branches of the seventh nerve, as well as on its hyoid branch, ganglionic enlargements are present at an early period of development.
The Glossopharyngeal and Vagus Nerves. Behind the ear there are formed a series of five nerves which pass down to respectively the first, second, third, fourth and fifth visceral arches.
For each arch there is thus one nerve, whose course lies close to the posterior margin of the preceding cleft, a second anterior branch being developed later. These nerves are connected with the brain (as I have determined by transverse sections) by roots at first attached to the dorsal summit, but eventually situated about half-way down the sides (Pl. 15, fig. 6) nearly opposite the level of the process which divides the ventricle of the hind-brain into a dorsal and a ventral moiety. The foremost of these nerves is the glossopharyngeal. The next four are, as has been shewn by Gegenbaur[290], equivalent to four independent nerves, but form, together with the glossopharyngeal, a compound nerve, which we may briefly call the vagus.
[Pg 426] This compound nerve by stage K attains a very complicated structure, and presents several remarkable and unexpected features. Since it has not been possible for me completely to elucidate the origin of all its various parts, it will conduce to clearness if I give an account of its structure during stage K or L, and then return to what facts I can mention with reference to its development. Its structure during these stages is represented on the diagram, Pl. 17, fig. 1. There are present five branches, viz. the glossopharyngeal and four branches of the vagus, arising probably by a considerably greater number of strands from the brain[291]. All the strands from the brain are united together by a thin commissure, Vg.com., continuous with the commissure of the posterior roots of the spinal nerves, and from this commissure the five branches are continued obliquely ventralwards and backwards, and each of them dilates into a ganglionic swelling. They all become again united together by a second thick commissure, which is continued backwards as the intestinal branch of the vagus nerve Vg.in. The nerves, however, are continued ventralwards each to its respective arch. From the hinder part of the intestinal nerve springs the lateral nerve n.l., at a point whose relations to the branches of the vagus I have not certainly determined.
The whole nerve-complex formed by the glossopharyngeal and the vagus nerves cannot of course be shewn in any single section. The various roots are shewn in Pl. 17, fig. 5. The dorsal commissure is represented in longitudinal section in Pl. 15, fig. 15b, com., and in transverse section in Pl. 17, fig. 2, Vg.com. The lower commissure continued as the intestinal nerve is shewn in Pl. 15, fig. 15a, Vg., and as seen in the living embryo in Pl. 15, figs. 1 and 2. The ganglia are seen in Pl. 15, fig. 6, Vg. The junction of the vagus and glossopharyngeal nerves is shewn in Pl. 15, fig. 10. My observations have not taught me much with reference to the origin of the two commissures, viz. the dorsal one and the one which forms the intestinal branch of the vagus. Very possibly they originate as a single commissure which becomes longitudinally segmented. It deserves to be noticed that the dorsal commissure has a long stretch, from [Pg 427] the last branch of the vagus to the first spinal nerve, during which it is not connected with the root of any nerve; vide fig. 15b, com. This space probably contained originally the now lost branches of the vagus. In many transverse sections where the dorsal commissure might certainly be expected to be present it cannot be seen, but this is perhaps due to its easily falling out of the sections. I have not been able to prove that the commissure is continued forwards into the auditory nerve.
The relation of the branches of the vagus and glossopharyngeal to the branchial clefts requires no special remark. It is fundamentally the same in the embryo as in the adult. The branches at the posterior side of the clefts are the first to appear, those at the anterior side of the clefts being formed subsequently to stage K.
One of the most interesting points with reference to the vagus is the number of separate strands from the brain which unite to form it. The questions connected with these have been worked out in a masterly manner, both from an anatomical and a theoretical standpoint, by Professor Gegenbaur[292]. It has not been possible for me to determine the exact number of these in my embryos, nor have I been able to shew whether they are as numerous at the earliest appearance of the vagus as at a later embryonic period. The strands are connected (Pl. 17, fig. 5) with separate ganglionic centres in the brain, though in several instances more than one strand is connected with a single centre. In an embryo between stage O and P more than a dozen strands are present. In an adult Scyllium I counted twelve separate strands, but their number has been shewn by Gegenbaur to be very variable. It is possible that they are remnants of the roots of the numerous primary branches of the vagus which have now vanished; and this perhaps is the explanation of their variability, since in the case of all organs which are on the way to disappear variability is a precursor of disappearance.
A second interesting point is the presence of the two connecting commissures spoken of above. It was not till comparatively late in my investigations that I detected the dorsal one. This has clearly the same characters as the dorsal commissure already [Pg 428] described as connecting the roots of all the spinal nerves, and is indeed a direct prolongation of this. It becomes gradually thinner and thinner, and finally ceases to be observable by about the close of stage L. It is of importance as shewing the similarity of the branches of the vagus to the dorsal roots of the spinal nerves. The ventral of the two commissures persists in the adult as the common stem from which all the branches of the vagus successively originate, and is itself continued backwards as the intestinal branch of the vagus. The glossopharyngeal nerve alone becomes eventually separated from the succeeding branches. Stannius and Gegenbaur have, as was mentioned above, detected in adult Elasmobranchii roots which join the vagus, and which resemble the anterior or ventral roots of spinal nerves; and I have myself described one such root in the adult Scyllium. I have searched for these in my embryos, but without obtaining conclusive results. In the earliest stages I can find no trace of them, but I have detected in stage L one anterior root on debatable border-land, which may conceivably be the root in question, but which I should naturally have put down for the root of a spinal nerve. Are the roots in question to be regarded as proper roots of the vagus, or as ventral roots of spinal nerves whose dorsal roots have been lost? The latter view appears to me the most probable one, partly from the embryological evidence furnished by my researches, which is clearly opposed to the existence of anterior roots in the brain, and partly from the condition of these roots in Echinorhinus, in which they join the succeeding spinal nerves and not the vagus[293]. The similar relations of the apparently homologous branch or branches in many Osseous Fish may also be used as an argument for my view.
If, as seems probable, the roots in question become the hypoglossal nerve, this nerve must be regarded as formed from the anterior roots of one or more spinal nerves. Without embryological evidence it does not however seem possible to decide whether the hypoglossal nerve contains elements only of anterior roots or of both anterior and posterior roots.
[Pg 429] Mesoblast of the Head.
Body-Cavity and Myotomes of the Head.—During stage F the appearance of a cavity on each side in the mesoblast of the head was described. (Vide Pl. 10, figs. 3b and 6, pp.) These cavities end in front opposite the blind anterior extremity of the alimentary canal; behind they are continuous with the general body-cavity. I propose calling them the head-cavities. The cavities of the two sides have no communication with each other.
Coincidently with the formation of an outgrowth from the throat to form the first visceral cleft, the head-cavity on each side becomes divided into a section in front of the cleft and a section behind the cleft (vide Pl. 15, figs. 4a and 4b, pp.); and during stage H it becomes, owing to the formation of a second cleft, divided into three sections: (1) a section in front of the first or hyomandibular cleft; (2) a section in the hyoid arch between the hyomandibular cleft and the hyobranchial or first branchial cleft; (3) a section behind the first branchial cleft.
The section in front of the hyomandibular cleft stands in a peculiar relation to the two branches of the fifth nerve. The ophthalmic branch of the fifth lies close to the outer side of its anterior part, the mandibular branch close to the outer side of its posterior part. During stage I this front section of the head-cavity grows forward, and becomes divided, without the intervention of a visceral cleft, into an anterior and posterior division. The anterior lies close to the eye, and in front of the commencing mouth involution, and is connected with the ophthalmic branch of the fifth nerve. The posterior part lies completely within the mandibular arch, and is closely connected with the mandibular division of the fifth nerve.
As the rudiments of the successive visceral clefts are formed, the posterior part of the head-cavity becomes divided into successive sections, there being one section for each arch. Thus the whole head-cavity becomes on each side divided into (1) a premandibular section; (2) a mandibular section; (3) a hyoid section; (4) sections in the branchial arches.
The first of these divisions forms a space of a considerable size, with epithelial walls of somewhat short columnar cells. It [Pg 430] is situated close to the eye, and presents a rounded or sometimes triangular figure in sections (Pl. 15, figs. 7, 9b and 16b, 1pp.). The ophthalmic branch of the fifth nerve passes close to its superior and outer wall.
Between stages I and K the anterior cavities of the two sides are prolonged ventralwards and meet below the base of the fore-brain (Pl. 15, fig. 8, 1pp.). The connection between the two cavities appears to last for a considerable time, and still persists at the close of stage L. The anterior or premandibular pair of cavities are the only parts of the body-cavity within the head which unite ventrally. In the trunk, however, the primitively independent lateral halves of the body-cavity always unite in this way. The section of the head-cavity just described is so similar to the remaining posterior sections that it must be considered as equivalent to them.
The next division of the head-cavity, which from its position may be called the mandibular cavity, presents during the stages I and K a spatulate shape. It forms a flattened cavity, dilated dorsally, and produced ventrally into a long thin process parallel to the hyomandibular gill-cleft, Pl. 15, fig. 1pp. and fig. 7, 9b and 15a, 2pp. Like the previous space it is lined by a short columnar epithelium.
The fifth nerve, as has already been mentioned, bifurcates over its dorsal summit, and the mandibular branch of that nerve passes down on its posterior and outer side. The mandibular aortic arch is situated close to its inner side, Pl. 15, fig. 7. Towards the close of this period the upper part of the cavity atrophies. Its lower part also becomes much narrowed, but its walls of columnar cells persist and lie close to one another. The outer or somatic wall becomes very thin indeed, the splanchnic wall, on the other hand, thickens and forms a layer of several rows of elongated cells. This thicker wall is on its inner side separated from the surrounding tissue by a small space lined by a membrane-like structure. In each of the remaining arches there is a segment of the original body-cavity fundamentally similar to that in the mandibular arch. A dorsal dilated portion appears, however, to be present in the third or hyoid section alone, and even there disappears by the close of stage K. The cavities in the posterior parts of the head become much reduced [Pg 431] like those in its anterior part, though at rather a later period. Their walls however persist, and become more columnar. In Pl. 15, fig. 13b, pp., is represented the cavity in the last arch but one, at a period when the cavity in the mandibular arch has become greatly reduced. It occupies the same position on the outer side of the aortic trunk of its arch as does the cavity in the mandibular arch (Pl. 15, fig. 7, 2pp). In Torpedo embryos the head-cavity is much smaller, and atrophies earlier than in the embryos of Pristiurus and Scyllium.
It has been shewn that, with the exception of the most anterior, the divisions of the body-cavity in the head become atrophied, not so however their walls. The cells forming these become elongated, and by stage N become distinctly developed into muscles. Their exact history I have not followed in its details, but they almost unquestionably become the musculus constrictor superficialis and musculus interbranchialis[294]; and probably also musculus levator mandibuli and other muscles of the front part of the head.
The most anterior cavity close to the eye remains unaltered much longer than the remaining cavities, and its two halves are still in communication at the close of stage L. I have not yet succeeded in tracing the subsequent fate of its walls, but think it probable that they develop into the muscles of the eye. The morphological importance of the sections of the body-cavity in the head cannot be over-estimated, and the fact that the walls become developed into the muscular system of the head renders it almost certain that we must regard them as equivalent to the muscle-plates of the body, which originally contain, equally with those of the head, sections of the body-cavity. If this determination is correct, there can be no doubt that they ought to serve as valuable guides to the number of segments which have coalesced to form the head. This point is, however, discussed in a subsequent section.
General mesoblast of the head.—In stage G no mesoblast is present in the head, except that which forms the walls of the head-cavity.
During stage H a few cells of undifferentiated connective [Pg 432] tissue appear around the stalk of the optic vesicle, and in the space between the front end of the alimentary tract and the base of the brain in the angle of the cranial flexure. They are probably budded off from the walls of the head-cavities. Their number rapidly increases, and they soon form an investment surrounding all the organs of the head, and arrange themselves as a layer, between the walls of the roof of the fore and mid-brain and the external skin. At the close of stage K they are still undifferentiated and embryonic, each consisting of a large nucleus surrounded by a very delicate layer of protoplasm produced into numerous thread-like processes. They form a regular meshwork, the spaces of which are filled up by an intercellular fluid.
I have not worked out the development of the cranial and visceral skeleton; but this has been made the subject of an investigation by Mr Parker, who is more competent to deal with it than any other living anatomist. His results were in part made known in his lectures before the Royal College of Surgeons[295], and will be published in full in the Transactions of the Zoological Society.
All my efforts have hitherto failed to demonstrate any segmentation in the mesoblast of the head, other than that indicated by the sections of the body-cavity before-mentioned; but since these, as above stated, must be regarded as equivalent to muscle-plates, any further segmentation of mesoblast could not be anticipated. To this statement the posterior part of the head forms an apparent exception. Not far behind the auditory involution there are visible at the end of period K a few longitudinal muscles, forming about three or four muscle-plates, the ventral part of which is wanting. I have not the means of deciding whether they properly belong to the head, or may not really be a part of the trunk system of muscles which has, to a certain extent, overlapped the back part of the head, but am inclined to accept the latter view. These cranial muscle-plates are shewn in Pl. 15, fig. 15b, and in Pl. 17, fig. 2.
[Pg 433] Notochord in the Head.
The notochord during stage G is situated for its whole length close under the brain, and terminates opposite the base of the mid-brain. As the cranial flexure becomes greater and mesoblast is collected in the angle formed by this, the termination of the notochord recedes from the base of the brain, but remains in close contact with the front end of the alimentary canal. At the same time its terminal part becomes very much thinner than the remainder, ends in a point, and exhibits signs of a retrogressive metamorphosis. It also becomes bent upon itself in a ventral direction through an angle of 180°; vide Pl. 15, figs. 9a and 16a. In some cases this curvature is even more marked than is represented in these figures.
The bending of the end of the notochord is not directly caused by the cranial flexure, as is proved by the fact that the end of the notochord becomes bent through a far greater angle than does the brain. During the stages subsequent to K the ventral flexure of the notochord disappears, and its terminal part acquires by stage O a distinct dorsal curvature.
Hypoblast of the Head.
The only feature of the alimentary tract in the head which presents any special interest is the formation of the gill-slits and of the thyroid body. In the present section the development of the former alone is dealt with; the latter body will be treated in the section devoted to the general development of the alimentary tract.
The gill-slits arise as outgrowths of the lining of the throat towards the external skin. In the gill-slits of Torpedo I have observed a very slight ingrowth of the external skin towards the hypoblastic outgrowth in one single case. In all other cases observed by me, the outgrowth from the throat meets the passive external skin, coalesces with it, and then, by the dissolution of the wall separating the lumen of the throat from the exterior, a free communication from the throat outwards is effected; vide Pl. 15, figs. 5a and b, and 13b. Thus it happens [Pg 434] that the walls lining the clefts are entirely formed of hypoblast. The clefts are formed successively[296], the anterior appearing first, and it is not till after the rudiments of three have appeared, that any of them become open to the exterior.
In stage K, four if not five are open to the exterior, and the rudiments of six, the full number, have appeared[297]. Towards the close of stage K there arise, from the walls of the 2nd, 3rd and 4th clefts, very small knob-like processes, the rudiments of the external gills. These outgrowths are formed both by the lining of the gill-cleft and by the adjoining mesoblast[298].
From the mode of development of the gill-clefts, it appears that their walls are lined externally by hypoblast, and therefore that the external gills are processes of the walls of the alimentary tract, i.e. are covered by an hypoblastic, and not an epiblastic layer. It should be remembered, however, that after the gill-slits become open, the point where the hypoblast joins the epiblast ceases to be determinable, so that some doubt hangs over the above statement.
The identification of the layer to which the gills belong is not without interest. If the external gills have an epiblastic origin, they may be reasonably regarded[299] as homologous with the external gills of Annelids; but, if derived from the hypoblast, this view becomes, to say the least, very much less probable.
Segmentation of the Head.
The nature of the vertebrate head and its relation to the trunk forms some of the oldest questions of Philosophical Morphology.
The answers of the older anatomists to these questions are of a contradictory character, but within the last few years it has been more or less generally accepted that the head is, in part at least, merely a modified portion of the trunk, and composed, like [Pg 435] that, of a series of homodynamous segments[300]. While the researches of Huxley, Parker, Gegenbaur, Götte, and other anatomists, have demonstrated in an approximately conclusive manner that the head is composed of a series of segments, great divergence of opinion still exists both as to the number of these segments, and as to the modifications which they have undergone, especially in the anterior part of the head. The questions involved are amongst the most difficult in the whole range of morphology, and the investigations recorded in the preceding pages do not, I am very well aware, go far towards definitely solving them. At the same time my observations on the nerves and on the head-cavities appear to me to throw a somewhat new light upon these questions, and it has therefore appeared to me worth while shortly to state the results to which a consideration of these organs points. There are three sets of organs, whose development has been worked out, each of which presents more or less markedly a segmental arrangement:—(1) The cranial nerves; (2) the visceral clefts; (3) the divisions of the head-cavity.
The first and second of these have often been employed in the solution of the present problem, while the third, so far as is known, exists only in the embryos of Elasmobranchii.
The development of the cranial nerves has recently been studied with great care by Dr Götte, and his investigations have led him to adopt very definite views on the segments of head. The arrangement of the cranial nerves in the adult has frequently been used in morphological investigations about the skull, but there are to my mind strong grounds against regarding it as affording a safe basis for speculation. The most important of these depends on the fact that nerves are liable to the greatest modification on any changes taking place in the organs they supply. On this account it is a matter of great difficulty, amounting in many cases to actual impossibility, to determine the morphological significance of the different nerve-branches, or the nature of the fusions and separations which have taken place at the roots of the nerves. It is, in fact, only in those parts of the [Pg 436] head which have, relatively speaking, undergone but slight modifications, and which require no special elucidation from the nerves, that these sufficiently retain in the adult their primitive form to serve as trustworthy morphological guides.
I propose to examine separately the light thrown on the segmentation of the head by the development of (1) the nerves, (2) the visceral clefts, (3) the head-cavities; and then to compare the three sets of results so obtained.
The post-auditory nerves present no difficulties; they are all organized in the same fashion, and, as was first pointed out by Gegenbaur, form five separate nerves, each indicating a segment. A comparison of the post-auditory nerves of Scyllium and other typical Elasmobranchii with those of Hexanchus and Heptanchus proves, however, that other segments were originally present behind those now found in the more typical forms. And the presence in Scyllium of numerous (twelve) strands from the brain to form the vagus, as well as the fact that a large section of the commissure connecting the vagus roots with the posterior roots of the spinal nerves is not connected with the brain, appear to me to shew that all traces of the lost nerves have not yet vanished.
Passing forwards from the post-auditory nerves, we come to the seventh and auditory nerves. The embryological evidence brought forward in this paper is against regarding these nerves as representing two segments. Although it must be granted that my evidence is not conclusive against an independent formation of these two nerves, yet it certainly tells in favour of their originating from a common rudiment, and Marshall's results on the origin of the two nerves in Birds (published in the Journal of Anatomy and Physiology, Vol. XI. Part 3) support, I have reason to believe, the same conclusion. Even were it eventually to be proved that the auditory nerve originated independently of the seventh, the general relations of this nerve, embryological and otherwise, are such that, provisionally at least, it could not be regarded as belonging to the same category as the facial or glossopharyngeal nerves, and it has therefore no place in a discussion on the segmentation of the head.
The seventh nerve of the embryo (Pl. 17,
fig. 1, VII) is [Pg 437] formed by the
junction of three conspicuous branches, (1) an anterior dorsal branch which
takes a more or less horizontal course above the eye (VII. a); (2) a main
branch to the hyoid arch (VII. hy); (3) a
smaller branch to the posterior edge of the mandibular arch (VII. mn). The first of these branches can clearly be
nothing else but the typical ramus dorsalis,
of which however the
auditory may perhaps be a specialized part. The fact that this branch
pursues an anterior and not a directly dorsal course is probably to be
explained as a consequence of the cranial flexure. The two other branches
of the seventh nerve are the same as those present in all the posterior
nerves, viz. the branches to the two sides of
a branchial cleft, in the present instance the spiracle; the seventh nerve
being clearly the nerve of the hyoid arch.
The fifth nerve presents in the arrangement of its branches a similarity to the seventh nerve so striking that it cannot be overlooked. This similarity is at once obvious from an inspection of the diagram of the nerves on Pl. 17, fig. 1, V, or from an examination of the sections representing these nerves (Pl. 17, figs. 3 and 4). It divides like the seventh nerve into three main branches: (1) an anterior and dorsal branch (r. ophthalmicus profundus), whose course lies parallel to but ventral to that of the dorsal branch of the seventh nerve; (2) a main branch to the mandibular arch (r. maxillæ inferioris); and (3) an anterior branch to the palatine arcade (r. maxillæ superioris). I was at first inclined to regard the anterior branch of the fifth (ophthalmic) as representing a separate nerve, and was supported in this view by its relation to the most anterior of the head-cavities; but the unexpected discovery of an exactly similar branch in the seventh nerve has induced me to modify this view, and I am now constrained to view the fifth as a single nerve, whose branches exactly correspond with those of the seventh. The anterior branch of the fifth is, like the corresponding branch of the seventh, the ramus dorsalis, and the two other branches are the equivalent of the branches of the seventh, which fork over the spiracle, though in the case of the fifth nerve no distinct cleft is present unless we regard the mouth as such. Embryology thus appears to teach us that the fifth nerve is a single nerve supplying the mandibular arch, and not, as has been usually thought, a [Pg 438] complex nerve resulting from the coalescence of two or three distinct nerves. My observations do not embrace the origin or history of the third, fourth, and sixth nerves, but it is hardly possible to help suspecting that in these we have the nerve of one or more segments in front of that supplied by the fifth nerve; a view which well accords with the most recent morphological speculations of Professor Huxley[301].
From this enumeration of the nerves the optic nerve is excluded for obvious reasons, and although it has been shewn above that the olfactory nerve develops like the other nerves as an outgrowth from the brain, yet its very late appearance and peculiar relations are, at least for the present, to my mind sufficient grounds for excluding it from the category of segmental cranial nerves.
The nerves then give us indications of seven cranial segments, or, if the nerves to the eye-muscles be included, of at the least eight segments, but to these must be added a number of segments now lost, but which once existed behind the last of those at present remaining.
The branchial clefts have been regarded as guides to segmentation by Gegenbaur, Huxley, Semper, etc., and this view cannot I think be controverted. In Scyllium there are six clefts which give indications of seven segments, viz., the segments of the mandibular arch, hyoid arch, and of the five branchial arches. If, following the views of Dr Dohrn[302], we regard the mouth as representing a cleft, we shall have seven clefts and eight segments; and it is possible, as pointed out in Dr Dohrn's very suggestive pamphlet, that remnants of a still greater number of præoral clefts may still be in existence. Whatever may be the value of these speculations, such forms as Hexanchus and Heptanchus and Amphioxus make it all but certain that the ancestors of Vertebrates had a number of clefts behind those now developed.
The last group of organs to be dealt with for our present question is that of the Head-Cavities.
The walls of the spaces formed by the cephalic prolongations [Pg 439] of the body-cavity develop into muscles and resemble the muscle-plates of the trunk, and with these they must be identified, as has been already stated. As equivalent to the muscle-plates, they clearly are capable of serving as very valuable guides for determining the segmentation of the head. There are then a pair of these in front of the mandibular arch, a pair in the mandibular arch, and a pair in each succeeding arch. In all there are eight pairs of these cavities representing eight segments, the first of them præoral. As was mentioned above, each of the sections of the head-cavity (except perhaps the first) stands in a definite relation to the nerve and artery of the arch in which it is situated.
The comparative results of these three independent methods of determining the segmentation of the head are in the subjoined table represented in a form in which they can be compared:—
Table of the Cephalic Segments as determined by the Nerves, Visceral Arches, and Head-Cavities.
| Segments | Nerves | Visceral Arches | Head-Cavities or Cranial Muscle-Plates |
|---|---|---|---|
| Præoral 1 | 3rd and 4th and ? 6th nerves (perhaps representing more than one segment) |
(?) | 1st head cavity (in my figures 1pp.) |
| Postoral 2 | 5th nerve | Mandibular | 2nd head-cavity (in my figures 2pp.) |
| ——3 | 7th nerve | Hyoid | 3rd head-cavity |
| ——4 | Glossopharyngeal nerve | 1st branchial arch | 4th head-cavity |
| ——5 | 1st branch of vagus | 2nd branchial arch | 5th head-cavity |
| ——6 | 2nd branch of vagus | 3rd branchial arch | 6th head-cavity |
| ——7 | 3rd branch of vagus | 4th branchial arch | 7th head-cavity |
| ——8 | 4th branch of vagus | 5th branchial arch | 8th head-cavity |
In the above table the first column denotes the segments of the head as indicated by a comparison of the three sets of organs employed. The second column denotes the segments as [Pg 440] obtained by an examination of the nerves; the third column is for the visceral arches (which lead to the same results as, but are more convenient for our table than, the visceral clefts), and the fourth column is for the head-cavities. It may be noticed that from the second segment backwards the three sets of organs lead to the same results. The head-cavities indicate one segment in front of the mouth, and now that the ophthalmic branch of the fifth has been dethroned from its position as a separate nerve, the eye-nerves, or one of them, may probably be regarded as belonging to this segment. If the suggestion made above (p. 431), that the walls of the first cavity become the eye-muscles, be correct, the eye-nerves would perhaps after all be the most suitable nerves to regard as belonging to the segment of the first head-cavity.
EXPLANATION OF PLATES 15, 16, 17.
Plate 15. (The Head during stages G—K.)
Complete List of Reference Letters.
1aa, 2aa, etc. 1st, 2d, etc. aortic arch. acv. Anterior cardinal vein. al. Alimentary canal. ao. Aorta. au. Thickening of epiblast to form the auditory pit. aun. Auditory nerve. aup. Auditory pit. auv. Auditory vesicle. b. Wall of brain. bb. Base of brain. cb. Cerebellum. cer. Cerebrum. Ch. Choroid slit. ch. Notochord. com. Commissure connecting roots of vagus nerve. 1, 2, 3 etc. eg. External gills. ep. External epiblast. fb. Fore-brain. gl. Glossopharyngeal nerve. hb. Hind-brain. ht. Heart. hy. Hyaloid membrane. In. Infundibulum. l. Lens. M. Mouth involution. m. Mesoblast at the base of the brain. mb. Mid-brain. mn. v. Mandibular branch of fifth. ol. Olfactory pit. op. Eye. opn. Optic nerve. opv. Optic vesicle. opth V. Ophthalmic branch of fifth. p. Posterior root of spinal nerve. pn. Pineal gland. 1, 2 etc. pp. First, second, etc. section of body-cavity in the head. pt. Pituitary body. so. Somatopleure. sp. Splanchnopleure. spc. Spinal cord. Th. Thyroid body. v. Blood-vessel. iv. v. Fourth ventricle. v. Fifth nerve. Vc. Visceral cleft. Vg. Vagus. vii. Seventh or facial nerve.
Fig. 1. Head of a Pristiurus embryo of stage K viewed as a transparent object.
The points which deserve special attention are: (1) The sections of the body-cavity in the head (pp): the first or premandibular section being situated close to the eye, the second in the mandibular arch. Above this one the fifth nerve bifurcates. The third at the summit of the hyoid arch.
The cranial nerves and the general appearance of the brain are well shewn in the figure.
[Pg 441] The notochord cannot be traced in the living embryo so far forward as it is represented. It has been inserted according to the position which it is seen to occupy in sections.
Fig. 2. Head of an embryo of Scyllium canicula somewhat later than stage K, viewed as a transparent object.
The figure shews the condition of the brain; the branches of the fifth and seventh nerves (v. vii.); the rudiments of the semicircular canals; and the commencing appearance of the external gills as buds on both walls of 2nd, 3rd, and 4th clefts. The external gills have not appeared on the first cleft or spiracle.
Fig. 3. Section through the head of a Pristiurus embryo during stage G. It shews (1) the fifth nerve (v.) arising as an outgrowth from the dorsal summit of the brain. (2) The optic vesicles not yet constricted off from the fore-brain.
Figs. 4a and 4b. Two sections through the head of a Pristiurus embryo of stage I. They shew (1) the appearance of the seventh nerve. (2) The portion of the body-cavity belonging to the first and second visceral arches. (3) The commencing thickening of epiblast to form the auditory involution.
In 4b, the posterior of the two sections, no trace of an auditory nerve is to be seen.
Figs. 5a and 5b. Two sections through the head of a Torpedo embryo with 3 visceral clefts. Zeiss A, ocul. 1.
5a shews the formation of the thin roof of the fourth ventricle by a divarication of the two lateral halves of the brain.
Both sections shew the commencing formation of the thyroid body (th) at the base of the mandibular arch.
They also illustrate the formation of the visceral clefts by an outgrowth from the alimentary tract without any corresponding ingrowth of the external epiblast.
Fig. 6. Section through the hind-brain of a somewhat older Torpedo embryo. Zeiss A, ocul. 1.
The section shews (1) the attachment of a branch of the vagus to the walls of the hind-brain. (2) The peculiar form of the hind-brain.
Fig. 7. Transverse section through the head of a Pristiurus embryo belonging to a stage intermediate between I and K, passing through both the fore-brain and the hind-brain. Zeiss A, ocul. 1.
The section illustrates (1) the formation of the pituitary body (pt) from the mouth involution (m), and proves that, although the wall of the throat (al) is in contact with the mouth involution, there is by this stage no communication between the two. (2) The eye. (3) The sections of the body-cavity in the head (1pp, 2pp). (4) The fifth nerve (v.) and the seventh nerve (vii).
Fig. 8. Transverse section through the brain of a rather older embryo than fig. 7. It shews the ventral junction of the anterior sections of the body-cavity in the head (1pp).
Figs. 9a and 9b. Two longitudinal sections through the brain of a Pristiurus embryo belonging to a stage intermediate between I and K. Zeiss A, ocul. 1.
9a is taken through the median line, but is reconstructed from two sections. It shews (1) The divisions of the brain—The cerebrum and thalamencephalon in the fore-brain; the mid-brain; the commencing cerebellum in the hind-brain. (2) The relation of the mouth involution to the infundibulum. (3) The termination of the notochord.
[Pg 442] 9b is a section to one side of the same brain. It shews (1) The divisions of the brain. (2) The point of outgrowth of the optic nerves (opn). (3) The sections of the body-cavity in the head and the bifurcation of the optic nerve over the second of these.
Fig. 10. Longitudinal section through the head of a Pristiurus embryo somewhat younger than fig. 9. Zeiss a, ocul. 4. It shews the relation of the nerves and the junction of the fifth, seventh, and auditory nerves with the brain.
Fig. 11. Longitudinal section through the fore-brain of a Pristiurus embryo of stage K, slightly to one side of the middle line. It shews the deep constriction separating the thalamencephalon from the cerebral hemispheres.
Fig. 12. Longitudinal section through the base of the brain of an embryo of a stage intermediate between I and K.
It shews (1) the condition of the end of the notochord; (2) the relation of the mouth involution to the infundibulum.
Fig. 13a. Longitudinal and horizontal section through part of the head of a Pristiurus embryo rather older than K. Zeiss A, ocul. 1.
The figure contains the eye cut through in the plane of the choroid slit. Thus the optic nerve (opn) and choroid slit (ch) are both exhibited. Through the latter is seen passing mesoblast accompanied by a blood-vessel (v). Op represents part of the optic vesicle to one side of the choroid slit.
No mesoblast can be seen passing round the outside of the optic cup; and the only mesoblast which enters the optic cup passes through the choroid slit.
Fig. 13b. Transverse section through the last arch but one of the same embryo as 13a. Zeiss A, ocul. 1.
The figure shews (1) The mode of formation of a visceral cleft without any involution of the external skin. (2) The head-cavity in the arch and its situation in relation to the aortic arch.
Fig. 14. Surface view of the nasal pit of an embryo of same age as fig. 13, considerably magnified. The specimen was prepared by removing the nasal pit, flattening it out and mounting in glycerine after treatment with chromic acid. It shews the primitive arrangement of the Schneiderian folds. One side has been injured.
Figs. 15a and 15b. Two longitudinal and vertical sections through the head of a Pristiurus embryo belonging to stage K. Zeiss a, ocul. 3.
15a is the most superficial section of the two. It shews the constitution of the seventh and fifth nerves, and of the intestinal branch of the vagus. The anterior branch of the seventh nerve deserves a special notice.
15b mainly illustrates the dorsal commissure of the vagus nerve (com) continuous with the dorsal commissures of the posterior root of the spinal nerves.
Fig. 16. Two longitudinal and vertical sections of the head of a Pristiurus embryo belonging to the end of stage K. Zeiss a, ocul. 1.
16a passes through the median line of the brain and shews the infundibulum, notochord and pituitary body, etc.
The pituitary body still opens into the mouth, though the septum between the mouth and the throat is broken through.
16b is a more superficial section shewing the head-cavities pp 1, 2, 3, and the lower vagus commissure.
Complete List of Reference Letters.
auv. Auditory vesicle. cb. Cerebellum. cer. Cerebral hemispheres. ch. Notochord. cin. Internal carotid. ft. Fasciculi teretes. in. Infundibulum. lv. Lateral ventricle. mb. Mid-brain, or optic lobes. md. Medulla oblongata. mn. Mandible. ol. Olfactory pit. oll. Olfactory lobe. op. Eye. opn. Optic nerve. opth. Optic thalamus. pc. Posterior commissure. pcl. Posterior clinoid. pn. Pineal gland. pt. Pituitary body. rt. Restiform tracts. tv. Tela vasculosa of the roof of the fourth ventricle. iv. v. Fourth ventricle. vii. Seventh nerve. x. Rudiment of septum which will grow backwards and divide the unpaired cerebral rudiment into the two hemispheres.
Figs. 1a, 1b, 1c. Longitudinal sections of the brain of a Scyllium embryo belonging to stage L. Zeiss a, ocul. 1.
1a is taken slightly to one side of the middle line, and shews the general features of the brain, and more especially the infundibulum (in) and pituitary body (pt).
1b is through the median line of the pineal gland.
1c is through the median line of the base of the brain, and shews the notochord (ch) and pituitary body (pt); the latter still communicating with the mouth. It also shews the wide opening of the infundibulum in the middle line into the base of the brain.
Fig. 2. Section through the unpaired cerebral rudiment during stage O, to shew the origin of the olfactory lobe and the olfactory nerve. The latter is seen to divide into numerous branches, one of which passes into each Schneiderian fold. At its origin are numerous ganglion cells represented by dots. Zeiss a, ocul. 2.
Fig. 3. Horizontal section through the three lobes of the brain during stage O. Zeiss a, ocul. 2.
The figure shews (1) the very slight indications which have appeared by this stage of an ingrowth to divide the cerebral rudiment into two lobes (x): (2) the optic thalami united by a posterior commissure, and on one side joining the base of the mid-brain, and behind them the pineal gland: (3) the thin posterior wall of the cerebral rudiment with folds projecting into the cerebral cavity.
Figs. 4a, 4b, 4c. Views from the side, from above, and from below, of a brain of Scyllium canicula during stage P. In the view from the side the eye (op) has not been removed.
The bilobed appearance both of the mid-brain and cerebellum should be noticed.
Fig. 5. Longitudinal section of a brain of Scyllium canicula during stage P. Zeiss a, ocul. 2.
There should be noticed (1) the increase in the flexure of the brain accompanying a rectification of the cranial axis; (2) the elongated pineal gland, and (3) the structure of the optic thalamus.
Figs. 6a, 6b, 6c. Views from the side, from above, and from below, of a brain of Scyllium stellare during a slightly later stage than Q.
[Pg 444] Figs. 7a and 7b. Two longitudinal sections through the brain of a Scyllium embryo during stage Q. Zeiss a, ocul. 2.
7a cuts the hind part of the brain nearly through the middle line; while 7b cuts the cerebral hemispheres and pineal gland through the middle.
In 7a the infundibulum (1), cerebellum (2), the passage of the restiform tracts (rt) into the cerebellum (3), and the rudiments of the tela vasculosa (4) are shewn. In 7b the septum between the two lobes of the cerebral hemispheres (1), the pineal gland (2), and the relations of the optic thalami (3) are shewn.
Figs. 8a, 8b, 8c, 8d. Four transverse sections of the brain of an embryo slightly older than Q. Zeiss a, ocul. 1.
8a passes through the cerebral hemispheres at their junction with the olfactory lobes. On the right side is seen the olfactory nerve coming off from the olfactory lobe. At the dorsal side of the hemispheres is seen the pineal gland (pn).
8b passes through the mid-brain now slightly bilobed, and the opening into the infundibulum (in). At the base of the section are seen the optic nerves and their chiasma.
8c passes through the opening from the ventricle of the mid-brain into that of the cerebellum. Below the optic lobes is seen the infundibulum with the rudiments of the sacci vasculosi.
8d passes through the front end of the medulla, and shews the roots of the seventh pair of nerves, and the overlapping of the medulla by the cerebellum.
Plate 17.
Complete List of Reference Letters.
vii. a. Anterior branch of seventh nerve. ar. Anterior root of spinal nerve. auv. Auditory vesicle. cer. Cerebrum. ch. Notochord. ch. Epithelial layer of choroid membrane. gl. Glossopharyngeal nerve. vii.hy. Hyoid branch of seventh nerve. hym. Hyaloid membrane. ll. Lateral line. v. mn. Ramus mandibularis of fifth nerve. vii. mn. Mandibular (spiracular) branch of seventh nerve. v. mx. Ramus maxillæ superioris of fifth nerve. nl. Nervus lateralis. ol. Olfactory pit. op. Eye. v. opth. Ramus ophthalmicus of fifth nerve. pch. Parachordal cartilage. pfal. Processus falciformis. pp. Head cavity. pr. Posterior root of spinal nerve. rt. Retina. sp. Spiracle. v. Fifth nerve. vii. Seventh nerve. vc. Visceral cleft. vg. Vagus nerve. vg.br. Branchial branch of vagus. vgcom. Commissure uniting the roots of the vagus, and continuous with commissure uniting the posterior roots of the spinal nerves. vgr. Roots of vagus nerves in the brain. vgin. Intestinal branch of vagus. vh. Vitreous humour.
Fig. 1. Diagram of cranial nerves at stage L.
A description of the part of this referring to the vagus and glossopharyngeal nerves is given at p. 426. It should be noticed that there are only five strands indicated as springing from the spinal cord to form the vagus and glossopharyngeal nerves. It is however probable that there are even from the first a greater number of strands than this.
[Pg 445] Fig. 2. Section through the hinder part of the medulla oblongata, stage between K and L. Zeiss A, ocul. 2.
It shews (1) the vagus commissure with branches on one side from the medulla: (2) the intestinal branch of the vagus giving off a nerve to the lateral line.
Fig. 3. Longitudinal and vertical section through the head of a Scyllium embryo of stage L. Zeiss a, ocul. 2.
It shews the course of the anterior branch of the seventh nerve (vii.); especially with relation to the ophthalmic branch of the fifth nerve (v. oth).
Figs. 4a and 4b. Two horizontal and longitudinal sections through the head of a Scyllium embryo belonging to stage O. Zeiss a, ocul. 2.
4a is the most dorsal of the two sections, and shews the course of the anterior branch of the seventh nerve above the eye.
4b is a slightly more ventral section, and shews the course of the fifth nerve.
Fig. 5. Longitudinal and horizontal section through the hind-brain at stage O, shewing the roots of the vagus and glossopharyngeal nerves in the brain. Zeiss B, ocul. 2.
There appears to be one root in the brain for the glossopharyngeal, and at least six for the vagus. The fibres from the roots divide in many cases into two bundles before leaving the brain. Swellings of the brain towards the interior of the fourth ventricle are in connection with the first five roots of the vagus, and the glossopharyngeal root; and a swelling is also intercalated between the first vagus root and the glossopharyngeal root.
Fig. 6. Horizontal section through a part of the choroid slit at stage P. Zeiss B, ocul. 2.
The figure shews (1) the rudimentary processus falciformis (pfal) giving origin to the vitreous humour; and (2) the hyaloid membrane (hym) which is seen to adhere to the retina, and not to the vitreous humour or processus falciformis.
[269] Ent. d. Unke, p. 304.
[270] Das Gehirn d. Selachier, Leipzig, 1870.
[271] Proceedings of the Zoological Society, 1876, Pt. 1. pp. 30 and 31.
[272]
Anterior brain-mass with Sharks and Skates,
American Journal
of Science and Arts, Vol. XII. 1876.
[273] Entwicklungsgeschichte d. Unke.
[274] The engraver has not been very successful in rendering these membranes.
[275]
Embryologie d. Coloboms,
Sitz. d. k. Akad.
Wien, Bd. LXXI. 1875.
[276] Quarterly Journal of Microscopic Science, Oct. 1874.
[277] Entwicklungsgeschichte der Unke. Götte was the first to draw attention to this fact. His observations were then shewn to hold true for Elasmobranchii by myself, and subsequently for Birds by Mihalkowics.
[278] Arch. f. micr. Anat. Vol. XI.
[279]
W. Müller, Ueber Entwicklung and Bau d. Hypophysis u.
d. Processus infundibuli cerebri,
Jenaische Zeitschrift,
Bd. VI.
[280] In the presence of this continuous outgrowth of the brain from which spring the separate nerve stems of the vagus, may perhaps be found a reconciliation of the apparently conflicting statements of Götte and myself with reference to the vagus nerve. Götte regards the vagus as a single nerve, from its originating as an undivided rudiment; but it is clear from my researches that, for Elasmobranchii at least, this method of arguing will not hold good, since it would lead to the conclusion that all the spinal nerves were branches of one single nerve, since they too spring as processes from a continuous outgrowth from the brain!
[281] The conclusion here arrived at with reference to the anterior roots, is opposed to the observations of both Gegenbaur on Hexanchus, Jenaische Zeitschrift, Vol. VI., and of Jackson and Clarke on Echinorhinus, Journal of Anatomy and Physiology, Vol. X. These morphologists identify certain roots springing from the medulla below and behind the main roots of the vagus as true anterior roots of this nerve. The existence of these roots is not open to question, but without asserting that it is impossible for me to have failed to detect such roots had they been present in the embryo, I think I may maintain if these anterior roots are not present in the embryo, their identification as vagus roots must be abandoned; and they must be regarded as belonging to spinal nerves. This point is more fully spoken of at p. 428.
[282] Journal of Anatomy and Physiology, Vol. X. [This Edition, No. IX.]
[283] Nervensystem d. Fische, Rostock, 1849.
[284] Jenaische Zeitschrift, Vol. VI.
[285] Journal of Anatomy and Physiology, Vol. X.
[286] My results with reference to these roots accord exactly, so far as they go, with the more carefully worked out conclusions of Stannius, loc. cit. pp. 29 and 30.
[287] The root of the seventh nerve cannot properly be distinguished from this root.
[288] Loc. cit.
[289] Hexanchus, Gegenbaur, Jenaische Zeitschrift, Vol. VI.
[290] Loc. cit.
[291] In the diagram there are only five strands represented. This is due to the fact that I have not certainly made out their true number.
[292] Loc. cit.
[293] Vide Jackson and Clarke, loc. cit. The authors take a different view to that here advocated, and regard the ventral roots described by them as having originally belonged to the vagus.
[294]
Vide Vetter, Die Kiemen und
Kiefermusculatur d. Fische.
Jenaische
Zeitschrift, Vol. VII.
[295] A report of the lectures appeared in Nature.
[296] Vide Plate 8.
[297] The description of stage K and L, pp. 292 and 293, is a little inaccurate with reference to the number of the visceral clefts, though the number visible in the hardened embryos is correctly described.
[298] Vide on the development of the gills, Schenk, Sitz. d. k. Akad. Wien, Vol. LXXI, 1875.
[299] Vide Dohrn, Ursprung d. Wirbelthiere.
[300] Semper, in his most recent work, maintains, if I understand him rightly, that the head is in no sense a modified part of the trunk, but admits that it is segmented in a similar fashion to the trunk.
[301] Preliminary note upon the brain and skull of Amphioxus, Proc. of the Royal Society, Vol. XXII.
[302] Ursprung d. Wirbelthiere.
The present Chapter completes the history of the primitive alimentary canal, whose formation has already been described. In order to economise space, no attempt has been made to give a full account of the alimentary canal and its appendages, but only those points have been dealt with which present any features of special interest.
The development of the following organs is described in order.
(1) The solid œsophagus.
(2) The postanal section of the alimentary tract.
(3) The cloaca and anus.
(4) The thyroid body.
(5) The pancreas.
(6) The liver.
(7) The subnotochordal rod.
The solid œsophagus.
A curious point which has turned up in the course of my investigations is the fact that for a considerable period of embryonic life a part of the œsophagus remains quite solid and without a lumen. The part of the œsophagus to undergo this peculiar change is that which overlies the heart, and extends from the front end of the stomach to the branchial region. At first, this part of the œsophagus has the form of a tube with a well-developed lumen like the remainder of the alimentary [Pg 447] tract, but at a stage slightly younger than K its lumen becomes smaller, and finally vanishes, and the original tube is replaced by a solid rod of uniform and somewhat polygonal cells. A section of it in this condition is represented in Pl. 11, fig. 8a.
At a slightly later stage its outermost cells become more columnar than the remainder, and between stages K and L it loses its cylindrical form and becomes much more flattened. By stage L the external layer of columnar cells is more definitely established, and the central rounded cells are no longer so numerous (Pl. 18, fig. 4, sœs).
In the succeeding stages the solid part of the œsophagus immediately adjoining the stomach is carried farther back relatively to the heart and overlies the front end of the liver. A lumen is not however formed in it by the close of stage Q, and beyond that period I have not carried my investigations, and cannot therefore state the exact period at which the lumen reappears. The limits of the solid part of the œsophagus are very satisfactorily shewn in longitudinal and vertical sections.
The solidification of the œsophagus belongs to a class of embryological phenomena which are curious rather than interesting, and are mainly worth recording from the possibility of their turning out to have some unsuspected morphological bearings.
Up to stage Q there are no signs of a rudimentary air-bladder.
The postanal section of the alimentary tract.
An account has already been given (p. 307) of the posterior continuity of the neural and alimentary canals, and it was there stated that Kowalevsky was the discoverer of this peculiar arrangement. Since that account was published, Kowalevsky has given further details of his investigations on this point, and more especially describes the later history of the hindermost section of the alimentary tract. He says[303]:
The two germinal layers, epiblast and hypoblast, are continuous with each other at the border of the germinal disc. The primitive groove or [Pg 448] furrow appears at the border of the germinal disc and is continued from the upper to the lower side. By the closing of the groove there is formed the medullary canal above, while the part of the groove on the under surface directed below is chiefly converted into the hind end of the alimentary tract. The connection of the two tubes in Acanthias persists till the formation of the anus, and the part of the nervous tube which lies under the chorda passes gradually upwards to the dorsal side of the chorda, and persists there for a long time in the form of a large thin-walled vesicle.
The last part of the description beginning at The connection of
does not hold good for any of the genera which I have had an opportunity of
investigating, as will appear from the sequel.
In a previous section[304] the history of the alimentary tract was completed up to stage G.
In stage H the point where the anus will (at a very much later period) appear, becomes marked out by the alimentary tract sending down a papilliform process towards the skin. This is shewn in Pl. 8, figs. H and I, an.
That part of the alimentary tract which is situated behind this point may, for convenience, be called the postanal section. During stage H the postanal section begins to develop a terminal dilatation or vesicle, connected with the remainder of the canal by a narrower stalk. The relation in diameter between the vesicle and the stalk may be gathered by a comparison of figs. 3a and 3b, Pl. 11. The diameter of the vesicle represented in section in Pl. 11, fig. 3, is 0.328 Mm.
The walls both of the vesicle and stalk are formed of a fairly columnar epithelium. The vesicle communicates in front by a narrow passage (Pl. 11, fig. 3a) with the neural canal, and behind is continued into two horns (Pl. 11, fig. 2, al.) corresponding with the two caudal swellings spoken of above (p. 288). Where the canal is continued into these two horns, its walls lose their distinctness of outline, and become continuous with the adjacent mesoblast.
In the succeeding stages up to K the tail grows longer and longer, and with it grows the postanal section of the alimentary tract, without however altering in any of its essential characters.
[Pg 449] Its features at stage K are illustrated by an optical section of the tail of an embryo (Pl. 18, fig. 5) and by a series of transverse sections through the tail of another embryo in Pl. 18, figs. 6a, 6b, 6c, 6d. In the optical section there is seen a terminal vesicle (alv.) opening into the neural canal, and connected with the remainder of the alimentary tract. The terminal vesicle causes the end of the tail to be dilated, as is shewn in Pl. 8, fig. K. The length of the postanal section extending from the abdominal paired fins to the end of the tail (equal to rather less than one-third of the whole length of the embryo), may be gathered from the same figure.
The most accurate method of studying this part of the alimentary canal is by means of transverse sections. Four sections have been selected for illustration (Pl. 18, figs. 6a, 6b, 6c, and 6d) out of a fairly-complete series of about one hundred and twenty.
Posteriorly (fig. 6a) there is present a terminal vesicle .25 Mm. in diameter, and therefore rather smaller than in the earlier stage, whose walls are formed of columnar epithelium, and which communicates dorsally by a narrow opening with the neural canal; to this is attached a stalk in the form of a tube, also lined by columnar epithelium, and extending through about thirty sections (Pl. 18, fig. 6b). Its average diameter is about .084 Mm. Overlying its front end is the subnotochordal rod (fig. 6b, x.), but this does not extend as far back as the terminal vesicle.
The thick-walled stalk of the vesicle is connected with the cloacal section of the alimentary tract by a very narrow thin-walled tube (Pl. 18, 6c, al.). This for the most part has a fairly uniform calibre, and a diameter of not more than .035 Mm. Its walls are formed of a flattened epithelium. At a point not far from the cloaca it becomes smaller, and its diameter falls to .03 Mm. In front of this point it rapidly dilates again, and, after becoming fairly wide, opens on the dorsal side of the cloacal section of the alimentary canal just behind the anus (fig. 6d).
Near the close of stage K at a point shortly behind the anus, where the postanal section of the canal was thinnest in the early part of the stage, the alimentary canal becomes solid [Pg 450] (Pl. 11, fig. 9d), and a rupture here occurs in it at a slightly later period.
In stage L the posterior part of the postanal section of the canal is represented by a small rudiment near the end of the tail. The rudiment no longer has a terminal vesicle, nor does it communicate with the neural canal. It was visible in one series for about 40 sections, and was continued forwards by a few granular cells, lying between the aorta and the caudal vein. The portion of the postanal section of the alimentary tract just behind the cloaca, was in the same embryo represented by a still smaller rudiment of the dilated part which at an earlier period opened into the cloaca.
Later than stage L no trace of the postanal section of the alimentary canal has come under my notice, and I conclude that it vanishes without becoming converted into any organ in the adult. Since my preliminary account of the development of Elasmobranch Fishes was written, no fresh light appears to have been thrown on the question of the postanal section of the alimentary canal being represented in higher Vertebrata by the allantois.
The cloaca and anus.
Elasmobranchii agree closely with other Vertebrates in the formation of the cloaca and anus, and in the relations of the cloaca to the urinogenital ducts.
The point where the anus, or more precisely the external opening of the cloaca, will be formed, becomes very early marked out by the approximation of the wall of the alimentary tract and external skin. This is shewn for stages H and I in Pl. 8 an.
Between stages I and K the alimentary canal on either side of this point, which we may for brevity speak of as the anus, is far removed from the external skin, but at the anus itself the lining of the alimentary canal and the skin are in absolute contact. There is, however, no involution from the exterior, but, on the contrary, the position of the anus is marked by a distinct prominence. Opposite the anus the alimentary canal dilates and forms the cloaca.
[Pg 451] During stage K, just in front of the prominence of the anus, a groove is formed between two downgrowths of the body-wall. This is shewn in Pl. 11, fig. 9a. During the same stage the segmental ducts grow downwards to the cloaca, and open into it in the succeeding stage (Pl. 11, fig. 9b). Up to stage K the cloaca is connected with the præanal section of the alimentary canal in front, and the postanal section behind; the latter, however, by stage L, as has been stated above, atrophies, with the exception of a very small rudiment. In stage L the posterior part of the cloaca is on a level with the hind end of the kidneys, and is situated behind the posterior horns of the body-cavity, which are continued backwards to about the point where the segmental ducts open into the cloaca, and though very small at their termination rapidly increase in size anteriorly.
Nothing very worthy of note takes place in connection with the cloaca till stage O. By this stage we have three important structures developed. (1) An involution from the exterior to form the mouth of the cloaca or anus. (2) A perforation leading into the cloaca at the hind end of this. (3) The rudiments of the abdominal pockets. All of these structures are shewn in Pl. 19, figs. 1a, 1b, 1c.
The mouth of the cloaca is formed by an involution of the skin, which is deepest in front and becomes very shallow behind (Pl. 19, figs. 1a, 1b). At first only the mucous layer of the skin takes part in it, but when the involution forms a true groove, both layers of the skin serve to line it. At its posterior part, where it is shallowest, there is present, at stage O, a slit-like longitudinal perforation, leading into the posterior part of the cloaca (Pl. 19, fig. 1c) and forming its external opening. Elsewhere the wall of the cloaca and cloacal groove are merely in contact but do not communicate. On each side of the external opening of the cloaca there is present an involution (Pl. 19, fig. 1c, ab.p.) of the skin, which resembles the median cloacal involution, and forms the rudiment of an abdominal pocket. These two rudiments must not be confused with two similar ones, which are present in all the three sections represented, and mark out the line which separates the limbs from the trunk. These latter are not present in the succeeding stages. The abdominal pockets are only found in sections through the opening into [Pg 452] the cloaca, and are only visible in the hindermost of my three sections.
All the structures of the adult cloaca appear to be already constituted by stage O, and the subsequent changes, so far as I have investigated them, may be dealt with in very few words. The perforation of the cloacal involution is carried slowly forwards, so that the opening into the cloaca, though retaining its slit-like character, becomes continuously longer; by stage Q its size is very considerable. The cloacal involution, relatively to the cloaca, recedes backwards. In stage O its anterior end is situated some distance in front of the opening of the segmental duct into the cloaca; by stage P the front end of the cloacal involution is nearly opposite this opening, and by stage Q is situated behind it.
As I have shewn elsewhere[305], the so-called abdominal pores of Scyllium are simple pockets open to the exterior, but without any communication with the body-cavity. By stage Q they are considerably deeper than in stage O, and retain their original position near the hind end of the opening into the cloaca. The opening of the urinogenital ducts into the cloaca will be described in the section devoted to the urinogenital system.
In Elasmobranchii, as in other Vertebrata, that part of the cloaca which receives the urinogenital ducts, is in reality the hindermost section of the gut and not the involution of epiblast which eventually meets this. Thus the urinogenital ducts at first open into the alimentary canal and not to the exterior. This fact is certainly surprising, and its meaning is not quite clear to me.
The very late appearance of the anus may be noticed as a point in which Elasmobranchii agree with other Vertebrata, notably the Fowl[306]. The abdominal pockets, as might be anticipated from their structure in the adult, are simple involutions of the epiblast.
The thyroid body.
The earliest trace of the thyroid body has come under my notice in a Torpedo embryo slightly older than I. In this [Pg 453] embryo it appeared as a diverticulum from the ventral surface of the throat in the region of the mandibular arch, and extended from the border of the mouth to the point where the ventral aorta divided into the two aortic branches of the mandibular arch. In front it bounded a groove (Pl. 15, fig. 5a, Th.), directly continuous with the narrow posterior pointed end of the mouth and open to the throat, while behind it became a solid rod attached to the ventral wall of the œsophagus (Pl. 15, fig. 5b, Th.). In a Scyllium embryo belonging to the early part of stage K, the thyroid gland presented the same arrangement as in the Torpedo embryo just described, with the exception that no solid posterior section of it was present.
Towards the close of stage K the thyroid body begins to elongate and become solid, though it still retains its attachment to the wall of the œsophagus. The solidification is effected by the columnar cells which line the groove elongating and meeting in the centre. As soon as the lumen is by these means obliterated, small cells make their appearance in the interior of the body, probably budded off from the original columnar cells.
The gland continues to grow in length, and by stage L assumes a long sack-like form with a layer of columnar cells bounding it externally, and a core of rounded cells filling up its interior. Anteriorly it is still attached to the throat, and its posterior extremity lies immediately below the end of the ventral aorta. The cells of the gland contain numerous yellowish concretionary pigment bodies, which are also present in the later stages.
Up to stage P the thyroid gland retains its original position. Its form and situation are shewn in Pl. 19, fig. 3, th., in longitudinal and vertical section for a stage between O and P. The external layer of columnar cells has now vanished, and the gland is divided up by the ingrowth of connective-tissue septa into a number of areas or lobules—the rudiments of the future follicles. These lobules are perfectly solid without any trace of a lumen. A capillary network following the septa is present.
By stage Q the rudimentary follicles are more distinctly marked, but still without a lumen, and a connective-tissue sheath indistinctly separated from the surrounding tissue has been formed. My sections do not shew a junction between the gland [Pg 454] and the epithelium of the throat; but the two are so close together, that I am inclined to think that such a junction still exists. It is certainly present up to stage P.
Dr Müller[307], in his exhaustive memoir on the thyroid body, gives an account of its condition in two Acanthias embryos. In his earliest embryo (which, judging from the size, is perhaps about the same age as my latest) the thyroid body is disconnected from the throat, yet contains a lumen, and is not divided up into lobules. It is clear from this account, that there must be considerable differences of detail in the development of the thyroid body in Acanthias and Scyllium.
In the Bird Dr Müller's figures shew that the thyroid body develops in the region of the hyoid arch, whereas, in Elasmobranchii, it develops in the region of the mandibular arch. Dr Götte's[308] account of this body in Bombinator accords very completely with my own, both with reference to the region in which it develops, and its mode of development.
The pancreas.
The pancreas arises towards the close of stage K as a somewhat rounded hollow outgrowth from the dorsal side of that part of the gut which from its homologies may be called the duodenum. In the region where the pancreas is being formed the appearances presented in a series of transverse sections are somewhat complicated (Pl. 18, fig. 1), owing to the several parts of the gut and its appendages which may appear in a single section, but I have detected no trace of other than a single outgrowth to form the pancreas.
By stage L the original outgrowth from the gut has become elongated longitudinally, but transversely compressed: at the same time its opening into the duodenum has become somewhat narrowed.
Owing to these changes the pancreas presents in longitudinal and vertical section a funnel-shaped appearance (Pl. 19, fig. 4). From the expanded dorsal part of the funnel, especially from its anterior end, numerous small tubular diverticula grow out [Pg 455] into the mesoblast. The apex of the funnel leads into the duodenum. From this arrangement it results that at this period the original outgrowth from the duodenum serves as a receptacle into which each ductule of the embryonic gland opens separately. I have not followed in detail the further growth of the gland. It is, however, easy to note that while the ductules grow longer and become branched, vascular processes grow in between them, and the whole forms a compact glandular body in the mesentery on the dorsal side of the alimentary tract, and nearly on a level with the front end of the spiral valve. The funnel-shaped receptacle loses its original form, and elongating, assumes the character of a duct.
From the above account it follows that the glandular part of the pancreas, and not merely its duct, is derived from the original hypoblastic outgrowth from the gut. This point is extremely clear in my preparations, and does not, in spite of Schenk's observations to the contrary[309], appear to me seriously open to doubt.
The liver.
The liver arises during stage I as a ventral outgrowth from the duodenum immediately in front of the opening of the umbilical canal (duct of the yolk-sack) into the intestine. Almost as soon as it is formed this outgrowth develops two lateral diverticula opening into a median canal.
The two diverticula are the rudimentary lobes of the liver, and the median duct is the rudiment of the common bile-duct (ductus choledochus) and gall-bladder (Pl. 11, fig. 9).
By stage K the hepatic diverticula have begun to bud out a number of small hollow knobs. These rapidly increase in length and number, and form the so-called hepatic cylinders. They anastomose and unite together, so that by stage L there is constructed a regular network. As the cylinders increase in length their lumen becomes very small, but appears never to vanish (Pl. 19, fig. 5).
The mode of formation of the liver parenchyma by hollow and not solid outgrowths agrees with the suggestion made in [Pg 456] the Elements of Embryology, p. 133, and also with the results of Götte on the Amphibian liver. Schenk has thrown doubts upon the hypoblastic nature of the secreting tissue of the liver, but it does not appear to me, from my own investigations, that this point is open to question.
Coincidently with the formation of the hepatic network, the umbilical vein (Pl. 11, fig. 9, u.v.) which unites with the subintestinal or splanchnic vein (Pl. 11, fig. 8, V.) breaks up into a series of channels, which form a second network in the spaces of the hepatic network. These vascular channels of the liver appear to me to have from the first distinct walls of delicate spindle-shaped cells, and I have failed to find a stage similar to that described by Götte for Amphibians in which the blood-channels are simply lacunar spaces in the hepatic parenchyma.
The changes of the median duct of the liver are of rather a passive nature. By stage O its anterior end has dilated into a distinct gall-bladder, whose duct receives in succession the hepatic ducts, and so forms the ductus choledochus. The ductus choledochus opens on the ventral side of the intestine immediately in front of the commencement of the spiral valve.
It may be noted that the liver and pancreas are corresponding ventral and dorsal appendages of the part of the alimentary tract immediately in front of its junction with the yolk-sack.
The subnotochordal rod.
The existence of this remarkable body in Vertebrata was first made known by Dr Götte[310], who not only demonstrated its existence, but also gave a correct account of its development. Its presence in Elasmobranchii and mode of development were mentioned by myself in my preliminary account of the development of these fishes[311], and it has been independently observed and described by Professor Semper[312]. No plausible suggestion as to its function has hitherto been made, and it is therefore a matter of some difficulty to settle with what group [Pg 457] of organs it ought to be treated. In the presence of this difficulty it seemed best to deal with it in this chapter, since it is unquestionably developed from the wall of the alimentary canal.
At its full growth this body forms a rod underlying the notochord, and has nearly the same longitudinal extension as this. It is indicated in most of my sections by the letter x. We may distinguish two sections of it, the one situated in the head, the other in the trunk. The junction between the two occurs at the hind border of the visceral clefts.
The section in the trunk is the first to develop. It arises during stage H in the manner illustrated in Pl. 11, figs. 1 and 1a. The wall of the alimentary canal becomes thickened (Pl. 11, fig. 1) along the median dorsal line, or else produced into a ridge into which there penetrates a narrow prolongation of the lumen of the alimentary canal. In either case the cells at the extreme summit of the thickening become gradually constricted off as a rod, which lies immediately dorsal to the alimentary tract, and ventral to the notochord. The shape of the rod varies in the different regions of the body, but it is always more or less elliptical in section. Owing to its small size and soft structure it is easily distorted in the process of preparing sections.
In the hindermost part of the body its mode of formation differs somewhat from that above described. In this part the alimentary wall is very thick and undergoes no special growth prior to the formation of the subnotochordal rod; on the contrary, a small linear portion of the wall becomes scooped out along the median dorsal line, and eventually separates from the remainder as the rod in question. In the trunk the splitting off of the rod takes place from before backwards, so that the anterior part of it is formed before the posterior.
The section of the subnotochordal rod in the head would appear from my observations on Pristiurus to develop in the same way as in the trunk, and the splitting off from the throat proceeds from before backwards (Pl. 15, fig. 4a, x).
In Torpedo, this rod develops very much later in the head than in the trunk; and indeed my conclusion that it develops in the head at all is only based on grounds of analogy, since in [Pg 458] my oldest Torpedo embryo (just younger than K) there is no trace of it present. In a Torpedo embryo of stage I the subnotochordal rod of the trunk terminated anteriorly by uniting with the wall of the throat. The junction was effected by a narrow pedicle, so that the rod appeared mushroom-shaped in section, the stalk representing the pedicle of attachment.
On the formation of the dorsal aorta, the subnotochordal rod becomes separated from the wall of the gut and the aorta interposed between the two.
The subnotochordal rod attains its fullest development during stage K. Anteriorly it terminates at a point well in front of the ear, though a little behind the end of the notochord; posteriorly it extends very nearly to the extremity of the tail and is almost co-extensive with the postanal section of the alimentary tract, though it does not quite reach so far back as the caudal vesicle (Pl. 18, fig. 6b, x). In stage L it is still fairly large in the tail, though it has begun to atrophy anteriorly. We may therefore conclude that its atrophy, like its development, takes place from before backwards. In the succeeding stages I have failed to find any trace of it, and conclude, as does Professor Semper, that it disappears completely.
Götte[313] is of opinion that the subnotochordal rod is converted into the dorsal lymphatic trunk, and regards it as the anterior continuation of the postanal gut, which he believes to be also converted into a lymphatic trunk. My observations afford no support to these views, and the fact already mentioned, that the subnotochordal rod is nearly co-extensive with the postanal section of the gut, renders it improbable that both these structures are connected with the lymphatic system.
[Pg 459] EXPLANATION OF PLATE 18.
Complete List of Reference Letters.
Nervous System.
ar. Anterior root of spinal nerve. nc. Neural canal. pr. Posterior root of spinal nerve. spn. Spinal nerve. syg. Sympathetic ganglion.
Alimentary Canal.
al. Alimentary canal. alv. Caudal vesicle of the postanal gut. clal. Cloacal section of alimentary canal. du. Duodenum. hpd. Ductus choledochus. pan. pancreas. sœs. Solid œsophagus. spv. Intestine with rudiment of spiral valve. umc. Umbilical canal.
General.
ao. Dorsal aorta. aur. Auricle of heart. cav. Cardinal vein. ch. Notochord. eppp. Epithelial lining of the body-cavity. ir. Interrenal body. me. Mesentery. mp. Muscle-plate. mpl. Muscle-plate sending a prolongation into the limb. po. Primitive ovum. pp. Body-cavity. sd. Segmental duct. st. Segmental tube. ts. Tail swelling. vcau. Caudal vein. x. Subnotochordal rod.
Fig. 1. Transverse section through the anterior abdominal region of an embryo of a stage between K and L. Zeiss B, ocul. 2. Reduced one-third.
The section illustrates the junction of a sympathetic ganglion with a spinal nerve and the sprouting of the muscle-plates into the limbs (mpl).
Fig. 2. Transverse section through the abdominal region of an embryo belonging to stage L. Zeiss B, ocul. 2. Reduced one-third.
The section illustrates the junction of a sympathetic ganglion with a spinal nerve, and also the commencing formation of a branch from the aorta (still solid) which will pass through the sympathetic ganglion, and forms the first sign of the conversion of part of a sympathetic ganglion into one of the suprarenal bodies.
Fig. 3. Longitudinal and vertical section of an embryo of a stage between L and M, shewing the successive junctions of the spinal nerves and sympathetic ganglia.
Fig. 4. Section through the solid œsophagus during stage L. Zeiss A, ocul. 1. The section is taken through the region of the heart, so that the cavity of the auricle (aur) lies immediately below the œsophagus.
Fig. 5. Optical section of the tail of an embryo between stages I and K, shewing the junction between the neural and alimentary canals.
Fig. 6. Four sections through the caudal region of an embryo belonging to stage K, shewing the condition of the postanal section of the alimentary tract. Zeiss A, ocul. 2. An explanation of these figures is given on p. 449.
Fig. 7. Section through the interrenal body of a Scyllium embryo belonging to stage Q. Zeiss C, ocul. 2.
Fig. 8. Portion of a section of the interrenal body of an adult Scyllium. Zeiss C, ocul. 2.
[303] Archiv f. Mic. Anat. Vol. XIII. pp. 194, 195.
[305] This Edition, No. VII. p. 152.
[306] Vide Gasser, Entwicklungsgeschichte der Allantois, etc.
[307] Jenaische Zeitschrift, Vol. VI.
[308] Entwicklungsgeschichte d. Unke.
[309] Lehrbuch d. vergleichenden Embryologie.
[310] Archiv für Micros. Anatomie, Bd. V., and Entwicklungsgeschichte d. Unke.
[311] Quarterly Journal of Microscopic Science, Oct., 1874. [This Edition, No. V.]
[312]
Stammverwandtschaft d. Wirbelthiere u. Wirbellosen
and
Das Urogenitalsystem d. Plagiostomen,
Arb.
Zool.-Zoot. Institut. z. Würzburg, Bd. II.
[313] Entwicklungsgeschichte d. Unke, p. 775.
The present chapter deals with the early development of the heart, the development of the general circulatory system, especially the venous part of it, and the circulation of the yolk-sack. It also contains an account of two bodies which I shall call the suprarenal and interrenal bodies, which are generally described as vascular glands.
The heart.
The first trace of the heart becomes apparent during stage G, as a cavity between the splanchnic mesoblast and the wall of the gut immediately behind the region of the visceral clefts (Pl. 11, fig. 4, ht.).
The body-cavity in the region of the heart is at first double, owing to the two divisions of it not having coalesced; but even in the earliest condition of the heart the layers of splanchnic mesoblast of the two sides have united so as to form a complete wall below. The cavity of the heart is circumscribed by a more or less complete epithelioid (endothelial) layer of flattened cells, connected with the splanchnic wall of the heart by protoplasmic processes. The origin of this lining layer I could not certainly determine, but its connection with the splanchnic mesoblast suggests that it is probably a derivative of this[314]. In [Pg 461] front the cavity of the heart is bounded by the approximation of the splanchnic mesoblast to the wall of the throat, and behind by the stalk connecting the alimentary canal with the yolk-sack.
As development proceeds the ventral wall of the heart becomes bent inwards on each side on a level with the wall of the gut (Plate 11, fig. 4), and eventually becomes so folded in as to form for the heart a complete muscular wall of splanchnic mesoblast. The growth inwards of the mesoblast to form the dorsal wall of the heart does not, as might be expected, begin in front and proceed backwards, but commences behind and is gradually carried forwards.
From the above account it is clear that I have failed to find in Elasmobranchii any traces of two distinct cavities coalescing to form the heart, such as have been recently described in Mammals and Birds; and this, as well as the other features of the formation of the heart in Elasmobranchii, are in very close accordance with the careful description given by Götte[315] of the formation of the heart in Bombinator. The divergence which appears to be indicated in the formation of so important an organ as the heart between Pisces and Amphibians on the one hand, and Aves and Mammalia on the other, is certainly startling, and demands a careful scrutiny. The most complete observations on the double formation of the heart in Mammalia have been made by Hensen, Götte and Kölliker. These observations lead to the conclusion (1) that the heart arises as two independent splits between the splanchnic mesoblast and the hypoblast, each with an epithelioid (endothelial) lining. (2) That the heart is first formed at a period when the folding in of the splanchnopleure to form the throat has [Pg 462] not commenced, and when therefore it would be impossible for it to be formed as a single tube.
In Birds almost every investigator since von Baer has detected more or less clearly the coalescence of two halves to form the unpaired heart[316]. Most investigators have however believed that there was from the first an unpaired anterior section of the heart, and that only the posterior part was formed by the coalescence of two lateral halves. Professor Darlste His, and more recently Kölliker, have stated that there is no such unpaired anterior section of the heart. My own recent observations confirm their conclusions as to the double formation of the heart, though I find that the heart has from the first a Λ-shaped form. At the apex of the Λ the two limbs are only separated by a median partition and are not continuous with the aortic arches, which do not arise till a later period[317]. In the Bird the heart arises just behind the completed throat, and a double formation of the heart appears, in fact, in all instances to be most distinctly correlated with the non-closure of the throat, a non-closure which it must be noted would render it impossible for the heart to arise otherwise than as a double cavity.
In the instances in which the heart arises as a double cavity it is formed before the complete closure of the throat, and in those in which it arises as a single cavity it is formed subsequently to the complete formation of the throat. There is thus a double coincidence which renders the conclusion almost certain, that the formation of the heart as two cavities is a secondary change which has been brought about by variations in the period of the closing in of the wall of the throat.
If the closing in of the throat were deferred and yet the primitive time
of formation of the heart retained, it is clear that such a condition as
may be observed in Birds and Mammals must occur, and that the two halves of
the heart must be formed widely apart, and only eventually united on the
folding in of [Pg 463] the wall of the throat. We may then safely
conclude that the double formation of the heart has no morphological
significance, and does not, as might at first sight be supposed, imply that
the ancestral Vertebrate had two tubes in the place of the present unpaired
heart. I have spoken of this point at considerable length, on account of
the morphological importance which has been attached to the double
formation of the heart. But the views above enunciated are not expressed
for the first time. In the Elements of Embryology we say,
p. 64, The exact mode of development (of the heart) appears
according to our present knowledge to be very different in different cases;
and it seems probable that the differences are in fact the result of
variations in the mode of formation and time of closure of the alimentary
canal.
Götte again in his great work[318]
appears to maintain similar views, though I do not perfectly understand all
his statements. In my review of Kölliker's Embryology[319] this point is still more distinctly enunciated in the
following passage: The primitive wide separation and complete
independence of the two halves of the heart is certainly surprising; but we
are inclined, provisionally at least, to regard it as a secondary condition
due to the late period at which the closing of the throat takes place in
Mammals.
The general circulation.
The chief points of interest in connection with the general circulation centre round the venous system. The arterial arches present no peculiarities: the dorsal aorta, as in all other Vertebrates, is at first double (Pl. 11, fig. 6, ao), and, generally speaking, the arrangement of the arteries accords with what is already known in other forms. The evolution of the venous system deserves more attention.
The cardinal veins are comparatively late developments. There is at first one single primitive vein continuous in front with the heart and underlying the alimentary canal through its præanal and postanal sections. This vein is shewn in section in Pl. 11, fig. 8, V. It may be called either the subintestinal or [Pg 464] splanchnic vein. At the cloaca, where the gut enlarges and comes in contact with the skin, this vein is compelled to bifurcate (Pl. 18, fig. 6,d, v.cau.), and usually the two branches into which it divides are unequal in size. The two branches meet again behind the cloaca and take their course ventral to the postanal section of the gut, and terminate close to the end of the tail, Pl. 18, fig. 6,c, v.cau. In the tail they form what is usually known as the caudal vein. The venous system of Scyllium or Pristiurus, during the early parts of stage K, presents the simple constitution just described.
Before proceeding to describe the subsequent changes which take place in it, it appears to me worth pointing out the remarkable resemblance which the vascular system of an Elasmobranch presents at this stage to that of an ordinary Annelid and Amphioxus. It consists, as does the circulatory system, in Annelids, of a neural vessel (the aorta) and an intestinal vessel, the blood flowing backwards in the latter and forwards in the former. The two in Elasmobranchii communicate posteriorly by a capillary system, and in front by the arterial arches, connected like the similar vessels in Annelids with the branchiæ. Striking as is this resemblance, there is a still closer resemblance between the circulation of the Scyllium embryo at stage K and that of Amphioxus. The two systems are in fact identical except in very small details. The subintestinal vessel, absent or only represented by the caudal vein and in part by the ductus venosus in higher Vertebrates and adult Fish, forms the main and only posterior venous trunk of Amphioxus and the embryo Scyllium. The only noteworthy point of difference between Amphioxus and the embryo Scyllium is the presence of a portal circulation in the former, absent at this stage in the latter; but even this is acquired in Scyllium before the close of stage K, and does not therefore represent a real difference between the two types.
The cardinal veins make their appearance before the close of stage K, and very soon unite behind with the unpaired section of the caudal vein (Pl. 11, fig. 9b, p.cav. and v.). On this junction being effected retrogressive changes take place in the original subintestinal vessel. It breaks up in front into a number of smaller vessels; the lesser of the two branches connecting [Pg 465] it round the cloaca with the caudal vein first vanishes (Pl. 11, fig. 9a, v), and then the larger; and the two cardinals are left as the sole forward continuations of the caudal vein. This latter then becomes prolonged forwards, and the two posterior cardinals open into it some little distance in front of the hind end of the kidneys. By these changes and by the disappearance of the postanal section of the gut the caudal vein is made to appear as a superintestinal and not a subintestinal vessel, and as the direct posterior continuation of the cardinal veins. Embryology proves however that the caudal vein is a true subintestinal vessel[320], and that its connection with the cardinals is entirely secondary.
The invariably late appearance of the cardinal veins in the embryo and their absence in Amphioxus leads me to regard them as additions to the circulatory system which appeared in the Vertebrata themselves, and were not inherited from their ancestors. It would no doubt be easy to point to vessels in existing Annelids which might be regarded as their equivalent, but to do so would be in my opinion to follow an entirely false morphological scent.
The circulation of the yolk-sack.
The observations recorded on this subject are so far as I am acquainted with them very imperfect, and in most cases the arteries and veins appear to have been transposed.
Professor Wyman[321], however, gives a short description of the circulation in Raja Batis, in which he rightly identifies the arteries, though he regards the arterial ring which surrounds the vascular area as equivalent to the venous sinus terminalis of the Bird.
The general features of the circulation are clearly portrayed in the somewhat diagrammatic figures on Pl. 9, in which the arteries are represented red, and the veins blue[322].
[Pg 466] I shall follow the figures on this plate in my descriptions.
Fig. 1 represents my earliest stage of the circulation of the yolk-sack. At this stage there is visible a single aortic trunk passing forwards from the embryo and dividing into two branches. No venous trunk could be detected with the simple microscope, but probably venous channels were present in the thickened edge of the blastoderm.
In fig. 2 the circulation was greatly advanced[323]. The blastoderm has now nearly completely enveloped the yolk, and there remains only a small circular space (yk) not enclosed by it. The arterial trunk is present as before, and divides in front of the embryo into two branches which turn backwards and nearly form a complete ring round the embryo. In general appearance it resembles the sinus terminalis of the area vasculosa of the Bird, but in reality bears quite a different relation to the circulation. It gives off branches only on its inner side.
A venous system of returning vessels is now fully developed, and its relations are very remarkable. There is a main venous ring round the thickened edge of the blastoderm, which is connected with the embryo by a single stem which runs along the seam where the edges of the blastoderm have coalesced. Since the venous trunks are only developed behind the embryo, it is only the posterior part of the arterial ring which gives off branches.
The succeeding stage, fig. 3, is also one of considerable interest. The arterial ring has greatly extended, and now embraces nearly half the yolk, and sends off trunks on its inner side along its whole circumference.
More important changes have taken place in the venous system. The blastoderm has now completely enveloped the yolk, and as a result of this, the venous ring no longer exists, but at the point where it vanished there may be observed a number of smaller veins diverging in a brush-like fashion from the termination of the unpaired trunk which originally connected the venous ring with the heart. This point is indicated in the figure by the letter y. The brush-like divergence of the veins is [Pg 467] a still more marked feature in a blastoderm of a succeeding stage (fig. 4).
The circulation in the succeeding stage (fig. 4) (projected in my figure) only differs in details from that of the previous stage. The arterial ring has become much larger, and the portion of the yolk not embraced (x) by it is quite small. Instead of all the branches from the ring being of nearly equal size, two of them are especially developed. The venous system has undergone no important changes.
In fig. 5 the circulation is represented at a still later stage. The arterial ring has come to embrace the whole yolk, and as a result of this, has in its turn vanished as did the venous ring before it. At this stage of the circulation there is present a single arterial and a single venous trunk. The arterial trunk is a branch of the dorsal aorta, and the venous trunk originally falls into the heart together with the subintestinal or splanchnic vein, but on the formation of the liver enters this and breaks up into capillaries in it. The venous trunk leaves the body on the right side, and the arterial on the left.
The most interesting point to be noticed in connection with the yolk-sack circulation of Scyllium is the fact of its being formed on a completely different type to that of the Amniotic Vertebrates.
The Vascular Glands.
There are in Scyllium two structures which have gone under the name of the suprarenal body. The one of these is an unpaired rod-like body lying between the dorsal aorta and the caudal vein in the region of the posterior end of the kidneys. This body I propose to call the interrenal body. The other is formed by a series of paired bodies situated dorsal to the cardinal veins on branches of the aorta, and arranged segmentally. These bodies I shall call the suprarenal bodies. I propose treating the literature of these bodies together, since they have usually been dealt with in this way, and indeed regarded as parts of the same system. As I hope to shew in the sequel, the origin of these bodies is very different. The interrenal body appears to be [Pg 468] developed from the mesoblast; while my researches on the suprarenal bodies confirm the brilliant investigations of Leydig, shewing that they are formed out of the sympathetic ganglia.
The most important investigations on these bodies have been made by Leydig[324]. In his first researches, Rochen u. Haie, pp. 71, 72, he gives an account of the position and histology of what is probably my interrenal body[325].
The position and relations of the interrenal body vary somewhat
according to Leydig in different cases. He makes the following statement
about its histology. Fat molecules form the chief mass of the body,
which causes its white, or ochre-yellow colour, and one finds freely
embedded in them clear vesicular nuclei.
He then proceeds to state that
this structure is totally dissimilar to that of the Mammalian suprarenal
body, and gives it as his opinion that it is not the same body as this. In
his later researches[326] he abandons this opinion, and
adopts the view that the interrenal body is part of the same system as the
suprarenal bodies to be subsequently spoken of. Leydig describes the
suprarenal bodies as paired bodies segmentally arranged along the ventral
side of the spinal column situated on the successive arteriæ axillares, and
in close connection with one or more sympathetic ganglia. He finds them
formed of lobes, consisting of closed vesicles full of nuclei and cells.
Numerous nerve-fibres are also described as present. With reference to the
real meaning of these bodies he expresses a distinct view. He says[327], As the pituitary body is an integral part of the
brain, so are the suprarenal bodies part of the sympathetic system.
He
re-affirms with still greater emphasis the same view in his Fische u. Reptilien. Though these views have not obtained
much [Pg
469] acceptance, and the accuracy of the histological data on which
they are grounded has been questioned, yet I hope to shew in the sequel not
only that Leydig's statements are in the main true, but that development
proves his conclusions to have been well founded.
Stannius alludes[328] to both these bodies, and though he does not contribute much to Leydig's previous statements, yet he accepts Leydig's position with reference to the relation of the sympathetic and suprarenal bodies[329].
The general text-books of Histology, Kölliker's work, and Eberth's article in Stricker's Histology, do not give much information on this subject; but Eberth, without apparently having examined the point, questions the accuracy of Leydig's statements with reference to the anatomical relations of the sympathetic ganglia and suprarenal bodies.
The last author who has dealt with this subject is Professor Semper[330]. He records observations both on the anatomy and development of these organs. His anatomical observations are in the main confirmatory of those of Leydig, but he shews still more clearly than did Leydig the segmental arrangement of the suprarenal bodies. He definitely regards the interrenal and suprarenal bodies as parts of the same system, and states that in many forms they are continuous (p. 228):
Hier freilich gehen sie bei manchen
Formen...in einen Körper über, welcher zwischen den Enden d. beiden Nieren
liegend dicht an der einfachen Caudalvene sitzt.
With reference to their development he says: They arise then also
completely independently of the kidneys, as isolated segmentally arranged
groups of mesoderm cells between the convolutions of the segmental organs;
only anteriorly do they stretch beyond them, and extend quite up to the
pericardium.
To Semper's statements I shall return, but now pass on to my own observations. The paired suprarenal bodies are dealt with first.
[Pg 470] The suprarenal bodies.
My observations on these bodies in the adult Scyllium have only been made with specimens hardened in chromic acid, and there are many points which deserve a fuller investigation than I have been able to give them.
The general position and relations of the suprarenal bodies have been fully given by Leydig and Semper, and I have nothing to add to their statements. They are situated on branches of the aorta, segmentally arranged, and extend on each side of the vertebral column from close behind the heart to the posterior part of the body-cavity. The anterior pair are the largest, and are formed apparently from the fusion of two bodies[331]. When these bodies are examined microscopically, their connection with the sympathetic ganglia becomes at once obvious. Bound up in the same sheath as the anterior one is an especially large ganglion already alluded to by Leydig, and sympathetic ganglia are more or less distinctly developed in connection with all the others. There is however considerable irregularity in the development and general arrangement of the sympathetic ganglia, which are broken up into a number of small ganglionic swellings, on some of which an occasional extra suprarenal body is at times developed. As a rule it may be stated that there is a much smaller ganglionic development in connection with the posterior suprarenal bodies than with the anterior.
The different suprarenal bodies exhibit variations in structure mainly dependent on the ganglion cells and nerves in them, and their typical structure is best exhibited in a posterior one, in which there is a comparatively small development of nervous elements.
A portion of a section through one of these is represented on Pl. 19, fig. 6, and presents the following features. Externally there is present a fibrous capsule, which sends in the septa, imperfectly dividing up the body into a series of alveoli or lobes. Penetrating and following the septa there is a rich capillary network. The parenchyma of the body itself exhibits a well-marked [Pg 471] distinction in the majority of instances into a cortical and medullary substance. The cortical substance is formed of rather irregular columnar cells, for the most part one row deep, arranged round the periphery of the body. Its cells measure on about an average .03 Mm. in their longest diameter. The medullary substance is more or less distinctly divided into alveoli, and is formed of irregularly polygonal cells; and though it is difficult to give an estimate of their size on account of their irregularity, .021 Mm. may be taken as probably about the diameter of an average cell. The character of the cortical and medullary cells is nearly the same, and the cells of the two strata appear rather to differ in shape than in any other essential point. The protoplasm of both has a markedly yellow tinge, giving to the suprarenal bodies a yellowish brown colour. The nuclei are small compared to the size of the cells, being about .009 Mm. in both cortical and medullary cells. In the anterior suprarenal body there is a less marked distinction between the cortical and the medullary layers, and a less pronounced yellow coloration of the whole, than in the posterior bodies. The suprarenal bodies are often partially or completely surrounded by a lymphoid tissue, which is alluded to in the account of their development.
The most interesting features of my sections of the anterior bodies are the relations they bring to light between the sympathetic ganglia and the suprarenal bodies. In the case of one of the posterior suprarenal bodies, a small ganglion is generally found attached to both ends of the body, and invested in the same sheath; in addition to this a certain number of ganglion cells (very conspicuous by their size and other characters) are to be found scattered through the body. In the anterior suprarenal bodies the development of ganglion cells is very much greater. If a section is taken through the region where the large sympathetic ganglion (already mentioned) is attached to the body, one half of the section is composed mainly of sympathetic ganglion cells and nerve fibres, and the other of suprarenal tissue, but the former spread in considerable numbers into the latter. A transverse section through the suprarenal body in front of, or behind this point, is still more instructive. One of these is represented in Pl. 19, fig. 7. The suprarenal tissue is not [Pg 472] inserted, but fills up the whole space within the outline of the body. At one point a nerve (n) is seen to enter. In connection with this are a number of ganglion cells, the exact distribution of which has been reproduced. They are scattered irregularly throughout the suprarenal body, but are more concentrated at the smaller than at the large end. It is this small end which, in succeeding sections, is entirely replaced by a sympathetic ganglion. Wavy fibres (which I take to be nervous) are distributed through the suprarenal body in a manner which, roughly speaking, is proportional to the number of ganglion cells. At the large end of the body, where there are few nerve cells, the typical suprarenal structure is more or less retained. Where the nerve fibres are more numerous at the small end of the section, they give to the tissue a somewhat peculiar appearance, though the individual suprarenal cells retain their normal structure. In a section of this kind the ganglion and nerves are clearly so intimately united with the suprarenal body as not to be separable from it.
The question naturally arises as to whether there are cells of an intermediate character between the ganglion cells and the cells of the suprarenal body. I have not clearly detected any such, but my observations are of too limited a character to settle the point in an adverse sense.
The embryological part of my researches on these bodies is in reality an investigation of later development of the sympathetic ganglia. The earliest stages in the development of these have already been given[332], and I take them up here as they appear during stage L, and shall confine my description to the changes they undergo in the anterior part of the trunk. They form during stage L irregular masses of cells with very conspicuous branches connecting them with the spinal nerves (Pl. 18, fig. 3). There may be noticed at intervals solid rods of cells passing from the bodies to the aorta, Pl. 18, fig. 2. These rods are the rudiments of the aortic branches to which the suprarenal bodies are eventually attached.
In a stage between M and N the trunks connecting these bodies with the spinal nerves are much smaller and less easy to see than during stage L. In some cases moreover the nerves [Pg 473] appear to attach themselves more definitely to a central and inner part of the ganglia than to the whole of them. This is shewn in Pl. 19, fig. 8, and I regard it as the first trace of a division of the primitive ganglia into a suprarenal part and a ganglionic part. The branches from the aorta have now a definite lumen, and take a course through the centre of these bodies, as do the aortic branches in the adult.
By stage O these bodies have acquired a distinct mesoblastic investment, which penetrates into their interior, and divides it, especially in the case of the anterior bodies, into a number of distinct alveoli. These alveoli are far more distinct in some parts of the bodies than in others. The nerve-trunks uniting the bodies with the spinal nerves are (at least in specimens hardened in picric and chromic acids) very difficult to see, and I have failed to detect that they are connected with special parts of the bodies, or that the separate alveoli differ much as to the nature of their constituent cells. The aortic branches to the bodies are larger than in the previous stage, and the bodies themselves fairly vascular.
By stage Q (Pl. 19, fig. 9) two distinct varieties of cells are present in these bodies. One of these is large, angular, and strikingly resembles the ganglion cells of the spinal nerves at the same period. This variety is found in separate lobules or alveoli on the inner border of the bodies. I take them to be true ganglion cells, though I have not seen them in my sections especially connected with the nerves. The cells of the second variety are also aggregated in special lobules, and are very markedly smaller than the ganglionic cells. They form, I imagine, the cells of the true suprarenal tissue. At this and the earlier stage lymphoid tissue, like that surrounding the suprarenal bodies in the adult, is found adjacent to these bodies.
Stage Q forms my last embryonic stage, and it may perhaps be asked on what grounds I regard these bodies as suprarenal bodies at all and not as simple sympathetic ganglia.
My determination mainly rests on three grounds: (1) That a branch from the aorta penetrates these bodies and maintains exactly the same relations to them that the same branches of the aorta do in the adult to the true suprarenal bodies. (2) That the bodies are highly vascular. (3) That in my last stage they [Pg 474] become divided into a ganglionic and a non-ganglionic part, with the same relations as the ganglia and suprarenal tissue in the adult. These grounds appear to me to afford ample justification for my determinations, and the evidence adduced above appears to me to render it almost certain that the suprarenal tissue is a product of the primitive ganglion and not introduced from the mesoblast without, though it is not to be denied that a more complete investigation of this point than it has been possible for me to make would be very desirable.
Professor Semper states that he only made a very slight embryological investigation of these bodies, and probably has only carefully studied their later stages. He has accordingly overlooked the branches connecting them with the spinal nerves, and has not therefore detected the fact that they develop as parts of the sympathetic nervous system. I feel sure that if he re-examines his sections of younger embryos he will not fail to discover the nerve-branches described by me. His descriptions apart from this point accord fairly well with my own. The credit of the discovery that these bodies are really derivatives of the sympathetic nervous system is entirely Leydig's: my observations do no more than confirm his remarkable observations and well-founded conclusions.
Interrenal body.
My investigations on the interrenal body in the adult are even less complete than those on the suprarenal bodies. I find the body forming a small rod elliptical in section in the posterior region of the kidney between the dorsal aorta and unpaired caudal vein. Some little distance behind its front end (and probably not at its thickest point) it measured in one example, of which I have sections, a little less than a millimetre in its longest diameter. Anteriorly it overlaps the suprarenal bodies, and I failed to find any connection between them and it. On this point my observations do not accord with those of Professor Semper. I have however only been able to examine hardened specimens.
It is, vide Pl. 18, fig. 8, invested by a fairly thick tunica propria, which sends in septa, dividing it into rather well-marked [Pg 475] lobules or alveoli. These are filled with polygonal cells, which form the true parenchyma of the body. These cells are in my hardened specimens not conspicuous by the number of oil-globules they contain, as might have been expected from Leydig's description[333]. They are rather granular in appearance, and are mainly peculiar from the somewhat large size of the nucleus. The diameter of an average cell is about .015 Mm., and that of the nucleus about .01 to .012. The nuclei are remarkably granular. The septa of the body are provided with a fairly rich capillary network.
At the first glance there is some resemblance in structure between the tissues of the suprarenal and interrenal bodies, but on a closer inspection this resemblance resolves itself into both bodies being divided up into lobules by connective-tissue septa. There is in the interrenal body no distinction between cortical and medullary layers as in the suprarenal. The cells of the two bodies have very different characters, as is demonstrated by a comparison of the relative diameters of the nuclei and the cells. The cells of the suprarenal bodies are considerably larger than those of the interrenal (.021 to .03 as compared to .015), yet the nuclei of the larger cells of the former body do not equal in size those of the smaller cells of the latter (.009 as compared to .01).
My observations both on the coarser anatomy and on the histology of the interrenal body in the adult point to its being in no way connected with the suprarenal bodies, and are thus in accordance with the earlier and not the later views of Leydig.
The embryology of this body (under the title of suprarenal body) was first described in my preliminary account of the development of the Elasmobranch Fishes[334]. A short account of its embryonic structure was given, and I stated that although I had not fully proved the point, yet I believed it to be derived from the wall of the alimentary canal. As will be shewn in the sequel this belief was ill-founded, and the organ in question is derived from the mesoblast. Allusion has also been made to it [Pg 476] by Professor Semper, who figures it at an early stage of development, and implies that it arises in the mesoblast and in connection with the suprarenal body. It appears at stage K as a rod-like aggregate of mesoblast cells, rather more closely packed than their neighbours, between the two kidneys near their hinder ends (Plate 11, fig. 9a, su). The posterior and best marked part of it does not extend further forwards than the front end of the large intestine, and reaches backwards nearly as far as the hinder end of the kidneys. This part of the body lies between the caudal vein and dorsal aorta.
At about the point where the unpaired caudal vein divides into the two cardinals, the interrenal body becomes less well marked off from the surrounding tissue, though it may be traced forward for a considerable distance in the region of the small intestine. It retains up to stage Q its original extension, but the anterior part becomes quite definite though still of a smaller calibre than the posterior. In one of my examples of stage O the two divisions were separated by a small interval, and not as in other cases continuous. I have not determined whether this was an accidental peculiarity or a general feature. I have never seen any signs of the interrenal body becoming continuous with the suprarenal bodies, though, as in the adult, the two bodies overlap for a considerable distance.
The histology of the interrenal body in the embryonic periods is very simple. At first it is formed of cells differing from those around in being more circular and more closely packed. By stage L its cells have acquired a character of their own. They are still spherical or oval, but have more protoplasm than before, and their nucleus becomes very granular. At the same time the whole body becomes invested by a tunic of spindle-shaped mesoblast cells. By stage O it begins to be divided into a number of separate areas or lobes by septa formed of nucleated fibres. These become more distinct in the succeeding stages up to Q (Pl. 18, fig. 7), and in them a fair number of capillaries are formed.
From the above description it is clear that embryology lends no more countenance than does anatomy to the view that the interrenal bodies belong to the same system as the suprarenal, and it becomes a question with which (if of either) of these two [Pg 477] bodies the suprarenal bodies of the higher Vertebrata are homologous. This question I shall not attempt to answer in a definite way. My own decided belief is that the suprarenal bodies of Scyllium are homologous with the suprarenal bodies of Mammalia, and a good many points both in their structure and position might be urged in favour of this view. In the mean time, however, it appears to me better to wait before expressing a definite opinion till the embryonic development of the suprarenal bodies has been worked out in the higher Vertebrata.
EXPLANATION OF PLATE 19.
Complete List of Reference Letters.
Nervous System.
n. Nerve. spn. Spinal nerve. syg. Sympathetic ganglion.
Alimentary Canal.
cl. Cloaca. incl. Cloacal involution. œep. Œsophageal epithelium. pan. Pancreas. th. Thyroid body.
General.
abp. Abdominal pocket (pore). aur. Auricle. cav. Cardinal vein. cauv. Caudal vein. ly. Lymphoid tissue. mm. Muscles. od. Oviduct. pc. Pericardium. pp. body-cavity. sr. Suprarenal body. u. Ureter. vao. Ventral aorta (anterior continuation of bulbus arteriosus). ven. Ventricle. wd. Wolffian duct.
Figs. 1a, 1b, 1c. Three sections through the cloacal region of an embryo belonging to stage O. 1a is the anterior of the three sections. Zeiss A, ocul. 2. Reduced one-third.
1a shews the cloacal involution at its deepest part abutting on the cloacal section of the alimentary tract.
1b is a section through a point somewhat behind this close to the opening of the Wolffian ducts into the cloaca.
1c shews the opening to the exterior in the posterior part of the cloaca, and also the rudiments of the two abdominal pockets (abp).
Fig. 2. Section through the cloacal region of an embryo belonging to stage P. Zeiss A, ocul. 2.
The figure shews the solid anterior extremity of the cloacal involution.
Fig. 3. Longitudinal vertical section through the thyroid body in a stage between O and P. Zeiss a a, ocul. 1.
The figure shews the solid thyroid body (th) connected in front with throat, and terminating below the bulbus arteriosus.
[Pg 478] Fig. 4. Pancreas (pan) and adjoining part of the alimentary tract in longitudinal section, from an embryo between stages L and M. Zeiss A, ocul. 2.
Fig. 5. Portion of liver network of stage L. Zeiss C, ocul. 2. The section is intended to illustrate the fact that the tubules or cylinders of which the liver is composed are hollow and not solid. Between the liver tubules are seen blood spaces with distinct walls, and blood corpuscles in their interior.
Fig. 6. Section through part of one of the suprarenal bodies of an adult Scyllium hardened in chromic acid. Zeiss C, ocul. 2. The section shews the columnar cells forming the cortex and the more polygonal cells of the medulla.
Fig. 7. Transverse section through the anterior suprarenal body of an adult Scyllium. Zeiss B, ocul. 2. Reduced one-third. The tissue of the suprarenal body has not been filled in, but only the sympathetic ganglion cells which are seen to be irregularly scattered through the substance of the body. The entrance of the nerve (n) is shewn, and indications are given of the distribution of the nerve-fibres.
Fig. 8. Section through the sympathetic ganglion of a Scyllium embryo between stages M and N, shewing the connecting trunk between the suprarenal body and the spinal nerve (spn), and the appearance of an indication in the ganglion of a portion more directly connected with the nerve. Zeiss D, ocul. 2.
Fig. 9. Section through one of the anterior sympathetic ganglia of an embryo of stage Q, shewing its division into a true ganglionic portion (syg), and a suprarenal body (sr). Zeiss C, ocul. 2.
[314] From observations on the development of the heart in the Fowl, I have been able to satisfy myself that the epithelioid lining of the heart is derived from the splanchnic mesoblast. When the cavity of the heart is being formed by the separation of the splanchnic mesoblast from the hypoblast, a layer of the former remains close to the hypoblast, but connected with the main mass of the splanchnic mesoblast by protoplasmic processes. A second layer next becomes split from the splanchnic mesoblast, connected with the first layer by the above-mentioned protoplasmic processes. These two layers form the epithelioid lining of the heart; between them is the cavity of the heart, which soon loses the protoplasmic trabeculæ which at first traverse it.
[315] Bischoff has recently stated, Historisch-kritische Bemerkungen ii. d. Entwicklung d. Säugethiereier, that Götte has found a double formation of the heart in Bombinator. It may seem bold to question the accuracy of Bischoff's interpretation of writings in his own language, but I have certainly failed to gather this either from Dr Götte's text or figures.
[316] Vide Elements of Embryology, Foster and Balfour, pp. 64-66.
[317] Professor Bischoff (loc. cit.) throws doubts upon the double formation of the heart, and supports his views by Dr Foster's and my failure to find any trace of a double formation of the heart in the chick. Professor Bischoff must, I think, have misunderstood our description, which contains a clear account of the double formation of the heart.
[318] Entwicklungsgeschichte d. Unke, pp. 779, 780, 781.
[319] Journal of Anatomy and Physiology, Vol. X. p. 794.
[320] The morphological importance of this point is considerable. It proves, for instance, that the hæmal arches of the vertebræ in the tail (vide pp. 373 and 374) potentially, at any rate, encircle the gut and enclose the body-cavity as completely as the ribs which meet in the median ventral line may be said to do anteriorly.
[321] Memoirs of the American Academy of Arts and Sciences, Vol. IX.
[322] I may state that my determinations of the arrangement of the circulation were made by actual observation of the flow of the blood under the microscope.
[323] My figure may be compared with that of Leydig, Rochen und Haie, Plate III. fig. 6. Leydig calls the arterial ring the sinus terminalis, and appears to regard it as venous, but his description is so short that this point is not quite clear.
[324] Rochen und Haie and Untersuchung. ü. Fische u. Reptilien.
[325] I do not feel sure that Leydig's unpaired suprarenal body is really my interrenal body, or at any rate it alone. The point could no doubt easily be settled with fresh specimens, but these I unfortunately cannot at present obtain. My doubts rest partly on the fact that, in addition to my interrenal body, other peculiar masses of tissue (which may be called lymphoid in lieu of a better name) are certainly present around some of the larger vessels of the kidneys which are not identical in structure and development with my interrenal body, and partly that Stannius' statements (to be alluded to directly) rather indicate the existence of a second unpaired body in connection with the kidneys, though I do not fully understand his descriptions.
[326] Fische u. Reptilien, p. 14.
[327] Rochen u. Haie, p. 18.
[328] Vergleichende Anatomie, II. Auflage.
[329] Stannius' description is not quite intelligible, but appears to point to the existence of a third kind of body connected with the kidney. From my own observations (vide above), I am inclined to regard it as probable that such a third body exists.
[330]
Urogenitalsystem d. Plagiostomen.
Arb.
zool.-zoot. Inst. z. Würzburg, Vol. II.
[331] There is a very good figure of them in Semper's paper, Pl. XXI. fig. 3.
[332] Antea, pp. 394-396.
[333] Perhaps the body I am describing is not identical with Leydig's posterior suprarenal body. I do not, as mentioned above, feel satisfied that it is so from Leydig's description.
[334] Quarterly Journal of Microscopic Science, October, 1874. [This edition No. V.]
The earliest stages in the development of the excretory system have already been described in a previous chapter[335] of this memoir, and up to the present time no investigator, with the exception of Dr Alex. Schultz[336], has gone over the same ground. Dr Schultz' descriptions are somewhat brief, but differ from my own mainly in stating that the segmental duct arises from an involution instead of as a solid knob. This discrepancy is, I believe, due to Dr Schultz drawing his conclusions as to the development of the segmental duct from its appearance at a comparatively late stage. He appears to have been unacquainted with my earlier descriptions.
The adult anatomy and later stages in the development of the excretory organs form the subject of the present chapter, and stand in marked contrast to the earlier stages in that they have been dealt with in a magnificent monograph[337] by Professor Semper, whose investigations have converted this previously almost unknown field of vertebrate embryology into one of the most fully explored parts of the whole subject. Reference is frequently made to this monograph in the succeeding pages, but my references, numerous as they are, give no adequate idea of the completeness and thoroughness of Professor Semper's investigations. In Professor Semper's monograph are embodied the results of a considerable number of preliminary papers published by him in his Arbeiten and in the Centralblatt. The excretory organs of Elasmobranchii have also formed the subject [Pg 480] of some investigations by Dr Meyer[338] and by myself[339]. Their older literature is fully given by Professor Semper. In addition to the above-cited works, there is one other paper by Dr Spengel[340] on the Urinogenital System of Amphibians, to which reference will frequently be made in the sequel, and which, though only indirectly connected with the subject of this chapter, deserves special mention both on account of the accuracy of the investigations of which it forms the record, and of the novel light which it throws on many of the problems of the constitution of the urinogenital system of Vertebrates.
Excretory organs and genital ducts in the adult.
The kidneys of Scyllium canicula are paired bodies in contact along the median line. They are situated on the dorsal wall of the abdominal cavity, and extend from close to the diaphragm to a point a short way behind the anus. Externally, each appears as a single gland, but by the arrangement of its ducts may be divided into two distinct parts, an anterior and a posterior. The former will be spoken of as the Wolffian body, and the latter as the kidney, from their respective homology with the glands so named in higher Vertebrates. The grounds for these determinations have already been fully dealt with both by Semper[341] and by myself.
Externally both the Wolffian body and the kidney are more or less clearly divided into segments, and though the breadth of both glands as viewed from the ventral surface is fairly uniform, yet the hinder part of the kidney is very much thicker and bulkier than the anterior part and than the whole of the Wolffian body. In both sexes the Wolffian body is rather longer than the kidney proper. Thus in a male example, 33 centimetres [Pg 481] long, the two glands together measured 8¼ centimetres and the kidney proper only 3½. In the male the Wolffian bodies extend somewhat further forwards than in the female. Leaving the finer details of the glands for subsequent treatment, I pass at once to their ducts. These differ slightly in the two sexes, so that it will be more convenient to take the male and female separately.
A partly diagrammatic representation of the kidney and Wolffian body of the male is given on Pl. 20, fig. 1. The secretion of the Wolffian body is carried off by a duct, the Wolffian duct (w.d.), which lies on the ventral surface of the gland, and receives a separate ductule from each segment (Pl. 20, fig. 5). The main function of the Wolffian duct in the male is, however, that of a vas deferens. The testicular products are brought to it through the coils of the anterior segments of the Wolffian body by a number of vasa efferentia, the arrangement of which is treated of on pp. 487, 488. The section of the Wolffian duct which overlies the Wolffian body is much contorted, and in adult individuals at the generative period enormously so. The duct often presents one or two contortions beyond the hind end of the Wolffian body, but in the normal condition takes a straight course from this point to the unpaired urinogenital cloaca, into which it falls independently of its fellow of the opposite side. It receives no feeders from the kidney proper.
The excretion of the kidney proper is carried off not by a single duct, but by a series of more or less independent ducts, which, in accordance with Prof. Semper's nomenclature, will be spoken of as ureters. These are very minute, and their investigation requires some care. I have reason, from my examinations of this and other species of Elasmobranchii, to believe that they are, moreover, subject to considerable variations, and the following description applies to a definite individual. Nine or possibly ten distinct ureters, whose arrangement is diagrammatically represented in fig. 1, Pl. 20, were present on each side. It will be noticed that, whereas the five hindermost are distinct till close to their openings into the urinogenital cloaca, the four anterior ones appear to unite at once into a single duct, but are probably only bound up in a common sheath. The ureters fall into the common urinogenital cloaca, [Pg 482] immediately behind the opening of the Wolffian duct (so far as could be determined), by four apertures on each side. In a section made through the part of the wall of the cloaca containing the openings of the ureters of both sides, there were present on the left side (where the section passed nearer to the surface than on the right) four small openings posteriorly, viz. the openings of the ureters and one larger one anteriorly, viz. the opening of the Wolffian duct. On the other side of the section where the level was rather deeper, there were five distinct ducts cut through, one of which was almost on the point of dividing into two. This second section proves that, in this instance at least, the two ureters did not unite till just before opening into the urinogenital cloaca. The same section also appeared to shew that one of the ureters fell not into the cloaca but into the Wolffian duct.
As stated above both the Wolffian duct and the ureters fall into an unpaired urinogenital cloaca. This cloaca communicates at one end with the general cloaca by a single aperture situated at the point of a somewhat conspicuous papilla, just behind the anus (Pl. 20, fig. 1, o), and on the other it opens freely into a pair of bladders, situated in close contact with each other, on the ventral side of the kidney (Pl. 20, fig. 1, sb). To these bladders Professor Semper has given the name uterus masculinus, from having supposed them to correspond with the lower part of the oviducts of the female. This homology he now admits to be erroneous, and it will accordingly be better to drop the name uterus masculinus, for which may be substituted seminal bladder—a name which suits their function, since they are usually filled with semen at the generation season. The seminal bladders communicate with the urinogenital cloaca by wide openings, and it is on the borders of these openings that the mouths of the Wolffian duct and ureters must be looked for. My embryological investigations, though they have not been specially directed to this point, seem to shew that the seminal bladders do not arise during embryonic life, and are still absent in very young individuals. It seems probable that both the bladders and the urinogenital cloaca are products of the lower extremities of the Wolffian duct. The only other duct requiring any notice in the male is the rudimentary oviduct. As was first [Pg 483] shewn by Semper, rudiments of the upper extremities of the oviducts, with their abdominal openings, are to be found in the male in the same position as in the female, on the front surface of the liver.
In the female the same ducts are present as in the male, viz. the Wolffian duct and the ureters. The part of the Wolffian duct which receives the secretion of the Wolffian body is not contorted, but is otherwise similar to the homologous part of the Wolffian duct in the male. The Wolffian ducts of the two sides fall independently into an unpaired urinal cloaca, but their lower ends, instead of remaining simple as in the male, become dilated into urinary bladders. Vide Pl. 20, fig. 2. There were nine ureters in the example dissected, whose arrangement did not differ greatly from that in the male—the hinder ones remaining distinct from each other, but a certain amount of fusion, the extent of which could not be quite certainly ascertained, taking place between the anterior ones. The arrangement of the openings of these ducts is not quite the same as in the male. A somewhat magnified representation of it is given in Pl. 20, fig. 3, o.u. The two Wolffian ducts meet at so acute an angle that their hindermost extremities are only separated by a septum. In the region of this septum on the inner walls of the two Wolffian ducts were situated the openings of the ureters, of which there were five on each side arranged linearly. In a second example, also adult, I found four distinct openings on each side similarly arranged to those in the specimen described. Professor Semper states that all the ureters in the female unite into a single duct before opening into the Wolffian duct. It will certainly surprise me to find such great variations in different individuals of this species as is implied by the discrepancy between Professor Semper's description and my own.
The main difference between the ureters in the male and female consists in their falling into the urinogenital cloaca in the former and into the Wolffian duct in the latter. Since, however, the urinogenital cloaca is a derivative of the Wolffian duct, this difference between the two sexes is not a very important one. The urinary cloaca opens, in the female, into the general cloaca by a median papilla of somewhat smaller dimensions than the corresponding papilla in the male. Seminal [Pg 484] bladders are absent in the female, though possibly represented by the bladder-like dilatations of the Wolffian duct. The oviducts, whose anatomy is too well known to need description, open independently into the general cloaca.
Since the publication of Professor Semper's researches on the urinogenital system of Elasmobranch fishes, it has been well known that, in most adult Elasmobranchii, there are present a series of funnel-shaped openings, leading from the perivisceral cavity, by the intermediation of a short canal, into the glandular tubuli of the kidney. These openings are called by Professor Semper, Segmentaltrichter, and by Dr Spengel, in his valuable work on the urogenital system of Amphibia, Nephrostomen. In the present work the openings will be spoken of as segmental openings, and the tubes connected with them as segmental tubes. Of these openings there are a considerable number in the adults of both sexes of Scy. canicula, situated along the inner border of each kidney. The majority of them belong to the Wolffian body, though absent in the extreme anterior part of this. In very young examples a few certainly belong to the region of the kidney proper. Where present, there is one for each segment[342]. It is not easy to make certain of their exact number. In one male I counted thirteen. In the female it is more difficult than in the male to make this out with certainty, but in one young example, which had left the egg but a short time, there appeared to be at least fourteen present. According to Semper there are thirteen funnels in both sexes—a number which fairly well agrees with my own results. In the male, rudiments of segmental tubes are present in all the anterior segments of the Wolffian body behind the vasa efferentia, but it is not till about the tenth segment that the first complete one is present. In the female a somewhat smaller number of the anterior segments, six or seven, are without segmental tubes, or only possess them in a rudimentary condition.
A typical segment of the Wolffian body or kidney, in the sense in which this term has been used above, consists of a number of factors, each of which will be considered in detail with reference to its variations. On Pl. 20, fig. 5, is represented [Pg 485] a portion of the Wolffian body with three complete segments and part of a fourth. If one of these be selected, it will be seen to commence with (1) a segmental opening, somewhat oval in form (st.o) and leading directly into (2) a narrow tube, the segmental tube, which takes a more or less oblique course backwards, and, passing superficially to the Wolffian duct (w.d), opens into (3) a Malpighian body (p.mg) at the anterior extremity of an isolated coil of glandular tubuli. This coil forms the fourth section of each segment, and starts from the Malpighian body. It consists of a considerable number of rather definite convolutions, and after uniting with tubuli from one or two (according to size of the segment) accessory Malpighian bodies (a.mg), smaller than the one into which the segmental tube falls, eventually opens by a (5) narrowish tube into the Wolffian duct at the posterior end of the segment. Each segment is completely isolated (except for certain rudimentary structures to be alluded to shortly) from the adjoining ones, and never has more than one segmental tube and one communication with the Wolffian duct.
The number and general arrangement of the segmental tubes have already been spoken of. Their openings into the body-cavity are, in Scyllium, very small, much more so than in the majority of Elasmobranchii. The general appearance of a segmental tube and its opening is somewhat that of a spoon, in which the handle represents the segmental tube, and the bowl the segmental opening. Usually amongst Elasmobranchii the openings and tubes are ciliated, but I have not determined whether this is the case in Scy. canicula, and Semper does not speak definitely on this point. From the segmental openings proceed the segmental tubes, which in the front segments have nearly a transverse direction, but in the posterior ones are directed more and more obliquely backwards. This statement applies to both sexes, but the obliquity is greater in the female than in the male.
As has been said, each segmental tube normally opens into a Malpighian body, from which again there proceeds the tubulus, the convolutions of which form the main mass of each segment. This feature can be easily seen in the case of the Malpighian bodies of the anterior part of the Wolffian gland in young [Pg 486] examples, and sometimes fairly well in old ones, of either sex[343]. There is generally in each segment a second Malpighian body, which forms the commencement of a tubulus joining that from the primary Malpighian body, and, where the segments are larger, there are three, and possibly in the hinder segments of the Wolffian gland and segments of the kidney proper, more than three Malpighian bodies.
The accessory Malpighian bodies, or at any rate one of them, appear to have curious relations to the segmental tubes. The necks of some of the anterior segmental tubes (Pl. 20, fig. 5) close to their openings into the primary Malpighian bodies are provided with a small knob of cells which points towards the preceding segment and is usually connected with it by a fibrous band. This knob is most conspicuous in the male, and in very young animals or almost ripe embryos. In several instances in a ripe male embryo it appeared to me to have a lumen, and to be continued directly forwards into the accessory Malpighian body of the preceding segment. One such case is figured in the middle segment on Pl. 20, fig. 5. In this embryo segmental tubes were present in the segments immediately succeeding those connected with the vasa efferentia, and at the same time these segments contained ordinary and accessory Malpighian bodies. The segmental tubes of these segments were not, however, connected with the Malpighian body of their proper segment, but instead, turned forwards and entered the segment in front of that to which they properly belonged. I failed to trace them quite definitely to the accessory Malpighian body of the preceding segment, but, in one instance at least, there appeared to me to be present a fibrous connection, which is shewn in the figure already referred to, Pl. 20, fig. 5, r.st. In any case it can hardly be doubted that this peculiarity of the foremost segmental tubes is related to what would seem to be the normal arrangement in the next few succeeding segments, where each segmental tube is connected with a Malpighian body in its own segment, and more or less distinctly with an accessory Malpighian body in the preceding segment.
[Pg 487] In the male the anterior segmental tubes, which even in the embryo exhibit signs of atrophy, become in the adult completely aborted (as has been already shewn by Semper), and remain as irregular tubes closed at both ends, which for the most part do not extend beyond the Wolffian duct (Pl. 20, fig. 4, r.st). In the adult, the first two or three segments with these aborted tubes contain only accessory Malpighian bodies; the remaining segments, with aborted segmental tubes, both secondary and primary Malpighian bodies. In neither case are the Malpighian bodies connected with the aborted tubes.
The Malpighian bodies in Scyllium present no special peculiarities. The outer layer of their capsule is for the most part formed of flattened cells; but, between the opening of the segmental tube and the efferent tubulus of the kidney, their cells become columnar. Vide Pl. 20, fig. 5. The convoluted tubuli continuous with them are, I believe, ciliated in their proximal section, but I have not made careful investigations with reference to their finer structure. Each segment is connected with the Wolffian duct by a single tube at the hinder end of the segment. In the kidney proper, these tubes become greatly prolonged, and form the ureters.
It has already been stated that the semen is carried by vasa efferentia from the testes to the anterior segments of the Wolffian body, and thence through the coils of the Wolffian body to the Wolffian duct. The nature of the vasa will be discussed in the embryological section of this chapter: I shall here confine myself to a simple description of their anatomical relations. The consideration of their connections naturally falls under three heads: (1) the vasa efferentia passing from the testes to the Wolffian body, (2) the mode in which these are connected with the Wolffian body, and (3) with the testis.
In Pl. 20, fig. 4, drawn for me from nature by my friend Mr Haddon, are shewn the vasa efferentia and their junctions both with the testes and the kidney. This figure illustrates better than any description the anatomy of the various parts. Behind there are two simple vasa efferentia (v.e.) and in front a complicated network of vasa, which might be regarded as formed of either two or four main vessels. It will be shewn in the sequel that it is really formed of four distinct vessels. [Pg 488] Professor Semper states that there is but a single vas efferens in Scyllium canicula, a statement which appears to me unquestionably erroneous. All the vasa efferentia fall into a longitudinal duct (l.c), which is connected in succession with the several segments of the Wolffian body (one for each vas efferens) which appertain to the testis. The hind end of the longitudinal duct is simple, and ends blindly close to its junction with the last vas efferens; but in front, where the vasa efferentia are complicated, the longitudinal duct also has a complicated constitution, and forms a network rather than a simple tube. It typically sends off a duct to join the coils of the Wolffian body between each pair of vasa efferentia, and is usually swollen where this duct parts from it. A duct similar to this has been described by Semper as Nierenrandcanal in several Elasmobranchii, but its existence is expressly denied in the case of Scyllium! It is usually found in Amphibia, as we know from Bidder and Spengel's researches. Spengel calls it Längscanal des Hoden; the vessels from it into the kidney he calls vasa efferentia, and the vessels to it, which I speak of as vasa efferentia, he calls Quercanale.
The exact mode of junction of the separate vasa efferentia with the testis is difficult to make out on account of the opacity of the basal portion of the testis. My figure shews that there is a network of tubes (formed of four main tubes connected by transverse branches) which is a continuation of the anterior vasa efferentia, and joined by the two posterior ones. These tubes receive the tubuli coming from the testicular ampullæ. The whole network may be called, with Semper, the testicular network. While its general relations are represented in my figure, the opacity of the testes was too great to allow of all the details being with certainty filled in.
The kidneys of Scyllium stellare, as might be expected, closely resemble those of Scy. canicula. The ducts of the kidney proper, have, in the former species, a larger number of distinct openings into the urinogenital cloaca. In two male examples I counted seven distinct ureters, though it is not impossible that there may have been one or two more present. In one of my examples the ureters had seven distinct openings into the cloaca, in the other five openings. In a female I counted eleven ureters opening into the Wolffian duct by seven distinct openings. [Pg 489] In the remaining parts of the excretory organs the two species of Scyllium resemble each other very closely.
As may be gathered from Prof. Semper's monograph, the excretory organs of Scyllium canicula are fairly typical for Elasmobranchii generally. The division into kidney and Wolffian body is universal. The segmental openings may be more numerous and larger, e.g. Acanthias and Squatina, or absent in the adult, e.g. Mustelus and Raja. Bladder-like swellings of the Wolffian duct in the female appear to be exceptional, and seminal bladders are not always present. The variations in the ureters and their openings are considerable, and in some cases all the ureters are stated to fall into a single duct, which may be spoken of as the ureter par excellence[344], with the same relations to the kidneys as the Wolffian duct bears to the Wolffian body. In some cases Malpighian corpuscles are completely absent in the Wolffian body, e.g. Raja.
The vasa efferentia of the testes in Scyllium are very typical, but there are some forms in which they are more numerous as well as others in which they are less so. Perhaps the vasa efferentia are seen in their most typical form in Centrina as described and figured (Pl. XXI) by Professor Semper, or in Squatina vulgaris, as I find it, and have represented it on Pl. 20, fig. 8. From my figure, representing the anterior part of the Wolffian body of a nearly ripe embryo, it will be seen that there are five vasa efferentia (v.e) connected on the one hand with a longitudinal canal at the base of the testes (n.t) and on the other with a longitudinal canal in the Wolffian body. Connected with the second longitudinal canal are four Malpighian bodies, three of them stalked and one sessile; from which again proceed tubes forming the commencements of the coils of the anterior segments of the Wolffian body. These Malpighian bodies are clearly my primary Malpighian bodies, but there are in Squatina, even in the generative segments, secondary Malpighian bodies. What Semper has described for Centrina and one or two other genera, closely correspond with what is present in Squatina.
[Pg 490] Development of the Segmental Tubes.
On p. 345, et seq. an account was given of the first formation of the segmental tubes and the segmental duct, and the history of these bodies was carried on till nearly the period at which it is taken up in the exhaustive Memoir of Professor Semper. Though the succeeding narration traverses to a great extent the same ground as Semper's Memoir, yet many points are treated somewhat differently, and others are dealt with which do not find a place in the latter. In the majority of instances, attention is called to points on which my results either agree with, or are opposed to, those of Professor Semper.
From previous statements it has been rendered clear that at first the excretory organs of Elasmobranchii exhibit no division into Wolffian body or kidney proper. Since this distinction is merely a question of the ducts, and does not concern the glandular tubuli, no allusion is made to its appearance in the present section, which deals only with the glandular part of the kidneys and not with their ducts.
Up to the close of stage K the urinogenital organs consist of a segmental duct opening in front into the body-cavity, and terminating blindly behind in close contact with the cloaca, and of a series of segmental tubes, each opening into the body-cavity on the inner side of the segmental duct, but ending blindly at their opposite extremities. It is with these latter that we have at present to deal. They are from the first directed obliquely backwards, and coil close round the inner and dorsal sides of the segmental duct. Where they are in contact (close to their openings into the body-cavity) with the segmental duct, the lumen of the latter diminishes and so comes to exhibit regular alternations of size. This is shewn in Pl. 12, fig. 18, s.d. At the points where the segmental duct has a larger lumen, it eventually unites with the segmental tubes.
The segmental tubes rapidly undergo a series of changes, the character of which may be investigated, either by piecing together transverse sections, or more easily from longitudinal and vertical sections. They acquire a Λ-shaped form with an anterior limb opening into the body-cavity and posterior limb, resting on a [Pg 491] dilated portion of the segmental duct. The next important change which they undergo consists in a junction being effected between their posterior limbs and the segmental duct. In the anterior part of the body these junctions appear before the commencement of stage L. A segmental tube at this stage is shewn in longitudinal section on Pl. 21, fig. 7a, and in transverse section on Pl. 18, fig. 2. In the former the actual openings into the body-cavity are not visible. In the transverse section only one limb of the Λ is met with on either side of the section; the limb opening into the body-cavity is seen on the left side, and that opening into the segmental duct on the right side. This becomes quite intelligible from a comparison with the longitudinal section, which demonstrates that it is clearly not possible to see more than a single limb of the Λ in any transverse section.
After the formation of their junctions with the segmental duct, other changes soon take place in the segmental tubes. By the close of stage L four distinct divisions may be noticed in each tube. Firstly, there is the opening into the body-cavity, with a somewhat narrow stalk, to which the name segmental tube will be strictly confined in the future, while the whole products of the original segmental tube will be spoken of as a segment of the kidney. This narrow stalk opens into a vesicle (Pl. 18, fig. 2, and 21, fig. 6), which forms the second division. From the vesicle proceeds a narrower section forming the third division, which during stage L remains very short, though in later stages it grows with great rapidity. It leads into the fourth division, which constitutes the posterior limb of the Λ, and has the form of a dilated tube with a narrow opening into the segmental duct.
The subsequent changes of each segment do not for the most part call for much attention. They consist mainly in the elongation of the third division, and its conversion into a coiled tubulus, which then constitutes the main mass of each segment of the kidney. There are, however, two points of some interest, viz. (1) the formation of the Malpighian bodies, and (2) the establishment of the connection between each segmental tube and the tubulus of the preceding segment which was alluded to in the description on p. 486. The development of the [Pg 492] Malpighian body is intimately linked with that of the secondary connection between two segments. They are both products of the metamorphosis of the vesicle which forms the termination of the segmental tube proper.
At about stage O this vesicle grows out in two directions (Pl. 21, fig. 10), viz. towards the segment in front (p.x) and posteriorly into the segment of which it properly forms a part (mg). That portion which grows backward remains continuous with the third division of its proper segment, and becomes converted into a Malpighian body. It assumes (Pl. 21, figs. 6 and 10) a hemispherical form, while near one edge of it is the opening from a segmental tube, and near the other the opening leading into a tubulus of the kidney. The two-walled hemisphere soon grows into a nearly closed sphere, with a central cavity into which projects a vascular tuft. For this tuft the thickened inner wall of cells forms a lining, and at the same time the outer wall becomes thinner, and formed of flattened cells, except in the interval between the openings of the segmental tube and kidney tubulus, where its cells remain columnar.
The above account of the formation of the Malpighian bodies agrees very well with the description which Pye[345] has given of the formation of these bodies in the embryonic Mammalian kidney. My statements also agree with those of Semper, in attributing the formation of the Malpighian body to a metamorphosis of part of the vesicle at the end of the segmental tube. Semper does not however enter into full details on this subject.
The elucidation of the history of the second outgrowth from the original vesicle towards the preceding segment is fraught with considerable difficulties, which might no doubt be overcome by a patient investigation of ample material, but which I have not succeeded in fully accomplishing.
The points which I believe myself to have determined are illustrated by fig. 10, Pl. 21, a longitudinal vertical section through a portion of the kidney between stages O and P. In this figure parts of three segments of the kidney are represented. In the hindermost of the three—the one to the right—there [Pg 493] is a complete segmental tube (s.t) which opens at its upper extremity into an irregular vesicle, prolonged behind into a body which is obviously a developing Malpighian body, m.g, and in front into a wide tube cut obliquely in the section and ending apparently blindly (p.x). In the preceding segment there is also a segmental tube (s.t) whose opening into the body-cavity passes out of the plane of the section, but which is again connected with a vesicle dilating behind into a Malpighian body (m.g) and in front into the irregular tube (p.x), as in the succeeding segment, but this tube is now connected (and this could be still more completely seen in the segment in front of this) with a vesicle which opens into the thick-walled collecting tube (fourth division) of the preceding segment close to the opening of the latter into the Wolffian duct. The fact that the anterior prolongation of the vesicle ends blindly in the hinder-most segment is due of course to its terminal part passing out of the plane of the section. Thus we have established between stages O and P a connection between each segmental tube and the collecting tube of the segment in front of that to which it properly belongs; and it further appears that in consequence of this each segment of the kidney contains two distinct coils of tubuli which only unite close to their common opening into the Wolffian duct!
This remarkable connection is not without morphological interest, but I am unfortunately only able to give in a fragmentary manner its further history. During the greater part of embryonic life a large amount of interstitial tissue is present in the embryonic kidneys, and renders them too opaque to be advantageously studied as a whole; and I have also, so far, failed to prepare longitudinal sections suitable for the study of this connection. It thus results that the next stage I have satisfactorily investigated is that of a nearly ripe embryo already spoken of in connection with the adult, and represented on Pl. 20, fig. 5. This figure shews that each segmental tube, while distinctly connected with the Malpighian body of its own segment, also sends out a branch towards the secondary Malpighian body of the preceding segment. This branch in most cases appeared to be rudimentary, and in the adult is certainly not represented by more than a fibrous band, but I fancy that I [Pg 494] have been able to trace it (though not with the distinctness I could desire) in surface views of the embryonic kidney of stage Q. The condition of the Wolffian body represented on Pl. 20, fig. 5 renders it probable that the accessory Malpighian body in each segment is developed in connection with the anterior growth from the original vesicle at the end of the segmental tube of the succeeding segment. How the third or fourth accessory Malpighian bodies, when present, take their origin I have not made out. It is, however, fairly certain that they form the commencement of two additional coils which unite, like the coil connected with the first accessory Malpighian body, with the collecting tube of the primitive coil close to its opening into the Wolffian duct or ureter.
The connection above described between two successive kidney segments appears to have escaped Professor Semper's notice, though I fancy that the peculiar vesicle he describes, loc. cit. p. 303, as connected with the end of each segmental tube, is in some way related to it. It seems possible that the secondary connection between the segmental tube and the preceding segment may explain a peculiar observation of Dr Spengel[346] on the kidney of the tailless Amphibians. He finds that, in this group, the segmental tubes do not open into Malpighian bodies, but into the fourth division of the kidney tube. Is it not just possible that in this case the primitive attachment of the segmental tubes may have become lost, and a secondary attachment, equivalent to that above described, though without the development of a secondary Malpighian body, have been developed? In my embryos the secondary coil of the segmental tubes opens, as in the Anura, into the fourth section of a kidney tubulus.
Development of the Müllerian and Wolffian ducts.
The formation of the Müllerian and Wolffian ducts out of the original segmental duct has been dealt with in a masterly manner by Professor Semper, but though I give my entire assent to his general conclusions, yet there are a few points on [Pg 495] which I differ from him. These are for the most part of a secondary importance; but they have a certain bearing on the homology between the Müllerian duct of higher Vertebrates and that of Elasmobranchii. The following account refers to Scy. canicula, but so far as my observations go, the changes in Scy. stellare are nearly identical in character.
I propose treating the development of these ducts in the two sexes separately, and begin with the female.
Shortly before stage N a horizontal split arises in the segmental duct[347], commencing some little distance from its anterior extremity, and extending backwards. This split divides the duct into a dorsal section and a ventral one. The dorsal section forms the Wolffian duct, and receives the openings of the segmental tubes, and the ventral one forms the Müllerian duct or oviduct, and is continuous with the unsplit anterior part of the primitive segmental duct, which opens into the body-cavity. The nature of the splitting may be gathered from the woodcut, fig. 6, p. 511, where x represents the line along which the segmental duct is divided. The splitting of the primitive duct extends slowly backwards, and thus there is for a considerable period a single duct behind, which bifurcates in front. A series of transverse sections through the point of bifurcation always exhibits the following features. Anteriorly two separate ducts are present, next two ducts in close juxtaposition, and immediately behind this a single duct. A series of sections through the junction of two ducts is represented on Plate 21, figs. 1A, 1B, 1C, 1D.
In my youngest example, in which the splitting had commenced, there were two separate ducts for only 14 sections, and in a slightly older one for about 18. In the second of these embryos the part of the segmental duct anterior to the front end of the Wolffian duct, which is converted directly into the oviduct, extended through 48 sections. In the space included in these 48 sections at least five, and I believe six, segmental tubes with openings into the body-cavity were present. These segmental tubes did not however unite with the oviduct, or at best, but one or two rudimentary junctions were visible, and the evidence of my earlier embryos appears to shew that the segmental [Pg 496] tubes in front of the Wolffian duct never become in the female united with the segmental duct. The anterior end of the Wolffian duct is very much smaller than the oviduct adjoining it, and as the reverse holds good in the male, an easy method is afforded of distinguishing the two sexes even at the earliest period of the formation of the Wolffian duct.
Hitherto merely the general features of the development of the oviduct and Wolffian duct have been alluded to, but a careful inspection of any good series of sections, shewing the junction of these two ducts, brings to light some features worth noticing in the formation of the oviduct. It might have been anticipated that, where the two ducts unite behind as the segmental duct, their lumens would have nearly the same diameter, but normally this appears to be far from the case.
To illustrate the formation of the oviduct I have represented a series of sections through a junction in an embryo in which the splitting into two ducts had only just commenced (Pl. 21, fig. 1), but I have found that the features of this series of sections are exactly reproduced in other series in which the splitting has extended as far back as the end of the small intestine. In the series represented (Pl. 21) 1A is the foremost section, and 1D the hindermost. In 1A the oviduct (od) is as large or slightly larger than the Wolffian duct (w.d), and in the section in front of this (which I have not represented) was considerably the larger of the two ducts. In 1B the oviduct has become markedly smaller, but there is no indication of its lumen becoming united with that of the Wolffian duct—the two ducts, though in contact, are distinctly separate. In 1C the walls of the two ducts have fused, and the oviduct appears merely as a ridge on the under surface of the Wolffian duct, and its lumen, though extremely minute, shews no sign of becoming one with that of the Wolffian duct. Finally, in 1D the oviduct can merely be recognised as a thickening on the under side of the segmental duct, as we must now call the single duct, but a slight bulging downwards of the lumen of the segmental duct appears to indicate that the lumens of the two ducts may perhaps have actually united. But of this I could not be by any means certain, and it seems quite possible that the lumen of the oviduct never does open into that of the segmental duct.
[Pg 497] The above series of sections goes far to prove that the posterior part of the oviduct is developed as a nearly solid ridge split off from the under side of the segmental duct, into which at the utmost a very small portion of the lumen of the latter is continued. One instance has however occurred amongst my sections which probably indicates that the lumen of the segmental duct may sometimes, in the course of the formation of the oviduct and Wolffian duct, become divided into two parts, of which that for the oviduct, though considerably smaller than that for the Wolffian duct, is not so markedly so as in normal cases (Pl. 21, fig. 2).
Professor Semper states that the lumen of the part of the oviduct split off from the hindermost end of the segmental duct becomes continuously smaller, till at last close to the cloaca it is split off as a solid rod of cells without a lumen, and thus it comes about that the oviduct, when formed, ends blindly, and does not open into the cloaca till the period of sexual maturity. My own sections do not include a series shewing the formation of a terminal part of the oviduct, but Semper's statements accord precisely with what might probably take place if my account of the earlier stages in the development of the oviduct is correct. The presence of a hymen in young female Elasmobranchii was first made known by Putmann and Garman[348], and subsequently discovered independently by Semper[349].
The Wolffian duct appears to receive its first segmental tube at its anterior extremity.
In the male the changes of the original segmental duct have a somewhat different character to those in the female, although there is a fundamental agreement between the two sexes. As in the female, a horizontal split makes its appearance a short way behind the front end of the segmental duct, and divides this into a dorsal Wolffian duct and a ventral Müllerian duct, the latter continuous with the anterior section of the segmental duct, which carries the abdominal opening. The differences in development between the two sexes are, in spite of a general similarity, [Pg 498] very obvious. In the first place, the ventral portion split off from the segmental duct, instead of being as in the female larger in front than the Wolffian duct, is very much smaller; while behind it does not form a continuous duct, but in some parts a lumen is present, and in others again absent (Pl. 21, fig. 6). It does not even form an unbroken cord, but is divided in disconnected portions. Those parts with a lumen do not appear to open into the Wolffian duct.
The process of splitting extends gradually backwards, so that there is a much longer rudimentary Müllerian duct by stage O than by stage N. By stage P the posterior portions of the Müllerian ducts have vanished. The anterior parts remain, as has been already stated, till adult life. A second difference between the male and female depends on the fact that, in the male, the splitting of the segmental duct into Müllerian duct and Wolffian duct never extends beyond the hinder extremity of the small intestine. A third and rather important point of difference consists in the splitting commencing far nearer the front end of the segmental duct in the male than in the female. In the female it was shewn that about 48 sections intervened between the front end of the segmental duct and the point where this became split, and that this region included five or six segmental tubes. In the male the homologous space only occupies about 7 to 12 sections, and does not contain the rudiment of more than a single segmental tube. Although my sections have not an absolutely uniform thickness, yet the above figures suffice to shew in a conclusive manner that the splitting of the segmental duct commences far further forwards in the male than in the female. This difference accounts for two facts which were mentioned in connection with the excretory organs of the adult, viz. (1) the greater length of the Wolffian body in the male than in the female, and (2) the fact that although a nearly similar number of segmental tubes persist in the adults of both sexes, yet that in the male there are five or six more segments in front of the first fully developed segmental opening than in the female.
The above description of the formation of the Müllerian duct in the male agrees very closely with that of Professor Semper for Acanthias. For Scyllium however he denies, as it appears to [Pg 499] me erroneously, the existence of the posterior rudimentary parts of the Müllerian duct. He further asserts that the portions of the Müllerian duct with a lumen open into the Wolffian duct. The most important difference, however, between Professor Semper's and my own description consists in his having failed to note that the splitting of the segmental duct commences much further forwards in the male than in the female.
I have attempted to shew that the oviduct in the female, with the exception of the front extremity, is formed as a nearly solid cord split off from the ventral surface of the segmental duct, and not by a simple splitting of the segmental duct into two equal parts. If I am right on this point, it appears to me far easier to understand the relationship between the oviduct or Müllerian duct of Elasmobranchii and the Müllerian duct of Birds, than if Professor Semper's account of the development of the oviduct is the correct one. Both Professor Semper and myself have stated our belief in the homology of the ducts in the two cases, but we have treated their relationship in a very different way. Professor Semper[350] finds himself compelled to reject, on theoretical grounds, the testimony of recent observers on the development of the Müllerian duct in Birds, and to assert that it is formed out of the Wolffian duct, or, according to my nomenclature, 'the segmental duct.' In my account[351], the ordinary statements with reference to the development of the Müllerian duct in Birds are accepted; but it is suggested that the independent development of the Müllerian duct may be explained by the function of this duct in the adult having, as it were, more and more impressed itself upon the embryonic development, till finally all connection, even during embryonic life, between the oviduct and the segmental duct (Wolffian duct) became lost.
Since finding what a small portion of the segmental duct became converted into the Müllerian duct in Elasmobranchii, I have reexamined the development of the Müllerian duct in the Fowl, in the hope of finding that its posterior part might develop nearly in the same manner as in Elasmobranchii, at the expense of a thickening of cells on the outer surface of the Wolffian duct. [Pg 500] I have satisfied myself, in conjunction with Mr Sedgwick, that this is not the case, and that the general account is in the main true; but at the same time we have obtained evidence which tends to shew that the cells which form the Müllerian duct are in part derived from the walls of the Wolffian duct. We propose giving a full account of our observations on this point, so that I refrain from mentioning further details here. It may however be well to point out that, apart from observations on the actual development of the Müllerian duct in the Bird, the fact of its abdominal opening being situated some way behind the front end of the Wolffian duct, is of itself a sufficient proof that it cannot be the metamorphosed front extremity of the Wolffian (= segmental) duct, in the same way that the abdominal opening of the Müllerian duct is the front extremity of the segmental duct in Elasmobranchii.
Although the evidence I can produce in the case of the Fowl of a direct participation of the Wolffian duct in the formation of the Müllerian is not of an absolutely conclusive kind, yet I am inclined to think that the complete independence of the two ducts, if eventually established as a fact, would not of itself be sufficient (as Semper is inclined to think) to disprove the identity of the Müllerian duct in Birds and Elasmobranchii.
We have, no doubt, almost no knowledge of the magnitude of the changes which can take place in the mode of development of the same organ in different types, yet this would have to be placed at a very low figure indeed in order to exclude the possibility of a change from the mode of development of the Müllerian duct in Elasmobranchii to that in Birds. We have, it appears to me, in the smallness of the portion of the segmental duct which goes to form the Müllerian duct in Elasmobranchii, evidence that a change has already appeared in this group in the direction of a development of the Müllerian duct independent of the segmental duct, and therefore of the Wolffian duct; and it has been in view of this consideration, that I have devoted so much attention to the apparently unimportant point of how much of the segmental duct was concerned in the formation of the Müllerian duct. An analogous change, in a somewhat different direction, would seem to be taking place in the development of the rudimentary Müllerian duct in the male Elasmobranchii.
[Pg 501] It is, perhaps, just worth pointing out, that the blindness of the oviduct of female Elasmobranchii, and its mode of development from an imperfect splitting of the segmental duct, may probably be brought into connection with the blindness of the extremity of the Müllerian duct or oviduct which so often occurs in both sexes of Sturgeons (Accipenser).
I may, perhaps, at this point, be permitted to say a few words about my original account of the development of the Wolffian duct This account was incorrect, and based upon a false interpretation of an imperfect series of sections, and I took the opportunity, in a general account of the urinogenital system of Vertebrates, to point out my mistake[352]. Professor Semper has, however, subsequently done me the honour to discuss, at considerable length, my original errors, and to attempt to explain them. Since it appears to me improbable that the continuation of such a discussion can be of much general interest, it will suffice to say now, that both Professor Semper's and my own original statements on the development of the Wolffian duct were erroneous; but that both of us have now recognised our mistakes; and that the first morphologically correct account of the development was given by him.
* * * * *
With reference to the formation of the urinal cloaca there is not much to say. The originally widely separated openings of the two Wolffian ducts gradually approximate in both sexes. By stage O (Pl. 19, fig. 1b) they are in close contact, and the lower ends of the two ducts actually coalesce at a somewhat later period, and open by a single aperture into the common cloaca. The papilla on which this is situated begins to make its appearance considerably before the actual fusion of the lower extremities of the two ducts.
Formation of Wolffian Body and Kidney proper.
Between stages L and M the hindermost ten or eleven segments of the primitive undivided excretory organ commence to undergo changes which result in their separation from the [Pg 502] anterior segments as a distinct gland, which was spoken of in the description of the adult as the kidney proper, while the unaltered preceding segments of the kidney were spoken of as the Wolffian body.
It will be remembered that each segment of the embryonic kidney consists of four divisions, the last or fourth of which opens into the Wolffian duct. The changes which take place in the hindermost ten or eleven segments, and cause them to become distinguished as the kidney proper, concern alone the fourth division of each segment, which becomes prolonged backwards, and its opening into the Wolffian duct proportionately shifted. These changes affect the foremost segments of the kidney much more than the hindermost, so that the fourth division in the foremost segments becomes very much longer than in the hindermost, and at last all the prolongations of the kidney segments come to open nearly on the same level, close to the cloacal termination of the Wolffian duct (Pl. 21, fig. 8). The prolongations of the fourth division of the kidney-segments have already (p. 481) been spoken of in the description of the adult as ureters, and this name will be employed for them in the present section.
The exact manner in which the changes, that have been briefly related, take place is rather curious, and very difficult to unravel without the aid of longitudinal sections. First of all, the junction between each segment of the kidney and the Wolffian duct becomes so elongated as to occupy the whole interval between the junctions of the two neighbouring segments. The original opening of each tube into the Wolffian duct is situated at the anterior end of this elongated attachment, the remaining part of the attachment being formed solely of a ridge of cells on the dorsal side of the Wolffian duct. The general character of this growth will be understood by comparing figs. 7a and 7b, Pl. 21—two longitudinal vertical sections through part of the kidneys. Fig. 7 a shews the normal junction of a segmental tube with the Wolffian duct in the Wolffian body, while in figure 7b (r.u) is shewn the modified junction in the region of the kidney proper in the same embryo. The latter of these figures (fig. 7b) appears to me to prove that the elongation of the attachments between the segmental tubes [Pg 503] and Wolffian duct takes place entirely at the expense of the former. Owing to the length of this attachment, every transverse section through the kidney proper at this stage either presents a solid ridge of cells closely adhering to the dorsal side of the Wolffian duct, or else passes through one of the openings into the Wolffian duct.
During stage M the original openings of the segmental tubes into the Wolffian duct appear to me to become obliterated, and at the same time the lumen of each ureter is prolonged into the ridge of cells on the dorsal wall of the duct.
Both of these changes are illustrated in my figures. The fact of the obliteration of the original opening into the Wolffian duct is shewn in longitudinal section in Pl. 21, fig. 9, u, but more conclusively in the series of transverse sections represented on Pl. 21, figs. 3A, 3B, 3C. In the hindermost of these (3C) is seen the solid terminal point of a ureter, while the same ureter possesses a lumen in the two previous sections, but exhibits no signs of opening into the Wolffian duct. Sections may however be met with which appear to shew that in some instances the ureters still continue to open into the Wolffian duct, but these I find to be rare and inconclusive, and am inclined to regard them as abnormalities. The prolongation of the lumen of the ureters takes place in a somewhat peculiar fashion. The lumen is not, as might be expected, completely circumscribed by the wall of the ureter, but only dorsally and to the sides. Ventrally it is closed in by the dorsal wall of the Wolffian duct. In other words, each ureter is at first an incomplete tube. This peculiarity is clearly shewn in the middle figure of the series on Pl. 21, fig. 3B.
During stages M and N the ureters elongate considerably, and, since the foremost ones grow the most rapidly, they soon come to overlap those behind. As each ureter grows in length it remains an incomplete tube, and its lumen, though proportionately prolonged, continues to present the same general relations as at first. It is circumscribed by its proper walls only dorsally and laterally; its floor being formed in the case of the front ureter by the Wolffian duct, and in the case of each succeeding ureter by the dorsal wall of the ureter in front. This is most easily seen in longitudinal sections, and is represented [Pg 504] on Pl. 21, fig. 9, or on a larger scale in fig. 9A. In the latter figure it is especially clear that while the wall on the dorsal side of the lumen of each ureter is continuous with the dorsal wall of the tubulus of its own segment, the wall on the ventral side is continuous with the dorsal wall of the ureter of the preceding segment. This feature in the ureters explains the appearance of transverse sections in which the ureters are not separate from each other, but form together a kind of ridge on the dorsal side of the Wolffian duct, in which there are a series of perforations representing the separate lumens of the ureters (Pl. 21, fig. 4). The peculiarities in the appearance of the dorsal wall of the Wolffian duct in fig. 9A, and the difference between the cells composing it and those of the ventral wall, become intelligible on comparing this figure with the representation of transverse section in figs. 3B and 3C, and especially in fig. 4. Most of the ureters continue to end blindly at the close of stage N, and appear to have solid posterior terminations like that of the Müllerian duct in Birds.
By stage O all the ureters have become prolonged up to the cloacal end of the Wolffian duct, so that the anterior one has a length equal to that of the whole kidney proper. For the most part they acquire independent openings into the end section of the Wolffian duct, though some of them unite together before reaching this. The general appearance of the hindermost of them between stages N and O is shewn in longitudinal and vertical section in Pl. 21, fig. 8, u.
They next commence to develop into complete and independent tubes by their side walls growing inwards and meeting below so as to completely enclose their lumen. This is seen already to have occurred in most of the posterior ureters in Pl. 21, fig. 8.
Before stage P the ureters cease to be united into a continuous ridge, and each becomes separated from its neighbours by a layer of indifferent tissue: by this stage, in fact, the ureters have practically attained very nearly their adult condition. The general features of a typical section through them are shewn on Pl. 21, fig. 5. The figure represents the section of a female embryo, not far from the cloaca. Below is the oviduct (od). Above this again is the Wolffian duct (w.d), and still dorsal to [Pg 505] this are four ureters (u). In female embryos more than four ureters are not usually to be seen in a single section. This is probably owing to the persistence, in some instances, of the intimate connection between the ureters found at an earlier stage of development, and results in a single ureter coming to serve as the collecting duct for several segments. A section through a male embryo of stage P would mainly differ from that through a female in the absence of the oviduct, and in the presence of probably six[353], instead of four, ureters.
The exact amount of fusion which takes place between the ureters, and the exact number of the ureters, cannot easily be determined from sections, but the study of sections is chiefly of value in shewing the general nature of the changes which take place in the process of attaining the adult condition.
It may be noticed, as a consequence of the above account, that the formation of the ureters takes place by a growth of the original segmental tubes, and not by a splitting off of parts of the wall of the Wolffian duct.
The formation of ureters in Scyllium, which has been only very cursorily alluded to by Professor Semper, appears to differ very considerably from that in Acanthias as narrated by him.
The Vasa Efferentia.
A comparison of the results of Professor Semper on Elasmobranchii, and Dr Spengel on Amphibians, suggests several interesting questions with reference to the development of the vasa efferentia, and the longitudinal canal of the Wolffian body.
Professor Semper was the first to describe the adult anatomy and development of vasa efferentia in Elasmobranchii, and the following extracts will fully illustrate his views with reference to them.
In[354] dem frühesten Stadium finden
sich wie früher angegeben ungefahr 34 Trichter in der Leibeshöhle, von
diesen gehen die 27 hintersten in die persistirenden Segmentaltrichter
über, von denen 4 beim erwachsenen Thiere auf dem Mesorchium stehen. [Pg 506]
Die übrigen 7 schliessen sich vollständig ab zu den erwähnten länglichen
und später mannigfach auswachsenden varicösen Trichterblasen; von diesen
sind es wiederum 3-4 welche untereinander in der Längsrichtung verwachsen
und dadurch den in der Basis der Hodenfalte verlaufenden Centralcanal des
Hodens bilden. Ehe aber diese Verwachsung zu einem mehr oder minder
geschlängelten Centralcanal vollständig wird, hat sich einmal das Lumen der
Trichterblasen fast vollständig geschlossen und ausserdem von ihnen aus
durch Verwachsung und Knospung die erste Anlage des rete vasculosum Halleri
gebildet (Taf. XX. Figs. 1, 2c). Es erstreckt sich nämlich
mehr oder minder weit in die Genitalfalte hinein ein unregelmässiges von
kleinen Zellen begränztes Canalnetz welches zweifellos mit dem noch nicht
ganz vollständigen Centralcanale des Hodens (Taf. XX. Fig. 2c) in
Verbindung steht. Von diesem letzteren aus gehen in regelmässigen Abständen
die Segmentalgänge (Taf. XX. Fig. 2 sg.)
gegen die Niere hin; da sie meist stark geneigt oder selbst geschlängelt
(bei 6ctm langen Embryonen)
gegen die Niere zu verlaufen, wo sie sich an die primären
Malpighi'schen Körperchen und deren Bildungsblasen ansetzen, so
kann ein verticaler Querschnitt auch nie einen solchen nun zum vas efferens
gewordenen Segmentalgang seiner ganzen Länge nach treffen. Gegen die
Trichterfurche zu aber steht namentlich am hinteren Theile der Genitalfalte
der Centralcanal häufig noch durch einen kurzen Zellstrang mit dem
Keimepithel der Trichterfurche in Verbindung; mitunter findet sich hier
sogar noch eine kleine Höhlung, Rest des ursprünglich hier vorhandenen
weiten Trichters
(Taf. XX. Fig. 3c).
And again: Dieser[355]
Gegensatz in der Umbildung der Segmentalgänge an der Hodenbasis scheint nun
mit einem anderen Hand in Hand zu gehen. Es bildet sich nämlich am
Innenrande der Niere durch Sprossung und Verwachsung der Segmentalgänge vor
ihrer Insertion an das primäre Malpighi'sche Körperchen ein Canal
beim Männchen aus, den ich als Nierenrandcanal oben bezeichnet
habe. Ich habe denselben bei Acanthias Centrina (Taf. XXI. Fig. 13) und Mustelus
(Taf. XV.
Fig. 8) gefunden. Bei Centrina ist er ziemlich lang und vereinigt
mindestens 7 Segmentalgänge, aber von diesen letzteren stehen nur 5 mit dem
[Pg
507] Hodennetz in Verbindung. Dort nun wo diese letzteren sich an
den Nierenrandcanal ansetzen (Taf. XXI. Fig. 13 sg.1-sg.5) findet sich jedesmal ein
typisch ausgebildetes Malpighi'sches Körperchen, mit dem aber nun
nicht mehr wie ursprünglich nur 2 Canäle verbunden sind (Taf. XXI. Fig. 14) sondern 3.
Einer dieser letzteren ist derjenige Ast des Nierenrandcanals welcher die
Verbindung mit dem nächst folgenden Segmentalgang zu besorgen hat. An den
Stellen aber wo sich an den Nierenrandcanal die hinteren blind gegen den
Hoden hin endenden Segmentalgänge ansetzen fehlen diese
Malpighi'schen Körperchen (Taf. XXI. Fig. 13 sg7) vollständig. Auch bei Mustelus (Taf.
XV. Figs. 8,
10) findet genau dasselbe Verhältniss statt; da aber hier nur 2 (oder 3)
Segmentalgänge zu vasa efferentia umgewandelt werden, so stehen hier am
kurzen Randcanal der Niere auch nur 2 oder 3 Malpighi'sche
Körperchen. Diese aber sind typisch ausgebildet
(Taf. XV. Fig. 10).
From these two extracts it is clear that Semper regards both the vasa efferentia, and central canal of the testis network, as well as the longitudinal canal of the Wolffian body, as products of the anterior segmental tubes.
The appearance of these various parts in the fully grown embryos or adults of such genera as Acanthias and Squatina strongly favours this view, but Semper appears to have worked out the development of these structures somewhat partially and by means of sections, a method not, in Scyllium at least, very suitable for this particular investigation. I myself at first unhesitatingly accepted Semper's views, and it was not till after the study of the paper of Dr Spengel on the Amphibian kidney that I came to have my doubts as to their accuracy. The arrangement of the parts in most Amphibians is strikingly similar to that in Elasmobranchii. From the testis come transverse canals corresponding with my vasa efferentia; these fall into a longitudinal canal of the kidneys, from which again, as in Squatina (Pl. 20, fig. 8), Mustelus and Centrina, canals (the vasa efferentia of Spengel) pass off to Malpighian bodies. So far there is no difficulty, but Dr Spengel has made the extremely important discovery, that in young Amphibians each Malpighian body in the region of the generative ducts, in addition to receiving the vasa efferentia, is connected with a fully developed segmental [Pg 508] tube opening into the body-cavity. In Amphibians, therefore, it is improbable that the vasa efferentia are products of the open extremities of the segmental tubes, considering that these latter are found in their unaltered condition at the same time as the vasa efferentia. When it is borne in mind how strikingly similar in most respects is the arrangement of the testicular ducts in Amphibia and Elasmobranchii, it will not easily be credited that they develop in entirely different methods. Since then we find in Amphibians fully developed segmental tubes in the same segments as the vasa efferentia, it is difficult to believe that in Elasmobranchii the same vasa efferentia have been developed out of the segmental tubes by the obliteration of their openings.
I set myself to the solution of the origin of the vasa efferentia by means of surface views, after the parts had been made transparent in creosote, but I have met with great difficulties, and so far my researches have only been partially successful. From what I have been able to see of Squatina and Acanthias, I am inclined to think that the embryos of either of these genera would form far more suitable objects for this research than Scyllium. I have had a few embryos of Squatina which were unfortunately too old for my purpose.
Very early the vasa efferentia are fully formed, and their arrangement in an embryo eight centimetres long is shewn in Pl. 20, fig. 6, v.e. It is there seen that there are six if not seven vasa efferentia connected with a longitudinal canal along the base of the testes (Semper's central canal of the testis), and passing down like the segmental tubes to spaces between the successive segments of the Wolffian body. They were probably connected by a longitudinal canal in the Wolffian body, but this could not be clearly seen. In the segment immediately behind the last vas efferens was a fully developed segmental tube. This embryo clearly throws no light on the question at issue except that on the whole it supports Semper's views. I further failed to make out anything from an examination of still younger embryos.
In a somewhat older embryo there was connected with the anterior vas efferens a peculiar structure represented on Pl. 20, fig. 7, r.st? which strangely resembled the opening of an ordinary segmental tube, but as I could not find it in the younger embryo, this suggestion as to its nature, is, at the best, [Pg 509] extremely hazardous. If, however, this body really is the remnant of a segmental opening, it would be reasonable to conclude that the vasa efferentia are buds from the segmental tubes as opposed to their openings; a mode of origin which is not incompatible with the discoveries of Dr Spengel. I have noticed a remnant, somewhat similar to that in the Scyllium embryo, close to the hindermost vas efferens in an embryo Squatina (Pl. 20, fig. 8, r.st?).
With reference to the development of the longitudinal canal of the Wolffian body, I am without observations, but it appears to me to be probably a further development of the outgrowths of the vesicles of each segmental tube, which were described in connection with the development of the segmental tubes, p. 492. Were an anterior outgrowth of one vesicle to meet and coalesce with the posterior outgrowth of the preceding vesicle, a longitudinal canal such as actually exists would be the result. The central canal of the base of the testes and the network connected with it in the adult (Pl. 20, fig. 4), appear to be derivatives of the vasa efferentia.
I am thus compelled to leave open the question of the real nature of the vasa efferentia, but am inclined to regard them as outgrowths from the anterior segmental tubes, though not from their open terminations.
* * * * *
My views upon the homologies of the various parts of the urinogenital system, the development of which has been described in the present chapter, have already been expressed in a paper on Urinogenital organs of Vertebrates[356]. Although Kölliker's[357] discovery of the segmental tubes in Aves, and the researches of Spengel[358], Gasser[359], Ewart[360] and others, have rendered necessary a few corrections in my facts, I still adhere in their entirety to the views expressed in that paper, and feel it unnecessary to [Pg 510] repeat them in this place. I conclude the chapter with a résumé of the development of the urinogenital organs in Elasmobranchii from their first appearance to their permanent condition.
* * * * *
Résumé.—The first trace of the urinary system makes its appearance as a knob springing from the intermediate cell-mass opposite the fifth protovertebra (woodcut, fig. 5A, p.d). This knob is the rudiment of the abdominal opening of the segmental duct, and from it there grows backwards to the level of the anus a solid column of cells, which constitutes the rudiment of the segmental duct itself (woodcut, fig. 5B, p.d). The knob projects towards the epiblast, and the column connected with it lies between the mesoblast and epiblast. The knob and column do not long remain solid, but the former acquires an opening into the body-cavity continuous with a lumen, which makes its appearance in the latter.
Fig. 5.
Two Sections of a Pristiurus Embryo with three visceral clefts.
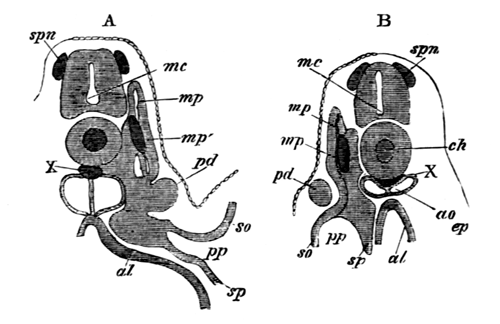
The sections illustrate the development of the segmental duct (pd) or primitive duct of the kidneys. In A (the anterior of the two sections) this appears as a solid knob (pd) projecting towards the epiblast. In B is seen a section of the column which has grown backwards from the knob in A.
spn. rudiment of a spinal nerve; mc. medullary canal; ch. notochord; X. string of cells below the notochord; mp. muscle-plate; mp´. specially developed portion of muscle-plate; ao. dorsal aorta; pd. segmental duct; so. somatopleure; sp. splanchnopleure; pp. pleuro-peritoneal or body-cavity; ep. epiblast; al. alimentary canal.
While the lumen is gradually pushing its way backwards along the solid rudiment of the segmental duct, the first traces [Pg 511] of the segmental tubes, or proper excretory organs, make their appearance in the form of solid outgrowths of the intermediate cell-mass, which soon become hollow and open into the body-cavity. Their blind ends curl obliquely backwards round the inner and dorsal side of the segmental duct. One segmental tube makes its appearance for each protovertebra, commencing with that immediately behind the abdominal opening of the segmental duct, the last tube being situated a short way behind the anus. Soon after their formation the blind ends of the segmental tubes open into the segmental duct, and each of them becomes divided into four parts. These are (woodcut 7) (1) a section carrying the abdominal opening or segmental tube proper, (2) a dilated vesicle into which this opens, (3) a coiled tubulus proceeding from (2) and terminating in (4), a wider portion opening into the segmental duct. At the same time, or shortly before this, each segmental duct unites with and opens into one of the horns of the cloaca, and also retires from its primitive position between the epiblast and mesoblast, and assumes a position close to the epithelium lining the body-cavity. The general features of the excretory organs at this period are diagrammatically represented on the woodcut, fig. 6. In this fig. p.d is the segmental duct and o its abdominal opening. s.t points to the segmental tubes, the finer details of whose structure are not represented in the diagram. The kidneys thus form at this period an unbroken gland composed of a series of isolated coiled [Pg 512] tubes, one extremity of each of which opens into the body-cavity, and the other into the segmental duct, which forms the only duct of the kidney, and communicates at one end with the body-cavity, and at the other with the cloaca.
Fig. 6.
Diagram of the primitive condition of the Kidney in an Elasmobranch Embryo.
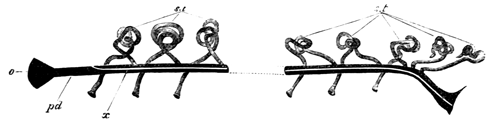
pd. segmental duct. It opens at o into the body-cavity and at its other extremity into the cloaca; x. line along which the division appears which separates the segmental duct into the Wolffian duct above and the Müllerian duct below; st. segmental tubes. They open at one end into the body-cavity, and at the other into the segmental duct.
The next important change concerns the segmental duct, which becomes longitudinally split into two complete ducts in the female, and one complete duct and parts of a second in the male. The manner in which this takes place is diagrammatically represented in woodcut 6 by the clear line x, and in transverse section in woodcut 7. The resulting ducts are the (1) Wolffian duct dorsally, which remains continuous with the excretory [Pg 513] tubules of the kidney, and ventrally (2) the oviduct or Müllerian duct in the female, and the rudiments of this duct in the male. In the female the formation of these ducts takes place by a nearly solid rod of cells, being gradually split off from the ventral side of all but the foremost part of the original segmental duct, with the short undivided anterior part of which duct it is continuous in front. Into it a very small portion of the lumen of the original segmental duct is perhaps continued (Pl. 21, fig. 1A, etc.). The remainder of the segmental duct (after the loss of its anterior section and the part split off from its ventral side) forms the Wolffian duct. The process of formation of the ducts in the male chiefly differs from that in the female in the fact of the anterior undivided part of the segmental duct, which forms the front end of the Müllerian duct, being shorter, and in the column of cells with which it is continuous being from the first incomplete.
Fig. 7.
Diagrammatic representation of a
transverse section of a Scyllium Embryo
illustrating the formation of
the Wolffian and Müllerian ducts by
the longitudinal splitting of the
segmental duct.
mc. medullary canal; mp. muscle-plate; ch. notochord; ao. aorta; cav. cardinal vein; st. segmental tube. On the one side the section passes through the opening of a segmental tube into the body-cavity. On the other this opening is represented by dotted lines, and the opening of the segmental tube into the Wolffian duct has been cut through; w.d. Wolffian duct; m.d. Müllerian duct. The section is taken through the point where the segmental duct and Wolffian duct have just become separate; gr. The germinal ridge with the thickened germinal epithelium; l. liver; i. intestine with spiral valve.
The tubuli of the primitive excretory organ undergo further important changes. The vesicle at the termination of each segmental tube grows forwards towards the preceding tubulus, and joins the fourth section of it close to the opening into the Wolffian duct (Pl. 21, fig. 10). The remainder of the vesicle becomes converted into a Malpighian body. By the first of these changes a connection is established between the successive segments of the kidney, and though this connection is certainly lost (or only represented by fibrous bands) in the anterior part of the excretory organs in the adult, and very probably in the hinder part, yet it seems most probable that traces of it are to be found in the presence of the secondary Malpighian bodies of the majority of segments, which are most likely developed from it.
Up to this time there has been no distinction between the anterior and posterior tubuli of the primitive excretory organ which alike open into the Wolffian duct. The terminal division of the tubuli of a considerable number of the hindermost of these (ten or eleven in Scyllium canicula), either in some species elongate, overlap, and eventually open by apertures (not usually so numerous as the separate tubes), on nearly the same level, into the hindermost section of the Wolffian duct in the female, or into the urinogenital cloaca, formed by the coalesced terminal [Pg 514] parts of the Wolffian ducts, in the male; or in other species become modified in such a manner as to pour their secretion into a single duct on each side, which opens in a position corresponding with the numerous ducts of the other type (woodcut, fig. 8). It seems that both in Amphibians and Elasmobranchii the type with a single duct, or approximations to it, are more often found in the females than in the males. The subject requires however to be more worked out in Elasmobranchii[361]. In both groups the modified posterior kidney-segments are probably equivalent to the permanent kidney of the amniotic Vertebrates, and for this reason the numerous ducts of the first group or single duct of the second were spoken of as ureters. The anterior tubuli of the primitive excretory organ retain their early relation to the Wolffian duct, and form the Wolffian body.
The originally separate terminal extremities of the Wolffian ducts always coalesce, and form a urinal cloaca, opening by a single aperture situated at the extremity of a median papilla behind the anus. Some of the abdominal openings of the segmental tubes in Scyllium, or in other cases all the openings, become obliterated.
In the male the anterior segmental tubes undergo remarkable modifications. There appear to grow from the first three or four or more of them (though the point is still somewhat obscure) branches, which pass to the base of the testis and there unite into a longitudinal canal, form a network, and receive the secretion of the testicular ampullæ (woodcut 9, nt). These ducts, the vasa efferentia, carry the semen to the Wolffian body, but before opening into the tubuli of this they unite into the longitudinal canal of the Wolffian body (l.c), from which pass off ducts equal in number to the vasa efferentia, each of which normally ends in a Malpighian body. From the Malpighian body so connected start the convoluted tubuli of what may be called the generative segments of the Wolffian body along which the semen is conveyed to the Wolffian duct (v.d). The Wolffian duct itself becomes much contorted and acts as vas deferens.
Diagram of the arrangement of
the Urinogenital Organs in an adult
Female Elasmobranch.
m.d. Müllerian duct; w.d. Wolffian duct; s.t. glandular tubuli; five of them are represented with openings into the body-cavity; d. duct of the posterior segmental tubes; ov. ovary.
In the woodcuts, figs. 8 and 9, are diagrammatically represented the chief constituents of the adult urinogenital organs in the two sexes. In the adult female, fig. 8, there are present the following parts:
(1) The oviduct or Müllerian duct (m.d) split off from the segmental duct of the kidneys. Each oviduct opens at its anterior extremity into the body-cavity, and behind the two oviducts have independent communications with the general cloaca.
(2) The Wolffian ducts (w.d), the other product of the segmental ducts of the kidneys. They end in front by becoming continuous with the tubulus of the anterior segment of the Wolffian body on each side, and unite behind to open by a common papilla into the cloaca. The Wolffian duct receives the secretion of the anterior part of the primitive kidney which forms the Wolffian body.
(3) The ureter (d) which carries off the secretion of the kidney proper. It is represented in my diagram in its most rare and differentiated condition as a single duct.
(4) The glandular tubuli (s.t), some of which retain their original openings into the body-cavity, and others are without them. They are divided into two groups, an anterior forming [Pg 516] the Wolffian body, which pour their secretion into the Wolffian duct, and a posterior group forming the kidney proper, which are connected with the ureter.
Fig. 9.
Diagram of the arrangement of
the Urinogenital Organs in an adult
Male Elasmobranch.
m.d. rudiment of Müllerian duct; w.d. Wolffian duct, marked vd in front and serving as vas deferens; st. glandular tubuli; two of them are represented with openings into the body-cavity; d. ureter; t. testis; nt. central canal at the base of the testis; VE. vasa efferentia; lc. longitudinal canal of the Wolffian body.
In the male the following parts are present (woodcut 9):
(1) The Müllerian duct (md), consisting of a small rudiment attached to the liver representing the foremost end of the oviduct of the female.
(2) The Wolffian duct (w.d) which precisely corresponds to the Wolffian duct of the female, but, in addition to functioning as the duct of the Wolffian body, also acts as a vas deferens (vd). In the adult male its foremost part has a very tortuous course.
(3) The ureter (d), which has the same fundamental constitution as in the female.
(4) The segmental tubes (st). The posterior of these have the same arrangement in both sexes, but in the male modifications take place in connection with the anterior ones to fit them to act as transporters of the testicular products.
Connected with the anterior ones there are present (1) the vasa efferentia (VE), united on the one hand with (2) the central canal in the base of the testis (nt), and on the other with the [Pg 517] longitudinal canal of the Wolffian body (l.c). From the latter are seen passing off the successive tubuli of the anterior segments of the Wolffian body in connection with which Malpighian bodies are typically present, though not represented in my diagram.
Postscript.
It was my original intention to have given an account of the development of the generative organs. In the course, however, of my work a number of novel and unexpected points turned up, which have considerably protracted my investigations, and it has appeared to me better no longer to delay the appearance of this monograph, but to publish elsewhere my results on the generative organs. In chapter VI. p. 349 et seq. the early stages of the generative organs are described, but in contemplation of the completion of the account no allusion was made to their literature, and more especially to Professor Semper's important contributions. I may perhaps say that I have been able to confirm the most important result to which he and other anatomists have nearly simultaneously arrived with respect to Vertebrates, viz. that the primitive ova give rise to both the male and female generative products.
[Pg 518] EXPLANATION OF PLATES 20 AND 21.
Complete List of Reference Letters.
amg. Accessory Malpighian body. cav. Cardinal vein. ge. Germinal epithelium. k. True kidney. l.c. Longitudinal canal of the Wolffian body connected with vasa efferentia. mg. Malpighian body. nt. Network and central canal at the base of the testis. o. External aperture of urinal cloaca. od. Oviduct or Müllerian duct of the female. od´. Müllerian duct of the male. ou. Openings of ureters in Wolffian duct in the female (fig. 3). pmg. Primary Malpighian body. px. Growth from vesicle at the end of a segmental tube to join the collecting tube of the preceding segment. rst. Rudimentary segmental tube. ru. Ureter commencing to be formed. sb. Seminal bladder. sd. Segmental duct. st. Segmental tube. sto. Opening of segmental tube into body-cavity. sur. Suprarenal body. t. Testis. u. Ureters. ve. Vas efferens. wb. Wolffian body. wd. Wolffian duct.
Plate 20.
Fig. 1. Diagrammatic representation of excretory organs on one side of a male Scyllium canicula, natural size.
Fig. 2. Diagrammatic representation of the kidney proper on one side of a female Scyllium canicula, natural size, shewing the ducts of the kidney and the dilated portion of the Wolffian duct.
Fig. 3. Opening of the ureters into the Wolffian duct of a female Scyllium canicula. The figure represents the Wolffian ducts (wd) with ventral portion removed so as to expose their inner surface, and shews the junction of the two W. ducts to form the common urinal cloaca, the single external opening of this (o), and openings of ureters into one Wolffian duct (ou).
Fig. 4. Anterior extremity of Wolffian body of a young male Scyllium canicula shewing the vasa efferentia and their connection with the kidneys and the testis. The vasa efferentia and longitudinal canal are coloured to render them distinct. They are intended to be continuous with the uncoloured coils of the Wolffian body, though this connection has not been very successfully rendered by the artist.
Fig. 5. Part of the Wolffian body of a nearly ripe male embryo of Scyllium canicula as a transparent object. Zeiss a a, ocul. 3. The figure shews two segmental tubes opening into the body-cavity and connected with a primary Malpighian body, and also, by a fibrous connection, with a secondary Malpighian body of the preceding segment. It also shews one segmental tube (rst) imperfectly connected with the accessory Malpighian body of the preceding segment of the kidney. The coils of the kidney are represented somewhat diagrammatically.
Fig. 6. Vasa efferentia of a male embryo of Scyllium canicula eight centimetres in length. Zeiss a a, ocul. 2.
There are seen to be at the least six and possibly seven distinct vasa going to as many segments of the Wolffian body and connected with a longitudinal canal in the base of the testis. They were probably also connected with a longitudinal canal in the Wolffian body, but this could not be clearly made out.
[Pg 519] Fig. 7. The anterior four vasa efferentia of a nearly ripe embryo. Connected with the foremost one is seen a body which looks like the remnant of a segmental tube and its opening (rst?).
Fig. 8. Testis and anterior part of Wolffian body of an embryo of Squatina vulgaris.
The figure is intended to illustrate the arrangement of the vasa efferentia. There are five of these connected with a longitudinal canal in the base of the testis, and with another longitudinal canal in the Wolffian body. From the second longitudinal canal there pass off four ducts to as many Malpighian bodies. Through the Malpighian bodies these ducts are continuous with the several coils of the Wolffian body, and so eventually with the Wolffian duct. Close to the hindermost vas efferens is seen a body which resembles a rudimentary segmental tube (rst?).
Plate 21.
Figs. 1A, 1B, 1C, 1D. Four sections from a female Scyllium canicula of a stage between M and N through the part where the segmental duct becomes split into Wolffian duct and oviduct. Zeiss B, ocul. 2. 1A is the foremost section.
The sections shew that the oviduct arises as a thickening on the under surface of the segmental duct into which at the utmost a very narrow prolongation of the lumen of the segmental duct is carried. The small size of the lumen of the Wolffian duct in the foremost section is due to the section passing through nearly its anterior blind extremity.
Fig. 2. Section close to the junction of the Wolffian duct and oviduct in a female embryo of Scyllium canicula belonging to stage N. Zeiss B, ocul. 2.
The section represented shews that in some instances the formation of the oviduct and Wolffian duct is accompanied by a division of the lumen of the segmental duct into two not very unequal parts.
Figs. 3A, 3B, 3C. Three sections illustrating the formation of a ureter in a female embryo belonging to stage N. Zeiss B, ocul. 2.
3A is the foremost section.
The figures shew that the lumen of the developing ureter is enclosed in front by an independent wall (fig. 3A), but that further back the lumen is partly shut in by the subjacent Wolffian duct, while behind no lumen is present, but the ureter ends as a solid knob of cells without an opening into the Wolffian duct.
Fig. 4. Section through the ureters of the same embryo as fig. 3, but nearer the cloaca. Zeiss B, ocul. 2.
The figure shews the appearance of a transverse section through the wall of cells above the Wolffian duct formed by the overlapping ureters, the lumens of which appear as perforations in it. It should be compared with fig. 9A, which represents a longitudinal section through a similar wall of cells.
Fig. 5. Section through the ureters, the Wolffian duct and the oviduct of a female embryo of Scy. canicula belonging to stage P. Zeiss B, ocul. 2.
Fig. 6. Section of part of the Wolffian body of a male embryo of Scyllium canicula belonging to stage O. Zeiss B, ocul. 2.
[Pg 520] The section illustrates (1) the formation of a Malpighian body (mg) from the dilatation at the end of a segmental tube, (2) the appearance of the rudiment of the Müllerian duct in the male (od´).
Figs. 7a, 7b. Two longitudinal and vertical sections through part of the kidney of an embryo between stages L and M. Zeiss B, ocul. 2.
7a illustrates the parts of a single segment of the Wolffian body at this stage, vide p. 491. The segmental tube and opening are not in the plane of the section, but the dilated vesicle is shewn into which the segmental tube opens.
7b is taken from the region of the kidney proper. To the right is seen the opening of a segmental tube into the body-cavity, and in the segment to the left the commencing formation of a ureter, vide p. 502.
Fig. 8. Longitudinal and vertical section through the posterior part of the kidney proper of an embryo of Scyllium canicula at a stage between N and O. Zeiss A, ocul. 2.
The section shews the nearly completed ureters, developing Malpighian bodies, &c.
Fig. 9. Longitudinal and vertical section through the anterior part of the kidney proper of the same embryo as fig. 8. Zeiss A, ocul. 2.
The figure illustrates the mode of growth of the developing ureters.
9A. More highly magnified portion of the same section as fig. 9.
Compare with transverse section fig. 4.
Fig. 10. Longitudinal and vertical section through part of the Wolffian body of an embryo of Scyllium canicula at a stage between O and P.
The section contains two examples of the budding out of the vesicle of a segmental tube to form a Malpighian body in its own segment and to unite with the tubulus of the preceding segment close to its opening into the Wolffian duct.
[335] Chapter VI. p. 345, et seq.
[336] Archiv f. Micr. Anat. Bd. XI.
[337]
Urogenital System d. Plagiostomen,
Semper,
Arbeiten, Vol. II.
[338] Sitzungsberichte d. Naturfor. Ges. Leipzig, 1875. No. 2.
[339]
Preliminary account of the development of Elasmobranch Fishes,
Quarterly Journal of Microscopical Science, 1874. Origin
and History of the Urinogenital Organs of Vertebrates,
Journal
of Anat. and Physiol.
Vol. X.
[340] Arbeiten, Semper, Vol. III.
[341] Though Professor Semper has come to the same conclusion as myself with respect to these homologies, yet he calls the Wolffian body Leydig's gland after its distinguished discoverer, and its duct Leydig's duct.
[342] The term segment will be more accurately defined below.
[343] My observations on this subject completely disprove, if it is necessary to do so after Professor Semper's investigations, the statement of Dr Meyer, that segmental tubes in Scyllium open into lymph organs.
[344] I feel considerable hesitation in accepting Semper's descriptions of the ureters and their openings. It has been shewn above that for Scyllium his statements are probably inaccurate, and in other instances, e.g. Raja, I cannot bring my dissections to harmonise with his descriptions.
[345] Journal of Anatomy and Physiology, Vol. IX.
[346] Loc. cit. pp. 85-89.
[347] For the development of the segmental duct, vide p. 345, et seq.
[348]
On the Male and Female Organs of Sharks and Skates, with special
reference to the use of the claspers,
Proceed. American Association for Advancement of
Science, 1874.
[349] Loc. cit.
[350] Loc. cit. pp. 412, 413.
[351]
The Urinogenital Organs of Vertebrates,
Journal of Anatomy
and Physiology, Vol. X.
p. 47. [This edition, p. 164.]
[352] Journal of Anatomy and Physiology, Vol X. 1875. [This edition, No. VII.]
[353] This at least holds good for one of my embryos at this stage, which is labelled Scy. canicula, but which may possibly be Scy. stellare.
[354] Loc. cit. p. 364.
[355] Loc. cit. p. 395.
[356] Journal of Anatomy and Physiology, Vol. X. [This edition, No. VII.]
[357] Entwicklungsgeschichte des Menschen u. der höheren Thiere.
[358] Loc. cit.
[359] Beiträge zur Entwicklungsg. d. Allantois d. Müller'schen Gänge u. d. Afters.
[360]
Abdominal Pores and Urogenital Sinus of Lamprey,
Journal of
Anatomy and Physiology, Vol. X. p. 488.
[361] The reverse of the above rule is the case with Raja, in the male of which a closer approximation to the single-duct type is found than in the female.
The brilliant discoveries of Strasburger and Auerbach have caused the attention of a large number of biologists to be turned to the phenomena accompanying the division of nuclei and the maturation and impregnation of the ovum. The results of the recent investigations on the first of these points formed the subject of an article by Mr Priestley in the sixteenth volume of this Journal, and the object of the present article is to give some account of what has so far been made out with reference to the second of them. The matters to be treated of naturally fall under two heads: (1) the changes attending the ripening of the ovum, which are independent of impregnation; (2) the changes which are directly due to impregnation.
Fig. 1.—Unripe ovum of Toxopneustes lividus (copied from Hertwig).
Every ovum as it approaches maturity is found to be composed (Fig. 1) of (1) a protoplasmic body or vitellus usually containing yolk-spherules in suspension; (2) of a germinal vesicle or nucleus, [Pg 522] containing (3) one or more germinal spots or nucleoli. It is with the germinal vesicle and its contents that we are especially concerned. This body at its full development has a more or less spherical shape, and is enveloped by a distinct membrane. Its contents are for the most part fluid, but may be more or less granular. Their most characteristic component is, however, a protoplasmic network which stretches from the germinal spot to the investing membrane, but is especially concentrated round the former (Fig. 1). The germinal spot forms a nearly homogeneous body, with frequently one or more vacuoles. It occupies an often excentric position within the germinal vesicle, and is usually rendered very conspicuous by its high refrangibility. In many instances it has been shewn to be capable of amœboid movements (Auerbach, and Os. Hertwig), and is moreover more solid and more strongly tinged by colouring reagents than the remaining constituents of the germinal vesicle. These peculiarities have caused the matter of which it is composed to be distinguished by Auerbach and Hertwig as nuclear substance.
In many instances there is only one germinal spot, or one main spot, and two or three accessory smaller spots. In other cases, e.g. Osseous Fish, there are a large number of nearly equal germinal spots. The eggs which have been most investigated with reference to the changes of germinal vesicle are those with a single germinal spot, and it is with these that I shall have more especially to deal in the sequel.
The germinal vesicle occupies in the first instance a central position in the ovum, but at maturity is almost always found in close proximity to the surface. Its change of position in a large number of instances is accomplished during the growth of the ovum in the ovary, but in other cases does not take place till the ovum has been laid.
The questions which many investigators have recently set themselves to answer are the two following:—(1) What becomes of the germinal vesicle when the ovum is ready to be impregnated? (2) Is any part of it present in the ovum at the commencement of segmentation? According to their answers to these questions the older embryologists roughly fall into two groups: (1) By one set the germinal vesicle is stated to completely disappear and not to be genetically connected with the subsequent nuclei [Pg 523] of the embryo. (2) According to the other set it remains in the ovum and by successive divisions forms the parent nucleus of all the nuclei in the body of the embryo. Though the second of these views has been supported by several very distinguished names the first view was without doubt the one most generally entertained, and Haeckel (though from his own observations he was originally a supporter of the second view) has even enunciated the theory that there exists an anuclear stage, after the disappearance of the germinal vesicle, which he regards as an embryonic repetition of the monad condition of the Protozoa.
While the supporters of the first view agree as to the disappearance of the germinal vesicle they differ considerably as to the manner of this occurrence. Some are of opinion that the vesicle simply vanishes, its contents being absorbed in the ovum; others that it is ejected from the ovum and appears as the polar cell or body, or Richtungskörper of the Germans—a small body which is often found situated in the space between the ovum and its membrane, and derives its name from retaining a constant position in relation to the ovum, and thus serving as a guide in determining the similar parts of the embryo through the different stages. The researches of Oellacher (15)[363] in this direction deserve special mention, as having in a sense formed the foundation of the modern views upon this subject. By a series of careful observations upon the egg of the trout and subsequently of the bird, he demonstrated that the germinal vesicle of the ovum, while still in the ovary, underwent partial degeneration and eventually became ejected. His observations were made to a great extent by means of sections, and the general accuracy of his results is fairly certain, but the nature of the eggs he worked on, as well as other causes, prevented his obtaining so deep an insight into the phenomena accompanying the ejection of the germinal vesicle as has since been possible. Lovén, Flemming (6), and others have been led by their investigations to adopt views similar in the main to Oellacher's. As a rule, however, it is held by believers in the disappearance of the germinal vesicle that it becomes simply absorbed, and many very accurate [Pg 524] accounts, so far as they go, have been given of the gradual atrophy of the germinal vesicle. The description of Kleinenberg (14) for Hydra, and Götte for Bombinator, may perhaps be selected as especially complete in this respect; in both instances the germinal vesicle commences to atrophy at a relatively early period.
Coming to the more modern period the researches of five workers, viz. Bütschli, E. van Beneden, Fol, Hertwig, and Strasburger have especially thrown light upon this difficult subject. It is now hardly open to doubt that while part of the germinal vesicle is concerned in the formation of the polar cell or cells, when such are present, and is therefore ejected from the ovum, part also remains in the ovum and forms a nuclear body which will be spoken of as the female pronucleus, the fate of which is recorded in the second part of this paper. The researches of Bütschli and van Beneden have been especially instrumental in demonstrating the relation between the polar bodies and the germinal vesicle, and those of Hertwig and Fol, in shewing that part of the germinal vesicle remained in the ovum. It must not, however, be supposed that the results of these authors are fully substantiated, or that all the questions connected with these phenomena are settled. The statements we have are in many points opposed and contradictory, and there is much that is still very obscure.
In the sequel an account is first given of the researches of the above-named authors, followed by a statement of those results which appear to me the most probable.
The researches of van Beneden (3 and 4) were made on the ovum of the rabbit and of Asterias, and from his observations on both these widely separated forms he has been led to conclude that the germinal vesicle is either ejected or absorbed, but that it has in no case a genetic connection with the first segmentation sphere. He gives the following description of the changes in the rabbit's ovum. The germinal vesicle is enclosed by a membrane, and contains one main germinal spot, and a few accessory ones, together with a granular material which he calls nucleoplasma, which affects, as is usual in nuclei, a reticular arrangement. The remaining space in the vesicle is filled by a clear fluid. As the ovum approaches maturity the germinal [Pg 525] vesicle assumes an excentric position, and fuses with the peripheral layer of the egg to constitute the cicatricular lens. The germinal spot next travels to the surface of the cicatricular lens and forms the nuclear disc: at the same time the membrane of the germinal vesicle vanishes though it probably unites with the nuclear disc. The nucleoplasma then collects into a definite mass and forms the nucleoplasmic body. Finally the nuclear disc assumes an ellipsoidal form and becomes the nuclear body. Nothing is now left of the original germinal vesicle but the nuclear body and the nucleoplasmic body both still situated within the ovum. In the next stage no trace of the germinal vesicle can be detected in the ovum, but outside it, close to the point where the modified remnants of the vesicle were previously situated, there is present a polar body which is composed of two parts, one of which stains deeply and resembles the nuclear body, and the other does not stain but is similar to the nucleoplasmic body. Van Beneden concludes that the polar bodies are the two ejected products of the germinal vesicle. In the case of Asterias, van Beneden has not observed the mode of formation of the polar bodies, and mainly gives an account of the atrophy of the germinal vesicle, but adds very little to what was already known to us from Kleinenberg's (14) earlier observations. He describes with precision the breaking up of the germinal spot into fragments and its eventual disappearance.
Though there are reasons for doubting the accuracy of all the above details on the ovum of the rabbit, nevertheless, the observations of van Beneden taken as a whole afford strong grounds for concluding that the formation of the polar cells is connected with the disappearance, partial or otherwise, of the germinal vesicle. A very similar account of the apparent disappearance of the germinal vesicle is given by Greeff (19) who states that the apparent disappearance of the germinal spot precedes that of the vesicle.
The observations of Bütschli are of still greater importance in this direction. He has studied with a view to elucidating the fate of the germinal vesicle, the eggs of Nephelis, Lymnæus, Cucullanus, and other Nematodes; and Rotifers. In all of these, with the exception of Rotifers, he finds polar bodies, and in this [Pg 526] respect his observations are of value as tending to shew the widespread existence of these structures. Negative results with reference to the presence of the polar bodies have, it may be remarked, only a very secondary value. Bütschli has made the very important discovery that in perfectly ripe eggs of Nephelis, Lymnæus and Cucullanus and allied genera a spindle, similar to that of ordinary nuclei in the act of division, appears close to the surface of the egg. This spindle he regards as the metamorphosed germinal vesicle, and has demonstrated that it takes part in the formation of the polar cells. He states that the whole spindle is ejected from the egg, and that after swelling up and forming a somewhat spherical mass it divides into three parts.
In the Nematodes generally, Bütschli has been unable to find the spindle modification of the germinal vesicle, but he states that the germinal vesicle undergoes degeneration, its outline becoming indistinct and the germinal spot vanishing. The position of the germinal vesicle continues to be marked by a clear space which gradually approaches the surface of the egg. When it is in contact with the surface a small spherical body, the remnant of the germinal vesicle, comes into view, and eventually becomes ejected. The clear space subsequently disappears. This description of Bütschli resembles in some respects that given by van Beneden of the changes in the rabbit's ovum, and not impossibly refers to a nearly identical series of phenomena. The discovery by Bütschli of the spindle and its relation to the polar body has been of very great value.
The publications of van Beneden, and more especially those of Bütschli, taken by themselves lead to the conclusion that the whole germinal vesicle is either ejected or absorbed. Nearly simultaneously with their publications there appeared, however, a paper by Oscar Hertwig (11) on the eggs of one of the common sea urchins (Toxopneustes lividus), in which he attempted to shew that part of the germinal vesicle, at any rate, was concerned in the formation of the first segmentation nucleus. He believed (though he has himself now recognised that he was in error on the point) that no polar cell was formed in Toxopneustes, and that the whole germinal vesicle was absorbed, with the exception of the germinal spot which remained in the egg as the female pronucleus.
[Pg 527] The following is the summary which he gives of his results, pp. 357-8.
At the time when the egg is mature the germinal
vesicle undergoes a retrogressive metamorphosis and becomes carried towards
the surface of the egg by the contraction of the protoplasm. Its membrane
becomes dissolved and its contents disintegrated and finally absorbed by
the yolk. The germinal spot appears, however, to remain unaltered and to
continue in the yolk and to become the permanent nucleus of the ripe ovum
capable of impregnation.
After the publication of Bütschli's monograph, O. Hertwig (12) continued his researches on the ova of Leeches (Hæmopis and Nephelis), and not only added very largely to our knowledge of the history of the germinal vesicle, but was able to make a very important rectification in Bütschli's conclusions. The following is a summary of his results:—The germinal vesicle, as in other cases, undergoes a form of degeneration, though retaining its central position; and the germinal spot breaks up into fragments. The stages in which this occurs are followed by one when, on a superficial examination, the ovum appears to be absolutely without a nucleus; but there can be demonstrated by means of reagents in the position previously occupied by the germinal vesicle a spindle nucleus with the usual suns at its poles, which Hertwig believes to be a product of the fragments of the germinal spot. This spindle travels towards the periphery of the ovum and then forms the spindle observed by Bütschli. At the point where one of the apices of the spindle lies close to the surface a small protuberance arises which is destined to form the first polar cell. As the protuberance becomes more prominent one half of the spindle passes into it. The spindle then divides in the normal manner for nuclei, one half remaining in the protuberance, the other in the ovum, and finally the protuberance becomes a rounded body united to the egg by a narrow stalk. It is clear that if, as there is every reason to think, the above description is correct, the polar cell is formed by a simple process of cell-division and not, as Bütschli believed, by the forcible ejection of the spindle.
The portion of the spindle in the polar cell becomes a mass of granules, and that in the ovum becomes converted without [Pg 528] the occurrence of the usual nuclear stage into a fresh spindle. A second polar cell is formed in the same manner as the first one, and the first one subsequently divides into two. The portion of the spindle which remains in the egg after the formation of the second polar cell reconstitutes itself into a nucleus—the female pronucleus—and travelling towards the centre of the egg undergoes a fate which will be spoken of in the second part of this paper.
The most obscure part of Hertwig's work is that which concerns the formation of the spindle on the atrophy of the germinal vesicle, and his latest paper, though it gives further details on this head, does not appear to me to clear up the mystery. Though Hertwig demonstrates clearly enough that this spindle is a product of the metamorphoses of the germinal vesicle, he does not appear to prove the thesis which he maintains, that it is the metamorphosed germinal spot.
Fol, to whom we are indebted in his paper on the development of Geryonia (7) for the best of the earlier descriptions of the phenomena which attend the maturation of the egg, and later for valuable contributions somewhat similar to those of Bütschli with reference to the development of the Pteropod egg (8), has recently given us a very interesting account of what takes place in the ripe egg of Asterias glacialis (9). In reference to the formation of the polar cells, his results accord closely with those of Hertwig, but he differs considerably from this author with reference to the preceding changes in the germinal vesicle. He believes that the germinal spot atrophies more or less completely, but that in any case its constituents remain behind in the egg, though he will not definitely assert that it takes no share in the formation of the spindle at the expense of which both the polar cells and the female pronucleus are formed. The spindle with its terminal suns arises, according to him, from the contents of the germinal vesicle, loses its spindle character, travels to the surface, and reacquiring a spindle character is concerned in the formation of the polar cells in the way described by Hertwig.
Giard (10) gives a somewhat different account of the behaviour of the germinal vesicle in Psammechinus miliaris. At maturity the contents of the germinal vesicle and spot mix [Pg 529] together and form an amœboid mass, which, assuming a spindle form, divides into two parts, one of which travels towards the centre of the egg and forms the female pronucleus, the other remains at the surface and gives origin to two polar cells, both of which are formed after the egg is laid. What Giard regards as the female pronucleus is perhaps the lower of the two bodies which take the place of the original germinal vesicle as described by Fol. Vide the account of Fol's observations on p. 531.
Strasburger, from observations on Phallusia, accepts in the main Hertwig's conclusion with reference to the formation of the polar bodies, but does not share Hertwig's view that either the polar bodies or female pronucleus are formed at the expense of the germinal spot alone. He has further shewn that the so-called canal-cell of conifers is formed in the same manner as the polar cells, and states his belief that an equivalent of the polar cells is widely distributed in the vegetable subkingdom.
This sketch of the results of recent researches will, it is hoped, suffice to bring into prominence the more important steps by which the problems of this department of embryology have been solved. The present aspects of the question may perhaps be most conveniently displayed by following the history of a single ovum. For this purpose the eggs of Asterias glacialis, which have recently formed the subject of a series of beautiful researches by Fol (9), may conveniently be selected.
The ripe ovum (Fig. 2), when detached from the ovary, is formed of a granular vitellus without a vitelline membrane, but enveloped in a mucilaginous coat. It contains an excentrically situated germinal vesicle and germinal spot. In the former is present the usual protoplasmic reticulum. As soon as the ovum reaches the sea water the germinal vesicle commences to undergo a peculiar metamorphosis. It exhibits frequent changes of form, its membrane becomes gradually absorbed and its outline indented and indistinct, and finally its contents become to a certain extent confounded with the vitellus (Fig. 3).
The germinal spot at the same time loses its clearness of outline and gradually disappears from view.
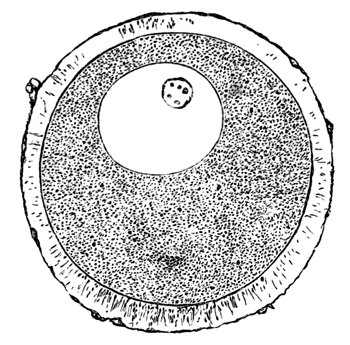
Fig. 2.—Ripe ovum of Asterias glacialis enveloped in a mucilaginous envelope, and containing an excentric germinal vesicle and germinal spot (copied from Fol).
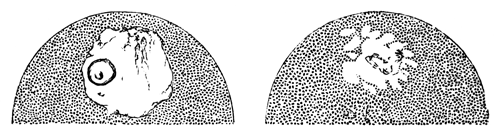
Fig. 3.—Two successive stages in the gradual metamorphosis of the germinal vesicle and spot of the ovum of Asterias glacialis immediately after it is laid (copied from Fol).
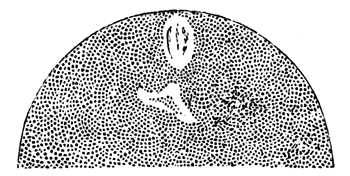
Fig. 4.—Ovum of Asterias glacialis, shewing the clear spaces in the place of the germinal vesicle. Fresh preparation (copied from Fol).
At a slightly later stage in the place of the original germinal vesicle there may be observed in the fresh ovum two clear spaces (fig. 4), one ovoid and nearer the surface, and the second more irregular in form and situated rather deeper in the vitellus. By treatment with reagents the first clear space is found to be formed of a spindle with two terminal suns on the lower side of which is a somewhat irregular body (Fig. 5). The second clear space by the same treatment is shewn to contain a round body. [Pg 531] Fol concludes that the spindle is formed out of part of the germinal vesicle and not of the germinal spot, while he sees in the round body present in the lower of the two clear spaces the metamorphosed germinal spot. He will not, however, assert that no fragment of the germinal spot enters into the formation of the spindle. It may be observed that Fol is here obliged to fill up (so far at least as his present preliminary account enables me to determine) a lacuna in his observations in a hypothetical manner, and O. Hertwig's (13) most recent observations on the ovum of the same or an allied species of Asterias tend to throw some doubt upon Fol's interpretations.
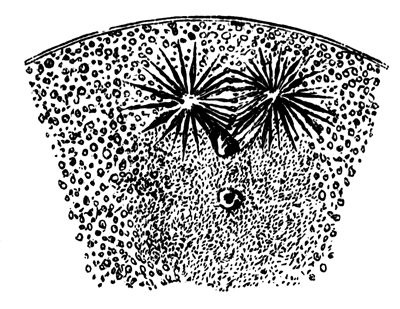
Fig. 5.—Ovum of Asterias glacialis, at the same stage as Fig. 4, treated with picric acid (copied from Fol).
The following is Hertwig's account of the changes in the germinal vesicle. A quarter of an hour after the egg is laid the protoplasm on the side of the germinal vesicle towards the surface of the egg develops a prominence which presses inwards the wall of the vesicle. At the same time the germinal spot develops a large vacuole, in the interior of which is a body consisting of nuclear substance, and formed of a firmer and more refractive material than the remainder of the germinal spot. In the above-mentioned prominence towards the germinal vesicle, first one sun is formed by radial striæ of protoplasm, and then a second makes its appearance, while in the living ovum the germinal spot appears to have vanished, the outline of the germinal vesicle to have become indistinct, and its contents to have mingled with the surrounding protoplasm. Treatment with reagents demonstrates that in the process of disappearance of the germinal spot the nuclear mass in the vacuole forms a [Pg 532] rod-like body, the free end of which is situated between the two suns which occupy the prominence of the germinal vesicle. At a slightly later period granules may be seen at the end of the rod and finally the rod itself vanishes. After these changes there may be demonstrated by the aid of reagents a spindle between the two suns, which Hertwig believes to grow in size as the last remnants of the germinal spot gradually vanish, and he maintains, as before mentioned, that the spindle is formed at the expense of the germinal spot. Without following Hertwig so far as this[364] it may be permitted to suggest that his observations tend to shew that the body noticed by Fol in the median line, on the inner side of his spindle, is in reality a remnant of the germinal spot and not, as Fol supposes, part of the germinal vesicle. Considering how conflicting is the evidence before us it seems necessary to leave open for the present the question as to what parts of the germinal vesicle are concerned in forming the first spindle.
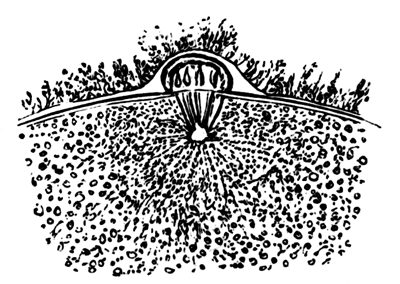
Fig. 6.—Portion of the ovum of Asterias glacialis, shewing the spindle formed from the metamorphosed germinal vesicle projecting into a protoplasmic prominence of the surface of the egg. Picric acid preparation (copied from Fol).
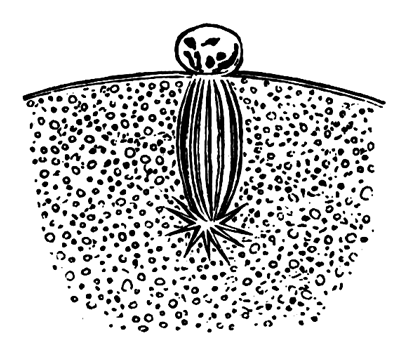
Fig. 7.—Portion of the ovum of Asterias glacialis at the moment of the detachment of the first polar body and the withdrawal of the remaining part of the spindle within the ovum. Picric acid preparation (copied from Fol).
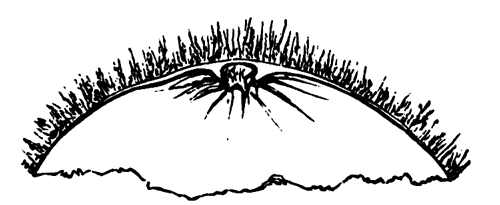
Fig. 8.—Portion of the ovum of Asterias glacialis, with the first polar body as it appears when living (copied from Fol).
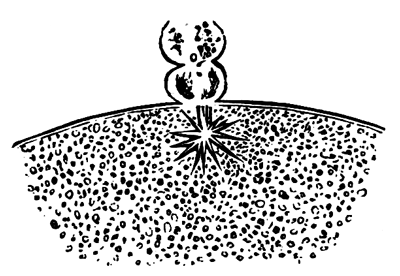
Fig. 9.—Portion of the ovum of Asterias glacialis immediately after the formation of the second polar body. Picric acid preparation (copied from Fol).
The spindle, however it be formed, has up to this time been situated with its axis parallel to the surface of the egg, but not long after the stage last described a spindle is found with one end projecting into a protoplasmic prominence which makes its appearance on the surface of the egg (Fig. 6). Hertwig believes that the spindle simply travels towards the surface, and while doing so changes the direction of its axis. Fol finds, however, that this is not the case, but that between the two conditions [Pg 533] of the spindle an intermediate one is found in which a spindle can no longer be seen in the egg, but its place is taken by a compact rounded body. He has not been able to arrive at a conclusion as to what meaning is to be attached to this occurrence. In any case the spindle which projects into the prominence on the surface of the egg divides it into two parts, one in the prominence and one in the egg (Fig. 7). The prominence itself with the enclosed portion of the spindle becomes partially constricted off from the egg as the first polar body (Fig. 8). The part of the spindle which remains in the egg becomes directly converted into a second spindle by the elongation of its fibres without passing through a typical nuclear condition. A second polar cell next becomes formed in the same manner as the first (Fig. 9), and [Pg 534] the portion of the spindle remaining in the egg becomes converted into two or three clear vesicles (Fig. 10) which soon unite to form a single nucleus, the female pronucleus (Fig. 11). The two polar cells appear to be situated between two membranes, the outer of which is very delicate and only distinct where it covers the polar cells, while the inner one is thicker and becomes, after impregnation, more distinct and then forms what Fol speaks of as the vitelline membrane. It is clear, as Hertwig has pointed out, that the polar bodies originate by a regular cell division and have the value of cells.
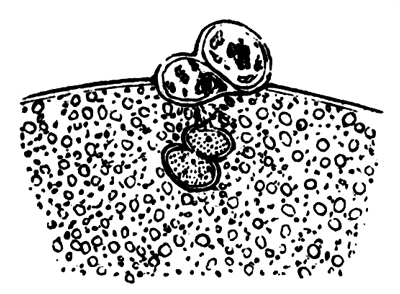
Fig. 10.—Portion of the ovum of Asterias glacialis after the formation of the second polar cell, shewing the part of the spindle remaining in the ovum becoming converted into two clear vesicles. Picric acid preparation (copied from Fol).
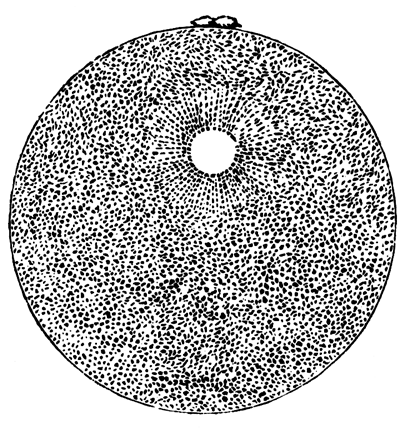
Fig. 11.—Ovum of Asterias glacialis with the two polar bodies and the female pronucleus surrounded by radial striæ, as seen in the living egg (copied from Fol).
Considering how few ova have been adequately investigated with reference to the behaviour of the germinal vesicle any general conclusions which may at present be formed are to be regarded as provisional, and I trust that this will be borne in mind by the reader in perusing the following paragraphs.
There is abundant evidence that at the time of maturation of the egg the germinal vesicle undergoes peculiar changes, which are, in part at least, of a retrogressive character. These changes may begin considerably before the egg has reached the period of maturity, or may not take place till after it has been laid. They consist in appearance of irregularity and obscurity in the outline of the germinal vesicle, the absorption of its membrane, the partial absorption of its contents in the yolk, and the breaking up and disappearance of the germinal spot. The exact fate of the single germinal spot, or the numerous spots where they are present, is still obscure; and the observations of Oellacher on the trout, and to a certain extent my own on the skate, tend to shew that the membrane of the germinal vesicle may in some cases be ejected from the egg, but this conclusion cannot be accepted without further confirmation.
The retrogressive metamorphosis of the germinal vesicle is followed in a large number of instances by the conversion of what remains into a striated spindle similar in character to a nucleus previous to division. This spindle travels to the surface and undergoes division to form the polar cell or cells in the manner above described. The part which remains in the egg forms eventually the female pronucleus.
The germinal vesicle has up to the present time only been observed to undergo the above series of changes in a certain number of instances, which, however, include examples from several divisions of the Cœlenterata, the Echinodermata, and the Mollusca, and also some of the Vermes (Nematodes, Hirudinea, Sagitta). It is very possible, not to say probable, that it is universal in the animal kingdom, but the present state of our knowledge does not justify us in saying so. It may be that in the case of the rabbit, and many Nematodes as described by van Beneden [Pg 536] and by Bütschli, we have instances of a different mode of formation of the polar cells.
The case of Amphibians, as described by Bambeke (2) and Hertwig (12) cannot so far be brought into conformity with our type, though observations are so difficult to make with such opaque eggs that not much reliance can be placed upon the existing statements. In both of these types of possible exceptions it is fairly clear that, whatever may be the case with reference to the formation of the polar cells, part of the germinal vesicle remains behind as the female pronucleus.
There are a large number of types, including the whole of the Rotifera[365] and Arthropoda, with a few doubtful exceptions, in which the polar cells cannot as yet be said to have been satisfactorily observed.
Whatever may be the eventual result of more extended investigation, it is clear that the formation of polar cells according to our type is a very constant occurrence. Its importance is also very greatly increased by the discovery by Strasburger of the existence of an analogous process amongst plants. Two questions about it obviously present themselves for solution: (1) What are the conditions of its occurrence with reference to impregnation? (2) What meaning has it in the development of the ovum or the embryo?
The answer to the first of these questions is not difficult to find. The formation of the polar bodies is independent of impregnation, and is the final act of the normal growth of the ovum. In a few types the polar cells are formed while the ovum is still in the ovary, as, for instance, in some species of Echini, Hydra, &c., but, according to our present knowledge, far more usually after the ovum has been laid. In some of the instances the budding off of the polar cells precedes, and in others follows impregnation; but there is no evidence to shew that in the later cases the process is influenced by the contact with the male element. In Asterias, as has been shewn by O. Hertwig, the [Pg 537] formation of the polar cells may indifferently either precede or follow impregnation—a fact which affords a clear demonstration of the independence of the two occurrences.
To the second of the two questions it does not unfortunately seem possible at present to give an answer which can be regarded as satisfactory.
The retrogressive changes in the membrane of the germinal vesicle which usher in the formation of the polar bodies may very probably be viewed as a prelude to a renewed activity of the contents of the vesicle; and are perhaps rendered the more necessary from the thickness of the membrane which results from a protracted period of passive growth. This suggestion does not, however, help us to explain the formation of polar cells by a process identical with cell division. The ejection of part of the germinal vesicle in the formation of the polar cells may probably be paralleled by the ejection of part or the whole of the original nucleus which, if we may trust the beautiful researches of Bütschli, takes place during conjugation in Infusoria as a preliminary to the formation of a fresh nucleus. This comparison is due to Bütschli, and according to it the formation of the polar bodies would have to be regarded as assisting, in some as yet unknown way, the process of regeneration of the germinal vesicle. Views analogous to this are held by Strasburger and Hertwig, who regard the formation of the polar bodies in the light of a process of excretion or removal of useless material. Such hypotheses do not unfortunately carry us very far.
I would suggest that in the formation of the polar cells part of the constituents of the germinal vesicle which are requisite for its functions as a complete and independent nucleus are removed to make room for the supply of the necessary parts to it again by the spermatic nucleus (vide p. 541). More light on this, as on other points, may probably be thrown by further investigations on parthenogenesis and the presence or absence of a polar cell in eggs which develop parthenogenetically. Curiously enough the two groups in which parthenogenesis most frequently occurs in the ordinary course of development (Arthropoda and Rotifera) are also those in which polar cells, with the possible exception mentioned above, of the parthenogenetic eggs of Lacenularia, are stated to be absent. This curious coincidence, [Pg 538] should it be confirmed, may perhaps be explained on the hypothesis, I have just suggested, viz. that a more or less essential part of the nucleus is removed in the formation of the polar cells; so that in cases, e.g. Arthropoda and Rotifera, where polar cells are not formed, and an essential part of the nucleus not therefore removed, parthenogenesis can much more easily occur than when polar cells are formed.
That the part removed in the formation of the polar cells is not absolutely essential, seems at first sight to follow from the fact of parthenogenesis being possible in instances where impregnation is the normal occurrence. The genuineness of all the observations on this head is too long a subject to enter into here[366], but after admitting, as we probably must, that there are genuine cases of parthenogenesis, it cannot be taken for granted without more extended observation that the occurrence of development in these rare instances may not be due to the polar cells not having been formed as usual, and that when the polar cells are formed the development without impregnation is less possible.
The remarkable observations of Professor Greeff (19) on the parthenogenetic development of the eggs of Asterias rubens tell, however, very strongly against this explanation. Greeff has found that under normal circumstances the eggs of this species of starfish will develop without impregnation in simple sea water. The development is quite regular and normal though much slower than in the case of impregnated eggs. It is not definitely stated that polar cells are formed, but there can be no doubt that this is implied. Professor Greeff's account is so precise and circumstantial that it is not easy to believe that any error can have crept in; but neither Hertwig nor Fol have been able to repeat his experiments, and we may be permitted to wait for further confirmation before absolutely accepting them.
[Pg 539] It is possible that the removal of part of the protoplasm of the egg in the formation of the polar cells may be a secondary process due to an attractive influence of the nucleus on the cell protoplasm, such as is ordinarily observed in cell division.
Impregnation of the Ovum.
A far greater amount of certainty appears to me to have been attained as to the effects of impregnation than as to the changes of the germinal vesicle which precede this, and there appears, moreover, to be a greater uniformity in the series of resulting phenomena. For convenience I propose to reverse the order hitherto adopted and to reserve the history of the literature and my discussion of disputed points till after my general account. Fol's paper on Asterias glacialis, is again my source of information. The part of the germinal vesicle which remains in the egg, after the formation of the second polar cell, becomes converted into a number of small vesicles (Fig. 10), which aggregate themselves into a single clear nucleus which gradually travels toward the centre of the egg and around which as a centre the protoplasm becomes radiately striated (Fig. 11). This nucleus is known as the female pronucleus[367]. In Asterias glacialis the most favourable period for fecundation is about an hour after the formation of the female pronucleus. If at this time the spermatozoa are allowed to come in contact with the egg, their heads soon become enveloped in the investing mucilaginous coat. A prominence, pointing towards the nearest spermatozoon, now arises from the superficial layer of protoplasm of the egg and grows till it comes in contact with the spermatozoon (Figs. 12 and 13), Under normal circumstances the spermatozoon, which meets the prominence, is the only one concerned in the fertilisation, and it makes its way into the egg by passing through the prominence. The tail of the spermatozoa, no longer motile, remains visible for some time after the head has bored its way in, but its place is soon taken by a pale conical body which is, however, probably in part a product of the metamorphosis of the tail itself (Fig. 14). This body vanishes in its turn.
Fig. 12.
Fig. 13.
Figs. 12 and 13.—Small portion of the ovum of Asterias glacialis. The spermatozoa are shewn enveloped in the mucilaginous coat. In Fig. 12 a prominence is rising from the surface of the egg towards the nearest spermatozoon; and in Fig. 13 the spermatozoon and prominence have met. From living ovum (copied from Fol).
At the moment of contact between the spermatozoon and the egg the outermost layer of the protoplasm of the latter raises itself as distinct membrane, which separates from the egg and prevents the entrance of any more spermatozoa. At the point where the spermatozoon entered a crater-like opening is left in the membrane (Fig. 14).
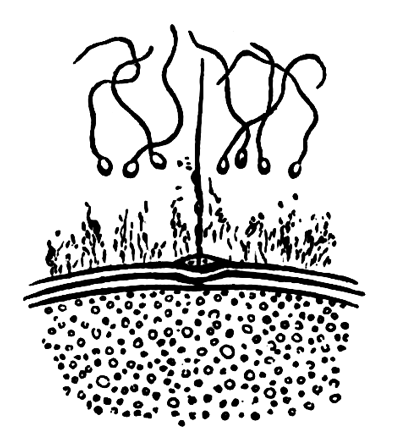
Fig. 14.—Portion of the ovum of Asterias glacialis after the entrance of a spermatozoon into the ovum. It shows the prominence of the ovum through which the spermatozoon has entered. A vitelline membrane with a crater-like opening has become distinctly formed. From living ovum (copied from Fol).
The head of the spermatozoon when in the egg forms a nucleus for which the name male pronucleus may be conveniently adopted. It grows in size by absorbing, it is said, material from the ovum, though this may be doubted, and around it is formed a clear space free from yolk-spherules. Shortly after its formation [Pg 541] the protoplasm in its neighbourhood assumes a radiate arrangement (Fig. 15). At whatever point of the egg the spermatozoon may have entered, it gradually travels towards the female pronucleus. This latter, around which the protoplasm no longer has a radial arrangement, remains motionless till it comes in contact with the rays of the male pronucleus, after which its condition of repose is exchanged for one of activity, and it rapidly approaches the male pronucleus, and eventually fuses with it (Fig. 16).
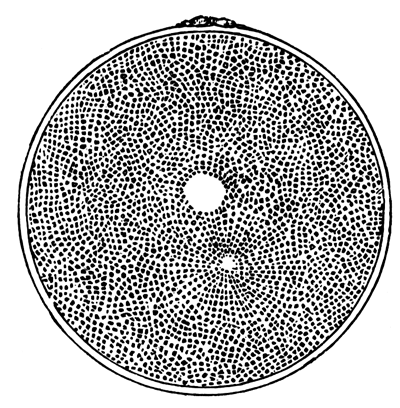
Fig. 15.—Ovum of Asterias glacialis, with male and female pronucleus and a radial striation of the protoplasm around the former. From living ovum (copied from Fol).

Fig. 16.—Three successive stages in the coalescence of the male and female pronucleus in Asterias glacialis. From the living ovum (copied from Fol).
The product of this fusion forms the first segmentation nucleus (Fig. 17), which soon, however, divides into the two nuclei of the two first segmentation spheres. While the two pronuclei are approaching one another the protoplasm of the egg exhibits amœboid movements.
Of the earlier observations on this subject there need perhaps only be cited one of E. van Beneden, on the rabbit's ovum, [Pg 542] shewing the presence of two nuclei before the commencement of segmentation. Bütschli was the earliest to state from observations on Rhabditis dolichura that the first segmentation nucleus arose from the fusion of two nuclei, and this was subsequently shewn with greater detail for Ascaris nigrovenosa, by Auerbach (1). Neither of these authors gave at first the correct interpretation of their results. At a later period Bütschli (5) arrived at the conclusion that in a large number of instances (Lymnæus, Nephelis, Cucullanus, &c.), the nucleus in question was formed by the fusion of two or more nuclei, and Strasburger at first made a similar statement for Phallusia, though he has since withdrawn it. Though Bütschli's statements depend, as it seems, upon a false interpretation of appearances, he nevertheless arrived at a correct view with reference to what occurs in impregnation. Van Beneden (3) described in the rabbit the formation of the original segmentation nucleus from two nuclei, one peripheral and the other central, and he gave it as his hypothetical view that the peripheral nucleus was derived from the spermatic element. It was reserved for Oscar Hertwig (11) to describe in Echinus lividus the entrance of a spermatozoon into the egg and the formation from it of the male pronucleus.
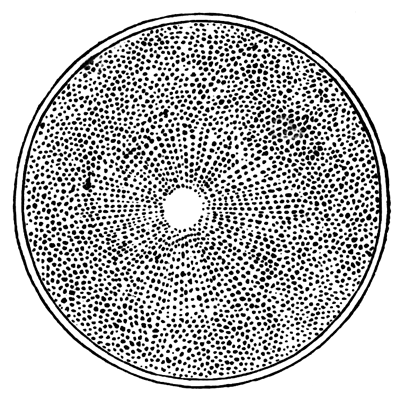
Fig. 17.—Ovum of Asterias glacialis, after the coalescence of the male and female pronucleus (copied from Fol).
Though there is a general agreement between the most recent observers, Hertwig, Fol, Selenka, Strasburger, &c., as to the main facts connected with the entrance of one spermatozoon into [Pg 543] the egg, the formation of the male pronucleus, and its fusion with the female pronucleus, there still exist differences of detail in the different descriptions which partly, no doubt, depend upon the difficulties of observation, but partly also upon the observations not having all been made upon the same species. Hertwig does not enter into details with reference to the actual entrance of the spermatozoon into the egg, but in his latest paper points out that considerable differences may be observed in occurrences which succeed impregnation, according to the relative period at which this takes place. When, in Asterias, the impregnation is effected about an hour after the egg is laid and previously to the formation of the polar cells, the male pronucleus appears at first to exert but little influence on the protoplasm, but after the formation of the second polar cell, the radial striæ around it become very marked, and the pronucleus rapidly grows in size. When it finally unites with the female pronucleus it is equal in size to the latter. In the case when the impregnation is deferred for four hours the male pronucleus never becomes so large as the female pronucleus. With reference to the effect of the time at which impregnation takes place, Asterias would seem to serve as a type. Thus in Hirudinea, Mollusca, and Nematodes impregnation normally takes place before the formation of the polar bodies is completed, and the male pronucleus is accordingly as large as the female. In Echinus, on the other hand, where the polar bodies are formed in the ovary, the male pronucleus is always small.
Selenka, who has investigated the formation of the male pronucleus in Toxopneustes variegatus, differs in certain points from Fol. He finds that usually, though not always, a single spermatozoon enters the egg, and that though the entrance may be effected at any part of the surface, it generally occurs at the point marked by a small prominence where the polar cell was formed. The spermatozoon first makes its way through the mucous envelope of the egg, within which it swims about, and then bores with its head into the polar prominence. The head of the spermatozoon on entering the egg becomes enveloped by the superficial protoplasm, and travels inward with its envelope, while the tail remains outside. As Fol has described, a delicate membrane becomes formed shortly after the entrance of the [Pg 544] spermatozoon. The head continues to make its way by means of rapid oscillations, till it has traversed about one eighth of the diameter of the egg, and then suddenly becomes still. The tail in the meantime vanishes, while the neck swells up and forms the male pronucleus. The junction of the male and female pronucleus is described by Fol and Selenka in nearly the same manner.
Giard gives an account of impregnation which is not easily brought into harmony with that of the other investigators. His observations were made on Psammechinus miliaris. At one point is situated a polar body and usually at the pole opposite to it a corresponding prominence. The spermatozoa on gaining access to the egg attach themselves to it and give it a rotatory movement, but according to Giard none of them penetrate the vitelline membrane which, though formed at an earlier period, now retires from the surface of the egg.
Giard believes that the prominence opposite the polar cells serves for the entrance of the spermatic material, which probably passes in by a process of diffusion. Thus, though he regards the male pronucleus as a product of impregnation, he does not believe it to be the head of a spermatozoon.
Both Hertwig and Fol have made observations on the result of the entrance into the egg of several spermatozoa. Fol finds that when the impregnation has been too long delayed the vitelline membrane is formed with comparative slowness and several spermatozoa are thus enabled to penetrate. Each spermatozoon forms a separate pronucleus with a surrounding sun; and several male pronuclei usually fuse with the female pronucleus. Each male pronucleus appears to exercise a repulsive influence on other male pronuclei, but to be attracted by the female pronucleus. When there are several male pronuclei the segmentation is irregular and the resulting larva a monstrosity. These statements of Fol and Hertwig are at first sight in contradiction with the more recent results of Selenka. In Toxopneustes variegatus Selenka finds that though impregnation is usually effected by a single spermatozoon yet that several may be concerned in the act. The development continues, however, to be normal if three or even four spermatozoa enter the egg almost simultaneously. Under such circumstances each spermatozoon forms a separate pronucleus and sun.
[Pg 545] It may be noticed that, while the observations of Fol and Hertwig were admittedly made upon eggs in which the impregnation was delayed till they no longer displayed their pristine activity, Selenka's were made upon quite fresh eggs; and it seems not impossible that the pathological symptoms in the embryos reared by the two former authors may have been due to the imperfection of the egg and not to the entrance of more than one spermatozoon. This, of course, is merely a suggestion which requires to be tested by fresh observations. We have not as yet a sufficient body of observations to enable us to decide whether impregnation is usually effected by a single spermatozoon, though in spite of certain conflicting evidence the balance would seem to incline towards the side of a single spermatozoon[368].
The discovery of Hertwig as to the formation of the male pronucleus throws a flood of light upon impregnation.
The act of impregnation is seen essentially to consist in the fusion of a male and female nucleus; not only does this appear in the actual fusion of the two pronuclei, but it is brought into still greater prominence by the fact that the female pronucleus is a product of the nucleus of a primitive ovum, and the male pronucleus is the metamorphosed head of the spermatozoon which is itself developed from the nucleus of a spermatic cell[369]. The spermatic cells originate from cells (in the case of Vertebrates at least) identical with the primitive ova, so that the fusion which takes place is the fusion of morphologically similar parts in the two sexes.
It must not, however, be forgotten, as Strasburger has pointed out, that part of the protoplasm of the generative cells of the two sexes also fuse, viz. the tail of the spermatozoon with the protoplasm of the egg. But there is no evidence that the former is of importance for the act of impregnation. The fact that impregnation mainly consists in the union of two nuclei gives an importance to the nucleus which would probably not have been accorded to it on other grounds.
[Pg 546] Hertwig's discovery is in no way opposed to Mr Darwin's theory of pangenesis and other similar theories, but does not afford any definite proof of their accuracy, nor does it in the meantime supply any explanation of the origin of two sexes or of the reasons for an embryo becoming male or female.
Summary.
In what may probably be regarded as a normal case the following series of events accompanies the maturation and impregnation of an egg:—
(1) Transportation of the germinal vesicle to the surface of the egg.
(2) Absorption of the membrane of the germinal vesicle and metamorphosis of the germinal spot.
(3) Assumption of a spindle character by the remains of germinal vesicle, these remains being probably largely formed from the germinal spot.
(4) Entrance of one end of the spindle into a protoplasmic prominence at the surface of the egg.
(5) Division of the spindle into two halves, one remaining in the egg, the other in the prominence. The prominence becomes at the same time nearly constricted off from the egg as a polar cell.
(6) Formation of a second polar cell in same manner as first, part of the spindle still remaining in the egg.
(7) Conversion of the part of the spindle remaining in the egg after the formation of the second polar cell into a nucleus—the female pronucleus.
(8) Transportation of the female pronucleus towards the centre of the egg.
(9) Entrance of one spermatozoon into the egg.
(10) Conversion of the head of the spermatozoon into a nucleus—the male pronucleus.
(11) Appearance of radial striæ round the male pronucleus which gradually travels towards the female pronucleus.
(12) Fusion of male and female pronuclei to form the first segmentation nucleus.
[Pg 547] List of important recent Publications on the Maturation and Impregnation of the Ovum.
1. Auerbach. Organologische Studien, Heft 2.
2. Bambeke. Recherches s. Embryologie des Batraciens.
Bull. de
l'Acad. royale de Belgique, 2me sér., t. LXI. 1876.
3. E. Van Beneden. La Maturation de l'Œuf des Mammifères.
Bull. de
l'Acad. royale de Belgique, 2me sér., t. XL, no. 12, 1875.
4. E. Van Beneden. Contributions à l'Histoire de la Vésicule Germinative, &c.
Bull. de l'Acad. royale de
Belgique, 2me sér., t. XLI,
no. 1, 1876.
5. Bütschli. Eizelle, Zelltheilung, und Conjugation der Infusorien.
6. Flemming. Studien in d. Entwicklungsgeschichte der Najaden.
Sitz. d. k. Akad. Wien, B. LXXI. 1875.
7. Fol. Die
erste Entwicklung des Geryonideneies.
Jenaische
Zeitschrift, Vol. VII.
8. Fol. Sur
le Développement des Pteropodes.
Archives de Zoologie Expérimentale
et Générale, Vols. IV and V.
9. Fol. Sur
le Commencement de l'Hénogénie.
Archives des Sciences Physiques
et Naturelles. Genève, 1877.
10. Giard. Note sur les premiers phénomènes du développement de l'Oursin. 1877.
11. Hertwig, Oscar. Beit. z. Kenntniss d. Bildung, &c., d. thier. Eies.
Morphologisches
Jahrbuch, Bd.I.
12. Hertwig, Oscar. Ibid. Morphologisches Jahrbuch, Bd.III, Heft. 1.
13. Hertwig, Oscar. Weitere Beiträge, &c.
Morphologisches Jahrbuch, Bd. III, Heft 3.
14. Kleinenberg. Hydra. Leipzig, 1872.
15. Oellacher, J. Beiträge zur Geschichte des Keimbläschens im Wirbelthiereie.
Archiv f. micr. Anat., Bd.VIII.
16. Selenka. Befruchtung u. Theilung des Eies von Toxopneustes variegatus (Vorläufige Mittheilung). Erlangen, 1877.
17. Strasburger. Ueber Zellbildung u. Zelltheilung. Jena, 1876.
18. Strasburger. Ueber Befruchtung u. Zelltheilung. Jena, 1878.
19. R. Greeff. Ueb. d. Bau u. d. Entwicklung d. Echinodermen.
Sitzun. der Gesellschaft z. Beförderung d. gesammten Naturwiss. z.
Marburg, No. 5. 1876.
[Pg 548]Postscript.—Two important
memoirs have appeared since this paper was in type. One of these by
Hertwig, Morphologisches Jahrbuch, Bd. IV, contains a full account with illustrations of
what was briefly narrated in his previous paper (13); the other by
Calberla, Der Befruchtungsvorgang beim Ei von Petromyzon
Planeri,
Zeit. für wiss. Zool., Bd. XXX, shews that the superficial layer of the egg is
formed by a coating of protoplasm free from yolk-spheres, which at one part
is continued inwards as a column, and contains the germinal vesicle. The
surface of this column is in contact with a micropyle in the egg-membrane.
Impregnation is effected by the entrance of the head of a single
spermatozoon (the tail remaining outside) through the micropyle, and then
along the column of clear protoplasm to the female pronucleus.
[362] From the Quarterly Journal of Microscopical Science, April, 1878.
[363] The numbers appended to authors' names refer to the list of publications at the end of the paper.
[364] Hertwig's full account of his observations, with figures, in the 4th vol. of the Morphologische Jahrbuch, has appeared since the above was written. The figures given strongly support Hertwig's views.
[365] Flemming (6) finds that, in the summer and probably parthenogenetic eggs of Lacinularia socialis, the germinal vesicle approaches the surface and becomes invisible, and that subsequently a slight indentation in the outline of the egg marks the point of its disappearance. In the hollow of the indentation Flemming believes a polar cell to be situated, though he has not definitely seen one.
[366] The instances quoted by Siebold from Hensen and Oellacher are not quite satisfactory. In Hensen's case impregnation would have been possible if we can suppose the spermatozoa to be capable of passing into the body-cavity through the open end of the uninjured oviduct; and though Oellacher's instances are more valuable, yet sufficient care seems hardly to have been taken, especially when it is not certain for what length of time spermatozoa may be able to live in the oviduct. For Oellacher's precautions, vide Zeit. für wiss. Zool. Bd. XXII. p. 202.
[367] According to Hertwig's most recent statement a nucleolus is present in this nucleus.
[368] The recent researches of Calberla on the impregnation of the ovum of Petromyzon Planeri support this conclusion.
[369] This seems the most probable view with reference to the nature of the head of the spermatozoon, though the point is not perhaps yet definitely decided.
(With Plates 24, 25, 26.)
The present paper records observations on the ovaries of but two types, viz., Mammalia and Elasmobranchii. The main points dealt with are three:—1. The relation of the germinal epithelium to the stroma. 2. The connection between primitive ova in Waldeyer's sense and the permanent ova. 3. The homologies of the egg membranes.
The second of these points seems to call for special attention after Semper's discovery that the primitive ova ought really to be regarded as primitive sexual cells, in that they give rise to the generative elements of both sexes.
The Development of the Elasmobranch Ovary.
The development of the Elasmobranch ovary has recently formed the subject of three investigations. The earliest of them, by H. Ludwig, is contained in his important work, on the 'Formation of the Ovum in the Animal Kingdom[371].' Ludwig arrives at the conclusion that the ovum and the follicular epithelium are both derived from the germinal epithelium, and enters into some detail as to their formation. Schultz[372], without apparently being acquainted with Ludwig's observations, has come to very similar results for Torpedo.
[Pg 550] Semper[373], in his elaborate memoir on the urogenital system of Elasmobranchii, has added very greatly to our knowledge on this subject. In a general way he confirms Ludwig's statements, though he shews that the formation of the ova is somewhat more complicated than Ludwig had imagined. He more especially lays stress on the existence of nests of ova (Ureiernester[TN11]), derived from the division of a single primitive ovum, and of certain peculiarly modified nuclei, which he compares to spindle nuclei in the act of division.
My own results agree with those of previous investigators, in attributing to the germinal epithelium the origin both of the follicular epithelium and ova, but include a number of points which I believe to be new, and, perhaps, of some little interest; they differ, moreover, in many important particulars, both as to the structure and development of the ovary, from the accounts of my predecessors.
The history of the female generative organs may conveniently be treated under two heads, viz. (1) the history of the ovarian ridge itself, and (2) the history of the ova situated in it. I propose dealing in the first place with the ovarian ridge.
The Ovarian ridge in Scyllium.—At the stage spoken of in my monograph on Elasmobranch Fishes as stage L, the ovarian ridge has a very small development, and its maximum height is about 0.1 mm. It exhibits in section a somewhat rounded form, and is slightly constricted along the line of attachment. It presents two surfaces, which are respectively outer and inner, and is formed of a layer of somewhat thickened germinal epithelium separated by a basement membrane from a central core of stroma. The epithelium is far thicker on the outer surface than on the inner, and the primitive ova are entirely confined to the former. The cells of the germinal epithelium are irregularly scattered around the primitive ova, and have not the definite arrangement usually characteristic of epithelial cells. Each of them has a large nucleus, with a deeply staining small nucleolus, and a very scanty protoplasm. In stage N the ovarian ridge has a pointed edge and narrower attachment than in stage L. Its greatest height is about 0.17 mm. There is more stroma, and the basement membrane is more distinct than before; in other respects no changes [Pg 551] worth recording have taken place. By stage P a distinction is observable between the right and left ovarian ridges; the right one has, in fact, grown more rapidly than the left, and the difference in size between the two ridges becomes more and more conspicuous during the succeeding stages, till the left one ceases to grow any larger, though it remains for a great part of life as a small rudiment.
The right ovarian ridge, which will henceforth alone engage our attention, has grown very considerably. Its height is now about 0.4 mm. It has in section (vide Pl. 24, fig. 1) a triangular form with constricted base, and is covered by a flat epithelium, except for an area on the outer surface, in length co-extensive with the ovarian ridge, and with a maximum breadth of about 0.25 mm. This area will be spoken of as the ovarian area or region, since the primitive ova are confined to it. The epithelium covering it has a maximum thickness of about 0.05 mm., and thins off rather rapidly on both borders, to become continuous with the general epithelium of the ovarian ridge. Its cells have the same character as before, and are several layers deep. Scattered irregularly amongst them are the primitive ova. The germinal epithelium in the ovarian region is separated by a basement membrane from the adjacent stroma.
In succeeding stages, till the embryo reaches a length of 7 centimetres, no very important changes take place. The ovarian region grows somewhat in breadth, though in this respect different embryos vary considerably. In two embryos of nearly the same age, the breadth of the ovarian epithelium was 0.3 mm. in the one and 0.35 mm. in the other. In the former of these embryos, the thickness of the epithelium was slightly greater than in the latter, viz. 0.09 mm. as compared with 0.08. In both the epithelium was sharply separated from the subjacent stroma. There were relatively more epithelial cells in proportion to primitive ova than at the earlier date, and the individual cells exhibited great variations in shape, some being oval, some angular, others very elongated, and many of them applied to part of an ovum and accommodating themselves to its shape. In some of the more elongated cells very deeply stained nuclei were present, which (in a favourable light and with high powers) exhibited the spindle modification of Strasburger with great [Pg 552] clearness, and must therefore be regarded as undergoing division. The ovarian region is at this stage bounded on each side by a groove.
In an embryo of seven centimetres (Pl. 24, fig. 2) the breadth of the ovarian epithelium was 0.5, but its height only 0.06 mm. It was still sharply separated from the subjacent stroma, though a membrane could only be demonstrated in certain parts. The amount of stroma in the ovarian ridge varies greatly in different individuals, and no reliance can be placed on its amount as a test of the age of the embryo. In the base of the ovarian ridge the cells were closely packed, elsewhere they were still embryonic.
My next stage (Pl. 24, fig. 3, and fig. 4), shortly before the time of the hatching of the embryo, exhibits in many respects an advance on the previous one. It is the stage during which a follicular covering derived from the germinal epithelium is first distinctly formed round the ova, in a manner which will be more particularly spoken of in the section devoted to the development of the ovum itself. The breadth of the ovarian region is 0.56 mm., and its greatest height close to the central border, 0.12 mm.—a great advance on the previous stage, mainly, however, due to the larger size of the ova.
The ovarian epithelium is still in part separated from the subjacent stroma by a membrane close to its dorsal and ventral borders, but elsewhere the separation is not so distinct, it being occasionally difficult within a cell or so to be sure of the boundary of the epithelium. The want of a clear line between the stroma and the epithelium is rendered more obvious by the fact that the surface of the latter is somewhat irregular, owing to projections formed by specially large ova, into the bays between which are processes of the stroma. In an ovary about this stage, hardened in osmic acid, the epithelium stains very differently from the subjacent stroma, and the line of separation between the two is quite sharp. A figure of the whole ovarian ridge, shewing the relation between the two parts, is represented on Pl. 24, fig. 5.
The layer of stroma in immediate contact with the epithelium is very different from the remainder, and appears to be destined to accompany the vascular growths into the epithelium, which [Pg 553] will appear in the next stage. The protoplasm of the cells composing it forms a loose reticulum with a fair number of oval or rounded nuclei, with their long axis for the most part parallel to the lower surface of the epithelium. It contains, even at this stage, fully developed vascular channels.
The remainder of the stroma of the ovarian ridge has now acquired a definite structure, which remains constant through life, and is eminently characteristic of the genital ridge of both sexes. The bulk of it (Pl. 24, fig. 3, str) consists of closely packed polygonal cells, of about 0.014 mm. with large nuclei of about 0.009. These cells appear to be supported by a delicate reticulum. The whole tissue is highly vascular, with the numerous capillaries; the nuclei in the walls of which stand out in some preparations with great clearness.
In the next oldest ovary, of which I have sections, the breadth of the ovarian epithelium is 0.7 mm. and its thickness 0.096. The ovary of this age was preserved in osmic acid, which is the most favourable reagent, so far as I have seen, for observing the relation of the stroma and epithelium. On Pl. 24, fig. 6, is represented a transverse section through the whole breadth of the ovary, slightly magnified to shew the general relations of the parts, and on Pl. 24, fig. 7, a small portion of a section more highly magnified. The inner surface of the ovarian epithelium is more irregular than in the previous stage, and it may be observed that the subjacent stroma is growing in amongst the ova. From the relation of the two tissues it is fairly clear that the growth which is taking place is a definite growth of the stroma into the epithelium, and not a mutual intergrowth of the two tissues. The ingrowths of the stroma are, moreover, directed towards individual ova, around which, outside the follicular epithelium, they form a special vascular investment in the succeeding stages. They are formed of a reticular tissue with comparatively few nuclei.
By the next stage, in my series of ovaries of Scy. canicula, important changes have taken place in the constitution of ovarian epithelium. Fig. 8, Pl. 24, represents a portion of the ovarian epithelium, on the same scale as figs. 1, 2, 3, &c., and fig. 9 a section through the whole ovarian ridge slightly magnified. Its breadth is now 1.3 mm., and its thickness 0.3 mm. [Pg 554] The ova have grown very greatly, and it appears to me to be mainly owing to their growth that the greater thickness of the epithelium is due, as well as the irregularity of its inner surface (vide fig. 9).
The general relation of the epithelium to the surrounding parts is much the same as in the earlier stage, but two new features have appeared—(1) The outermost cells of the ovarian region have more or less clearly arranged themselves as a kind of epithelial covering for the organ; and (2) the stroma ingrowths of the previous stage have become definitely vascular, and have penetrated through all parts of the epithelium.
The external layer of epithelium is by no means a very marked structure, the character of its cells varies greatly in different regions, and it is very imperfectly separated from the subjacent layer. I shall speak of it for convenience as pseudo-epithelium.
The greater part of the germinal epithelium forms anastomosing columns, separated by very thin tracts of stroma. The columns are, in the majority of instances, continuous with the pseudo-epithelium at the surface, and contain ova in all stages of development. Many of the cells composing them naturally form the follicular epithelium for the separate ova; but the majority have no such relation. They have in many instances assumed an appearance somewhat different from that which they presented in the last stage, mainly owing to the individual nuclei being more widely separated. A careful examination with a high power shews that this is owing to an increase in the amount of protoplasm of the individual cells, and it may be noted that a similar increase in the size of the bodies of the cells has taken place in the pseudo-epithelium and in the follicular epithelium of the individual ova.
The stroma ingrowths form the most important feature of the stage. In most instances they are very thin and delicate, and might easily be overlooked, especially as many of the cells in them are hardly to be distinguished, taken separately, from those of the germinal epithelium. These features render the investigation of the exact relation of the stroma and epithelium a matter of some difficulty. I have, however, been greatly assisted by the investigation of the ovary of a young example [Pg 555] of Scyllium stellare, 16½ centimètres in length, a section of which is represented in Pl. 25, fig. 26. In this ovary, although no other abnormalities were observable, the stroma ingrowths were exceptionally wide; indeed, quite without a parallel in my series of ovaries in this respect. The stroma most clearly divides up the epithelium of the ovary into separate masses, or more probably anastomosing columns, the equivalents of the egg-tubes of Pflüger. These columns are formed of normal cells of the germinal epithelium, which enclose ovarian nests and ova in all stages of development. A comparison of the section I have represented, with those from previous stages, appears to me to demonstrate that the relation of the epithelium and stroma has been caused by an ingrowth or penetration of the stroma into the epithelium, and not by a mutual intergrowth of the two tissues. Although the ovary, of which fig. 26 represents a section was from Scy. stellare, and the previous ovaries have been from Scy. canicula, yet the thickness of the epithelium may still be appealed to in confirmation of this view. In the previous stage the thickness was about 0.096 mm., in the present one it is about 0.16 mm., a difference of thickness which can be easily accounted for by the growth of the individual ova and the additional tracts of stroma. A pseudo-epithelium is more or less clearly formed, but it is continuous with the columns of epithelium. In the stroma many isolated cells are present, which appear to me, from a careful comparison of a series of sections, to belong to the germinal epithelium.
The thickness of the follicular epithelium on the inner side of the larger ova deserves to be noted. Its meaning is discussed on p. 567.
Quite a different interpretation to that which I have given has been put by Ludwig and Semper upon the parts of the ovary at this stage. My pseudo-epithelium is regarded by them as forming, together with the follicular epithelium of the ova, the sole remnant of the original germinal epithelium; and the masses of cells below the pseudo-epithelium, which I have attempted to shew are derived from the original germinal epithelium, are regarded as parts of the ingrowths of the adjacent stroma.
Ludwig has assumed this interpretation without having had an opportunity of working out the development of the parts, but [Pg 556] Semper attempts to bring forward embryological proofs in support of this position.
If the series of ovaries which I have represented be examined, it will
not, I think, be denied that the general appearances are very much in
favour of my view. The thickened patch of ovarian epithelium can apparently
be traced through the whole series of sections, and no indications of its
sudden reduction to the thin pseudo-epithelium are apparent. The most
careful examination that I have been able to make brings to light nothing
tending to shew that the general appearances are delusive. The important
difference between us refers to our views of the nature of the tissue
subjacent to the pseudo-epithelium. If my results be accepted, it is
clear that the whole ovarian region is an epithelium interpenetrated by
connective tissue ingrowths, so that the region below the pseudo-epithelium
is a kind of honeycomb or trabecular net-work of germinal epithelium,
developing ova of all stages and sizes, and composed of cells capable of
forming follicular epithelium for developing ova. Ludwig figures what he
regards as the formation of the follicular epithelium round primitive ova
during their passage into the stroma. It is quite clear to me, that his
figures of the later stages, 33 and 34, represent fully formed permanent
ova surrounded by a follicular epithelium, and that their situation in
contact with the pseudo-epithelium is, so to speak, an accident, and it is
quite possible that his figures 31 and 32 also represent fully formed ova;
but I have little hesitation in asserting that he has not understood the
mode of formation of the follicular epithelium, and that, though his
statement that it is derived from the germinal epithelium is quite correct,
his account of the process is completely misleading. The same criticism
does not exactly apply to Semper's statements. Semper has really observed
the formation of the follicular epithelium round young ova; but,
nevertheless, he appears to me to give an entirely wrong account of the
relation of the stroma to the germinal epithelium. The extent of the
difference between Semper's and my view may perhaps best be shewn by a
quotation from Semper, loc. cit., 465:—In females
the nests of primitive ova sink in groups into the stroma. In these groups
one cell enlarges till it becomes the ovum, the neighbouring cells [Pg 557] increase
and arrange themselves around the ova as follicle cells.
Although the histological changes which take place in the succeeding stages are not inconsiderable, they do not involve any fundamental change in the constitution of the ovarian region, and may be described with greater brevity than has been so far possible.
In a half-grown female, with an ovarian region of 3mm. in breadth, and 0.8mm. in thickness, the stroma of the ovarian region has assumed a far more formed aspect than before. It consists (Pl. 24, fig. 10) of a basis in most parts fibrous, but in some nearly homogeneous, with a fair number of scattered cells. Immediately below the pseudo-epithelium, there is an imperfectly developed fibrous layer, forming a kind of tunic, in which are imbedded the relatively reduced epithelial trabeculæ of the previous stages. They appear in sections as columns, either continuous with or independent of the pseudo-epithelium, formed of normal cells of the germinal epithelium, nests of ova, and permanent ova in various stages of development. Below this there comes a layer of larger ova which are very closely packed. A not inconsiderable number of the larger ova have, however, a superficial situation, and lie in immediate contact with the pseudo-epithelium. Some of the younger ova, enclosed amongst epithelial cells continuous with the pseudo-epithelium, are very similar to those figured by Ludwig. It is scarcely necessary to insist that this fact does not afford any argument in favour of his interpretations. The ovarian region is honeycombed by large vascular channels with distinct walls, and other channels which are perhaps lymphatic.
The surface of the ovarian region is somewhat irregular and especially marked by deep oblique transverse furrows. It is covered by a distinct, though still irregular pseudo-epithelium, which is fairly columnar in the furrows but flattened along the ridges. The cells of the pseudo-epithelium have one peculiarity very unlike that of ordinary epithelial cells. Their inner extremities (vide fig. 10) are prolonged into fibrous processes which enter the subjacent tissue, and bending nearly parallel to the surface of the ovary, assist in forming the tunic spoken of above. This peculiarity of the pseudo-epithelial cells seems [Pg 558] to indicate that they do not essentially differ from cells which have the character of undoubted connective tissue cells, and renders it possible that the greater part of the tunic, which has apparently the structure of ordinary connective tissue, is in reality derived from the original germinal epithelium, a view which tallies with the fact that in some instances the cells of the tunic appear as if about to assist in forming the follicular epithelium of some of the developing ova. In Raja, the similarity of the pseudo-epithelium to the subjacent tissue is very much more marked than in Scyllium. The pseudo-epithelium appears merely as the superficial layer of the ovarian tunic somewhat modified by its position on the surface. It is formed of columnar cells with vertically arranged fibres which pass into the subjacent layers, and chiefly differ from the ordinary fibres in that they still form parts of the cell-protoplasm enclosing the nucleus. In Pl. 25, fig. 34, an attempt is made to represent the relations of the pseudo-epithelium to the subjacent tissue in Raja. Ludwig's figures of the pseudo-epithelium of the ovary, in the regular form of its constituent cells, and its sharp separation by a basement membrane from the tissue below, are quite unlike anything which I have met with in my sections either of Raja or Scyllium.
Close to the dorsal border of the ovary the epithelial cells of the non-ovarian region have very conspicuous tails, extending into a more or less homogeneous substance below, which constitutes a peculiar form of tunic for this part of the ovarian ridge.
In the full-grown female the stroma of the ovarian region is denser and has a more fibrous aspect than in the younger animal. Below the pseudo-epithelium it is arranged in two or three more or less definite layers, in which the fibres run at right angles. It forms a definite ovarian tunic. The pseudo-epithelium is much more distinct, and the tails of its cells, so conspicuous in previous stages, can no longer be made out.
Formation of the permanent ova and the follicular epithelium.—In my monograph on the development of Elasmobranch Fishes an account was given of the earliest stages in the development of the primitive ova, and I now take up their development from [Pg 559] the point at which it was left off in that work. From their first formation till the stage spoken of in my monograph as P, their size remains fairly constant. The larger examples have a diameter of about 0.035 mm., and the medium-sized examples of about 0.03 mm. The larger nuclei have a diameter of about 0.16 mm., but their variations in size are considerable. If the above figures be compared with those on page 350 of my monograph on Elasmobranch Fishes, it will be seen that the size of the primitive ova during these stages is not greater than it was at the period of their very first appearance.
The ova (Pl. 24, fig. 1) are usually aggregated in masses, which might have resulted from division of a single ovum. The outlines of the individual ova are always distinct. Their protoplasm is clear, and their nuclei, which are somewhat passive towards staining reagents, are granular, with one to three nucleoli. I have noticed, up to stage P, the occasional presence of highly refractive spherules in the protoplasm of the primitive ova already described in my monograph (pp. 353, 354, Pl. 12, fig. 15). They seem to occur up to a later period than I at first imagined. Their want of constancy probably indicates that they have no special importance. Professor Semper has described similar appearances in the male primitive ova of a later period.
As to the distribution of the primitive ova in the germinal epithelium, Professor Semper's statement that the larger primitive ova are found in masses in the centre, and that the smaller ova are more peripherally situated is on the whole true, though I do not find this distribution sufficiently constant to lay so much stress on it as he does.
The passive condition of the primitive ova becomes suddenly broken during stage Q, and is succeeded by a period of remarkable changes. It has only been by the expenditure of much care and trouble that I have been able to elucidate to my own satisfaction what takes place, and there are still points which I do not understand.
Very shortly after stage Q, in addition to primitive ova with a perfectly normal nucleus, others may be seen in which the nucleus is apparently replaced by a deeply stained irregular body, smaller than the ordinary nuclei (Pl. 24, fig. 11, d.n.). [Pg 560] This body, by the use of high objectives, is seen to be composed of a number of deeply stained granules, and around it may be noticed a clear space, bounded by a very delicate membrane. The granular body usually lies close to one side of this membrane, and occasionally sends a few fine processes to the opposite side.
The whole body, i.e. all within the delicate membrane is, according to my view, a modified nucleus; as appears to me very clearly to be shewn by the fact that it occupies the normal position of a nucleus within a cell body. Semper, on the other hand, regards the contained granular body as the nucleus, which he compares with the spindles of Bütschli, Auerbach, &c.[374]. This interpretation appears to me, however, to be negatived by the position of these bodies. The manner in which Semper may, perhaps, have been led to his views will be obvious when the later changes of the primitive ova are described. The formation of these nuclei would seem to be due to a segregation of the constituents of the original nuclei; the solid parts becoming separated from the more fluid. As a rule, the modified nuclei are slightly larger than the original ones. In stage Q the following two tables shew the dimensions of the parts of three unmodified and of three modified nuclei taken at random.
Primitive ova with unmodified nuclei—
Nuclei.
0.014 mm.
0.012 mm.
0.01 mm.
Primitive ova with modified nuclei—
| Nuclei. | Granular Bodies in nuclei. |
|---|---|
| 0.018 mm. | 0.006 mm. |
| 0.018 mm. | 0.006 mm. |
| 0.012 mm. | 0.009 mm. |
For a slightly older stage than Q, the two annexed tables also shew the comparative size of the modified and unmodified nuclei:
[Pg 561]Unmodified nuclei of normal primitive ova—
0.014 mm.
0.016 mm.
0.014 mm.
0.016 mm.
0.016 mm.
Nuclei of primitive ova with modified nuclei—
| Nuclei. | Granular Bodies in nuclei. |
|---|---|
| 0.018 mm. | 0.008 mm. |
| 0.016 mm. | 0.008 mm. |
| 0.016 mm. | 0.010 mm. |
| 0.016 mm. | |
| 0.018 mm. |
These figures bring out with clearness the following points: (1) that the modified nuclei are slightly but decidedly larger on the average than the unmodified nuclei; (2) that the contained granular bodies are very considerably smaller than ordinary nuclei.
Soon after the appearance of the modified nuclei, remarkable changes take place in the cells containing them. Up to the time such nuclei first make their appearance the outlines of the individual ova are very clearly defined, but subsequently, although numerous ova with but slightly modified nuclei are still to be seen, yet on the whole the outlines of all the primitive ova are much less distinct than before; and this is especially the case with the primitive ova containing modified nuclei.
From cases in which three or four ova are found in a mass with modified nuclei, but in which the outline of each ovum is fairly distinct, it is possible to pass by insensible gradations to other cases in which two or three or more modified nuclei are found embedded in a mass of protoplasm in which no division into separate cells can be made out (fig. 14). For these masses I propose to employ the term nests. They correspond in part with the Ureiernester of Professor Semper.
Frequently they are found in hardened specimens to be enclosed in a membrane-like tunic which appears to be of the nature of coagulated fluid. These membranes closely resemble and sometimes are even continuous with trabeculæ which traverse the germinal epithelium. Ovaries differ considerably as [Pg 562] to the time and completeness of the disappearance of the outlines marking the separate cells, and although, so far as can be gathered from my specimens, the rule is that the outlines of the primitive ova with modified nuclei soon become indistinct, yet in one of my best preserved ovaries very large nests with modified nuclei are present in which the outline of each ovum is as distinct as during the period before the nuclei undergo these peculiar changes (Pl. 24, fig. 12). In the same ovary other nests are present in which the outlines of the individual ova are no longer visible. The section represented on Pl. 24, fig. 2, is fairly average as to the disappearance of the outlines of the individual ova.
It is clear from the above statements, that in the first instance the nests are produced by the coalescence of several primitive ova into a single mass or syncytium; though of course, the several separate ova of a nest may originally, as Semper believes, have arisen from the division of a single ovum. In any case there can be no doubt that the nests of separate ova increase in size as development proceeds; a phenomenon which is more reasonably explained on the view that the ova divide, than on the view that they continue to be freshly formed. The same holds true for the nests of nuclei and this, as well as other facts, appears to me to render it probable that the nests grow by division of the nuclei without corresponding division of the protoplasmic matrix. I cannot, however, definitely prove this point owing to my having found nests, with distinct outlines to the ova, as large as any without such outlines.
The nests are situated for the most part near the surface of the germinal epithelium. The smaller ones are frequently spherical, but the larger are irregular in form. The former are about 0.05 mm. in diameter; the latter reach 0.1 mm. Scattered generally, and especially in the deeper layers, and at the edges of the germinal epithelium, are still unmodified or only slightly modified primitive ova. These unmodified primitive ova are aggregated in masses, but in these masses the outlines of each ovum, though perhaps less clear than in the earlier period, are still distinct.
When the embryo reaches a length of seven centimètres, and even in still younger embryos, further changes are observable. [Pg 563] In the first place many of the modified nuclei acquire fresh characters, and it becomes necessary to divide the modified nuclei into two categories. In both of these the outer boundary of the nucleus is formed by a very delicate membrane, the space within which is perfectly clear except for the granular body. In the variety which now appears in considerable numbers the granular body has an irregular star-like form. The rays of the star are formed of fibres frequently knobbed at their extremities, and the centre of the star usually occupies an eccentric position. Typical examples of this form of modified nucleus, which may be spoken of as the stellate variety, are represented on Pl. 25, fig. 17; between it and the older granular variety there is an infinite series of gradations, many of which are represented on Pl. 24, figs. 12, 14, 15, 16. Certain of the stellate nuclei exhibit two centres instead of one, and in some cases, like that represented on Pl. 25, fig. 19, the stellate body of two nuclei is found united. Both of these forms are possibly modifications of the spindle-like form assumed by nuclei in the act of dividing, and may be used in proving that the nests increase in size by the division of the contained nuclei. In addition to the normal primitive ova, a few of which are still present, there are to be found, chiefly in the deeper layers of the germinal epithelium, larger ova differing considerably from the primitive ova. They form the permanent ova (Pl. 24, fig. 3, o). Their average diameter is 0.04 mm., compared with 0.03 mm., the diameter of original primitive ova. The protoplasm of which they are composed is granular, but at first a membrane can hardly be distinguished around them; their nucleus is relatively large, 0.02 - 0.027 mm. in diameter. It presents the characters ascribed by Eimer[375], and many other recent authors[376], to typical nuclei (vide Pl. 24, fig. 3, and Pl. 24, 25, figs. 13, 14, 15, 16, 17, 18). It is bounded by a distinct membrane, within which is a more or less central nucleolus from which a number of radial fibres which stain very deeply pass to the surface; here they form immediately internal to the membrane a network with granules at the nodal points. In some instances the regularity of the arrangement of these fibres is very great, in other instances [Pg 564] two central nucleoli are present, in which case the regularity is considerably interfered with. The points in which the youngest permanent ova differ from the primitive may be summed up as follows:—
(1) The permanent ova are larger, the smallest of them being larger than the average primitive ova in the proportion of four to three. (2) They have less protoplasm as compared to the size of the nucleus. (3) Their protoplasm is granular instead of being clear. (4) Their nucleus is clear with exception of a network of fibres instead of being granular as in the primitive ova. It thus appears that the primitive ova and permanent ova are very different in constitution, though genetically related in a way to be directly narrated.
The formation of permanent ova is at its height in embryos of about seven centimètres or slightly larger. The nests at this stage are for the most part of a very considerable size and contain a large number of nuclei, which have probably, as before insisted, originated from a division of the smaller number of nuclei present in the nests at an earlier stage. Figs. 14-18 are representations of nests at this period. The diameter of the nuclei is, on the whole, slightly greater than at an earlier stage. A series of measurements gave the following results:—
0.016 mm.
0.016 mm.
0.018 mm.
0.020 mm.
0.020 mm.
Both varieties of modified nuclei are common enough, though the stellate variety predominates. The nuclei are sometimes in very close contact, and sometimes separated by protoplasm, which in many instances is very slightly granular. In a large number of the nests nothing further is apparent than what has just been described, but in a very considerable number one or more nuclei are present, which exhibit a transitional character between the ordinary stellate nuclei of my second category, and the nuclei of permanent ova as above described; and in these nests the formation of permanent ova is taking place. Permanent ova in the act of development are indicated in my figures by the letters do. Many of the intermediate nuclei are more [Pg 565] definitely surrounded by granular protoplasm than the other nuclei of the nests, and accordingly have their outlines more sharply defined. Between nuclei of this kind, and others as large as those of the permanent ova, there are numerous transitional forms. The larger ones frequently lie in a mass of granular protoplasm projecting from the nest, and only united with it by a neck (Pl. 24, figs. 14 and 16). For prominences of this kind to become independent ova, it is only necessary for the neck to become broken through. Nests in which such changes are taking place present various characters. In some cases several nuclei belonging to a nest appear to be undergoing conversion into permanent ova at the same time. Such a case is figured on Pl. 25, figs. 17 and 18. In these cases the amount of granular protoplasm in the nest and around each freshly formed ovum is small. In the more usual cases only one or two permanent ova at the utmost are formed at the same time, and in these instances a considerable amount of granular protoplasm is present around the nucleus of the developing permanent ovum. In such instances it frequently happens several of the nuclei not undergoing conversion appear to be in the process of absorption, and give to the part of the nest in which they are contained a very hazy and indistinct aspect (Pl. 24, fig. 15). Their appearance leads me to adopt the view that while some of the nuclei of each nest are converted into the nuclei of the permanent ova, others break down and are used as the pabulum, at the expense of which the protoplasm of the young ovum grows.
It should, however, be stated, that after the outlines of the permanent ova have become definitely established, I have only observed in a single instance the inclusion of a nucleus within an ovum (Pl. 25, fig. 24). In many instances normal nuclei of the germinal epithelium may be so observed within the ovum.
The nuclei which are becoming converted into the nuclei of permanent ova gradually increase in size. The following table gives the diameter of four such nuclei:—
0.022 mm.
0.022 mm.
0.024 mm.
0.032 mm.
[Pg 566] These figures should be compared with those of the table on page 564.
The ova when first formed are situated either at the surface or in the deeper layers of the germinal epithelium. Though to a great extent surrounded by the ordinary cells of the germinal epithelium, they are not at first enclosed in a definite follicular epithelium. The follicle is, however, very early formed.
My observations lead me then to the conclusion that in a general way the permanent ova are formed by the increase of protoplasm round some of the nuclei of a nest, and the subsequent separation of the nuclei with their protoplasm from the nest as distinct cells—a mode of formation exactly comparable with that which so often takes place in invertebrate egg tubes.
Besides the mode of formation of permanent ova just described, a second one also seems probably to occur. In ovaries just younger than those in which permanent ova are distinctly formed, there are present primitive ova, with modified nuclei of the stellate variety, or nuclei sometimes even approaching in character those of permanent ova, which are quite isolated and not enclosed in a definite nest. The body of these ova is formed of granular protoplasm, but their outlines are very indistinct. Such ova are considerably larger than the normal primitive ova. They may measure 0.04 mm. In a slightly later stage, when fully formed permanent ova are present, isolated ones are not infrequent, and it seems natural to conclude that these isolated ova are the direct descendants of the primitive ova of the earlier stage. It seems a fair deduction that in some cases primitive ova undergo a direct metamorphosis into permanent ova by a modification of their nucleus, and the assumption of a granular character in their protoplasm, without ever forming the constituent part of a nest.
It is not quite clear to me that in all nests the coalescence of the protoplasm of the ova necessarily takes place, since some nests are to be found at all stages in which the ova are distinct. Nevertheless, I am inclined to believe that the fusion of the ova is the normal occurrence.
The mode of formation of the permanent ova may then, according to my observations, take place in two ways:—1. By the formation of granular protoplasm round the nucleus in a [Pg 567] nest, and the separation of the nucleus with its protoplasm as a distinct ovum. 2. By the direct metamorphosis of an isolated primitive ovum into a permanent ovum. The difference between these two modes of formation does not, from a morphological point of view, appear to be of great importance.
The above results appear clearly to shew that the primitive ova in the female are not to be regarded as true ova, but as the parent sexual cells which give rise to the ova: a conclusion which completely fits in with the fact that cells exactly similar to the primitive ova in the female give rise to the spermatic cells in the male.
Slightly after the period of their first formation the permanent ova become invested by a very distinct and well-marked, somewhat flattened, follicular epithelium (Pl. 24, fig. 3). Where the ova lie in the deeper layers of the germinal epithelium, the follicular epithelium soon becomes far more columnar on the side turned inwards, than on that towards the surface, especially when the inner side is in contact with the stroma (Pl. 24, fig. 7, and Pl. 25, figs. 24 and 26). This is probably a special provision for the growth and nutrition of the ovum.
There cannot be the smallest doubt that the follicular epithelium is derived from the general cells of the germinal epithelium—a point on which my results fully bear out the conclusions of Ludwig and Semper.
The larger ova themselves have a diameter of about 0.06 mm., and their nucleus of about 0.04 mm. The vitellus is granular, and provided with a distinct, though delicate membrane, which has every appearance of being a product of the ovum itself rather than of the follicular epithelium. The membrane would seem indeed to be formed in some instances even before the ovum has a definite investment of follicle cells. The vitellus is frequently vacuolated, but occasionally the vacuoles appear to be caused by a shrinking due to the hardening reagent. The nucleus has the same peculiar reticulate character as at first. Its large size, as compared with the ovum, is very noticeable.
With this stage the embryonic development of the ova comes to a close, though the formation of fresh ova continues till comparatively late in life. I have, however, two series of sections of ovaries preserved in osmic acid, from slightly larger embryos [Pg 568] than the one last described, about which it may be well to say a few words before proceeding to the further development of the permanent ova.
The younger of these ovaries was from a Scyllium embryo 10 centimètres long, preserved in osmic acid.
A considerable number of nests were present (Pl. 24, fig. 13), exhibiting, on the whole, similar characters to those just described.
A series of measurements of the nuclei in them were made, leading to the following results:—
0.014 mm.
0.014 mm.
0.016 mm.
0.016 mm.
0.018 mm.
0.018 mm.
Thus, if anything, the nuclei were slightly smaller than in the younger embryo. It is very difficult in the osmic specimens to make out clearly the exact outlines of the various structures, the nuclei in many instances being hardly more deeply stained than in the protoplasm around them. The network in the nuclei is also far less obvious than after treatment with picric acid. The permanent ova were hardly so numerous as in the younger ovary before described. A number of these were measured with the following results:—
| Ovum. | Nucleus. |
|---|---|
| 0.03 mm. | 0.014 mm. |
| 0.034 mm. | 0.018 mm. |
| 0.028 mm. | 0.016 mm. |
| 0.03 mm. | 0.02 mm. |
| 0.04 mm. | 0.02 mm. |
| 0.04 mm. | 0.02 mm. |
| 0.048 mm. | 0.02 mm. |
These figures shew that the nuclei of the permanent ova are smaller than in the younger embryo, and it may therefore be safely concluded that, in spite of the greater size of the embryo from which it is taken, the ovary now being described is in a more embryonic condition than the one last dealt with.
Though the permanent ova appeared to be formed from the nests in the manner already described, it was fairly clear from [Pg 569] the sections of this ovary that many of the original primitive ova, after a metamorphosis of the nucleus and without coalescing with other primitive ova to form nests, become converted directly into the permanent ova. Many large masses of primitive ova, or at least of ova with the individual outlines of each ovum distinct, were present. The average size of ova composing these was however small, the body measuring about 0.016 mm., and the nucleus 0.012 mm. Isolated ova with metamorphosed nuclei could also be found measuring 0.022, and their nuclei about 0.014 mm.
The second of the two ovaries, hardened in osmic acid, was somewhat more advanced than the ovary in which the formation of permanent ova was at its height. Fewer permanent ova were in the act of being formed, and many of these present had reached a considerable size, measuring as much as 0.07 mm. Nests of the typical forms were present as before, but the nuclei in them were more granular than at the earlier period, and on the average slightly smaller. A series measured had the following diameters:—
0.010 mm.
0.012 mm.
0.014 mm.
0.016 mm.
One of these nests is represented on Pl. 25, fig. 20. Many nests with the outlines of the individual ova distinct were also present.
On the whole it appeared to me, that the second mode of formation of permanent ova, viz. that in which the nest does not come into the cycle of development, preponderated to a greater extent than in the earlier embryonic period.
Post-embryonic Development of the Ova.—My investigations upon the post-embryonic growth and development of the ova, have for the most part been conducted upon preserved ova, and it has been impossible for me, on this account, to work out, as completely as I should have wished, certain points, more especially those connected with the development of the yolk.
Although my ovaries have been carefully preserved in a large number of reagents, including osmic acid, picric acid, chromic acid, spirit, bichromate of potash, and Müller's fluid, none of these have proved universally successful, and bichromate of potash [Pg 570] and Müller's fluid are useless. Great difficulties have been experienced in distinguishing the artificial products of these reagents. My investigations have led me to the result, that in the gradual growth of the ova with the age of the individual the changes are not quite identical with those during the rapid growth which takes place at periods of sexual activity, after the adult condition has been reached—a result to which His has also arrived, with reference to the ova of Osseous Fish. I propose dealing separately with the several constituents of the egg-follicle.
Egg membranes.—A vitelline membrane has been described by Leydig[377] in Raja, and an albuminous layer of the nature of a chorion[378] by Gegenbaur[379] in Acanthias—the membranes described in these two ways being no doubt equivalent.
Dr Alex. Schultz[380] has more recently investigated a considerable variety of genera and finds three conditions of the egg membranes. (1) In Torpedo, a homogeneous membrane, which is of the nature of a chorion. (2) In Raja, a homogeneous membrane which is, however, perforated. (3) In Squalidæ, a thick homogeneous membrane, internal to which is a thinner perforated membrane. He apparently regards the perforated inner membrane as a specialised part of the simple membrane found in Torpedo, and states that this membrane is of the nature of a chorion.
My own investigations have led me to the conclusion that though the egg-membranes can probably be reduced to single type for Elasmobranchii, yet that they vary with the stage of development of the ovum. Scyllium (stellare and canicula) and Raja have formed the objects of my investigation. I commence with the two former.
It has already been stated that in Scyllium, even before the follicular epithelium becomes formed, a delicate membrane round [Pg 571] the ovum can be demonstrated, which appears to me to be derived from the vitellus or body of the ovum, and is therefore of the nature of a vitelline membrane. It becomes the vitelline membrane of Leydig, the albuminous membrane of Gegenbaur, and homogeneous membrane of Schultz.
In a young fish (not long hatched) with ova of not more than 0.12 mm., this membrane, though considerably thicker than in the embryo, is not thick enough to be accurately measured. In ova of 0.5 mm. from a young female (Pl. 25, fig. 21) the vitelline membrane has a thickness of 0.002 mm. and is quite homogeneous[381]. Internally to it may be observed very faint indications of the differentiation of the outermost layer of the vitellus into the perforated or radially striated membrane of Schultz, which will be spoken of as zona radiata.
In an ovum of 1 mm. from the nearly full grown though not sexually mature female, the zona radiata has increased in thickness and definiteness, and may measure as much as 0.004 mm. It is always very sharply separated from the vitelline membrane, but appears to be more or less continuous on its inner border with the body of the ovum, at the expense of which it no doubt grows in thickness.
In ova above 1 mm. in diameter, both vitelline membrane and zona radiata, but especially the latter, increase in thickness. The zona becomes marked off from the yolk, and its radial striæ become easy to see even with comparatively low powers. In many specimens it appears to be formed of a number of small columns, as described by Gegenbaur and others. The stage of about the greatest development of both the vitelline membrane and zona radiata is represented on Pl. 25, fig. 22.
At this time the vitelline membrane appears frequently to exhibit a distinct stratification, dividing it into two or more successive layers. It is not, however, acted on in the same manner by all reagents, and with absolute alcohol appears at times longitudinally striated.
From this stage onwards, both vitelline membrane and zona gradually atrophy, simultaneously with a series of remarkable [Pg 572] changes which take place in the follicular epithelium. The zona is the first to disappear, and the vitelline membrane next becomes gradually thinner. Finally, when the egg is nearly ripe, the follicular epithelium is separated from the yolk by an immeasurably thin membrane—the remnant of the vitelline membrane—only visible in the most favourable sections (Pl. 25, fig. 23, vt.). When the egg becomes detached from the ovary even this membrane is no longer to be seen.
Both the vitelline membrane and the zona radiata are found in Raja, but in a much less developed condition than in Scyllium. The vitelline membrane is for a long time the only membrane present, but is never very thick (Pl. 25, fig. 31). The zona is not formed till a relatively much later period than in Scyllium, and is always delicate and difficult to see (Pl. 25, fig. 32). Both membranes atrophy before the egg is quite ripe; and an apparently fluid layer between the follicular epithelium and the vitellus, which coagulates in hardened specimens, is probably the last remnant of the vitelline membrane. It is, however, much thicker than the corresponding remnant in Scyllium.
Though I find the same membranes in Scyllium as Alexander Schultz did in other Squalidæ, my results do not agree with his as to Raja. Torpedo I have not investigated.
It appears to me probable that the ova in all Elasmobranch Fishes have at some period of their development the two membranes described at length for Scyllium. Of these the inner one, or zona radiata, will probably be admitted on all hands to be a product of the peripheral protoplasm of the egg.
The outer one corresponds with the membrane usually regarded in other Vertebrates as a chorion or product of the follicular epithelium, but, by tracing it back to its first origin, I have been led to reject this view of its nature.
The follicular epithelium.—The follicular epithelium in the eggs of Raja and Acanthias has been described by Gegenbaur[382]. He finds it flat in young eggs, but in the larger eggs of Acanthias more columnar, and with the cells wedged in so as to form a double layer. These observations are confirmed by Ludwig[383].
Alexander Schultz[384] states that in Torpedo, the eggs are at first enclosed in a simple epithelium, but that in follicles of [Pg 573] .008 mm. there appear between the original large cells of the follicle (which he describes as granulosa cells and derives from the germinal epithelium) a number of peculiar small cells. He states that these are of the same nature as the general stroma cells of the ovary, and believes that they originate in the stroma. When the eggs have reached 0.1 - 0.15 mm., he finds that the small and large cells have a very regular alternating arrangement.
Semper records but few observations on the follicular epithelium, but describes in Raja the presence of a certain number of large cells amongst smaller cells. He believes that they may develop into ova, and considers them identical with the larger cells described by Schultz, whose interpretations he does not, however, accept.
My own results accord to a great extent with those of Dr Schultz, as far as the structure of the follicular epithelium is concerned, but I am at one with Semper in rejecting Schultz's interpretations.
In Scyllium, as has already been mentioned, the follicular epithelium is at first flat and formed of a single layer of uniform cells, each with a considerable amount of clear protoplasm and a granular nucleus. It is bounded externally by a delicate membrane—the membrana propria folliculi of Waldeyer—and internally by the vitelline membrane. In the ovaries of very young animals the cells of the follicular epithelium are more columnar on the side towards the stroma than on the opposite side, but this irregularity soon ceases to exist.
In many cases the nuclei of the cells of the follicular epithelium exhibit a spindle modification, which shews that the growth of the follicular epithelium takes place by the division of its cells. No changes of importance are observable in the follicular epithelium till the egg has reached a diameter of more than 1 mm.
It should here be stated that I have some doubts respecting the completeness of the history of the epithelium recorded in the sequel. Difficulties have been met with in completely elucidating the chronological order of the occurrences, and it is possible that some points have escaped my observation.
The first important change is the assumption of a palisade-like character by the follicle cells, each cell becoming very narrow [Pg 574] and columnar and the nucleus oval (Pl. 25, fig. 28). In this condition the thickness of the epithelium is about 0.025 mm. The epithelium does not, however, become uniformly thick over the whole ovum, but in the neighbourhood of the germinal vesicle it is very flat and formed of granular cells with indistinct outlines, rather like the hypodermis cells of many Annelida. Coincidently with this change in the follicular epithelium the commencement of the atrophy of the membranes of the ovum, described in the last section, becomes apparent.
The original membrana propria folliculi is still present round the follicular epithelium, but is closely associated with a fibrous layer with elongated nuclei. Outside this there is now a layer of cells, very much like an ordinary epithelial layer, which may possibly be formed of cells of the true germinal epithelium (fig. 28, fe´). This layer, which will be spoken of as the secondary follicle layer, might easily be mistaken for the follicular epithelium, and it is possible that it has actually been so mistaken by Eimer, Clark, and Klebs, in Reptilia, and that the true follicular epithelium (in a flattened condition) has been then spoken of as the Binnenepithel.
In slightly older eggs the epithelial cells are no longer uniform or arranged as a single layer. The general arrangement of these cells is shewn in Pl. 25, fig. 29. A considerable number of them are more or less flask-shaped, with bulky protoplasm prolonged into a thin stem directed towards the vitelline membrane, with which, in many instances if not all, it comes in contact. These larger cells are arranged in several tiers. Intercalated between them are a number of elongated small cells with scanty protoplasm and a deeply staining nucleus, not very dissimilar to, though somewhat smaller than, the columnar cells of the previous stage. There is present a complete series of cells intermediate between the larger cells and those with a deeply stained nucleus, and were it not for the condition of the epithelium in Raja, to be spoken of directly, I should not sharply divide the cells into two categories. In surface views of the epithelium the division into two kinds of cells would not be suspected. There can, it appears to me, be no question that both varieties of cell are derived from the primitive uniform follicle cells.
[Pg 575] The fibrous layer bounding the membrana propria folliculi is thicker than in the last stage, and the epithelial-like layer (fe´) which bounds it externally is more conspicuous than before. Immediately adjoining it are vascular and lymph sinuses. The thickness of the follicular epithelium at this stage may reach as much as 0.04 mm., though I have found it sometimes considerably flatter. The cells composing it are, however, so delicate that it is not easy to feel certain that the peculiarities of any individual ovum are not due to handling. The absence of the peculiar columnar epithelium on the part of the surface adjoining the germinal vesicle is as marked a feature as in the earlier stage. When the egg is nearly ripe, and the vitelline membrane has been reduced to a mere remnant, the follicular epithelium is still very columnar (Pl. 25, fig. 23). The thickness is greater than in the last stage, being now about 0.045 mm., but the cells appear only to form a single definite layer. From the character of their nuclei, I feel inclined to regard them as belonging to the category of the smaller cells of the previous stage, and feel confirmed in this view by finding certain bodies in the epithelium, which have the appearance of degenerating cells with granular nuclei, which I take to be the flask-shaped cells which were present in the earlier stage.
I have not investigated the character of the follicular epithelium in the perfectly ripe ovum ready to become detached from the ovary. Nor can I state for the last-described stage anything about the character of the follicular epithelium in the neighbourhood of the germinal vesicle.
As to the relation of the follicular epithelium to the vitelline membrane, and the possible processes of its cells continued into the yolk, I can say very little. I find in specimens teased out after treatment with osmic acid, that the cells of the follicular epithelium are occasionally provided with short processes, which might possibly have perforated the vitelline membrane, but have met with nothing so clear as the teased out specimens figured by Eimer. Nothing resembling the cells within the vitelline membrane, as described by His[385] in Osseous Fish, and Lindgren in Mammalia, has been met with[386].
[Pg 576] My observations in Raja are not so full as those upon Scyllium, but they serve to complete and reconcile the observations of Semper and Schultz, and also to shew that the general mode of growth of the follicular epithelium is fundamentally the same in my representatives of the two divisions of the Elasmobranchii. In very young eggs, in conformity with the results of all previous observers, I find the follicular epithelium approximately uniform. The cells are flat, but extended so as to appear of an unexpected size in views of the surface of the follicle. This condition does not, however, last very long. A certain number of the cells enlarge considerably, others remaining smaller and flat. The differences between the larger and the smaller cells are more conspicuous in sections than in surface views, and though the distribution of the cells is somewhat irregular, it may still be predicted as an almost invariable rule that the smaller cells of the follicle will line that part of the surface of the ovum, near to which the germinal vesicle is situated. On Pl. 25, fig. 30, is shewn in section a fairly average arrangement of the follicle cells. Semper considers the larger cells of such a follicle to be probably primitive ova destined to become permanent ova. This view I cannot accept: firstly, because these cells only agree with primitive ova in being exceptionally large—the character of their nucleus, with its large nucleolus, being not very like that of a primitive ovum. Secondly, because they shade into ordinary cells of the follicle; and thirdly, because no evidence of their becoming ova has come before me, but rather the reverse, in that it seems probable that they have a definite function connected with the nutrition of the egg. To this point I shall return.
In the next stage the small cells have become still smaller. They are columnar, and are wedged in between the larger ones. No great regularity in distribution is as yet attained (Pl. 25, fig. 31). Such a regularity appears in a later stage (Pl. 25, fig. 32), which clearly corresponds with fig. 8 on Pl. 34 of Schultz's paper, and also with the stage of Scyllium in Pl. 25, fig. 29, though the distinction between the two kinds of cells is here far better marked than in Scyllium. The big cells have now become flask-shaped like those in Scyllium, and send a process down to the vitelline membrane. The smaller cells are arranged [Pg 577] in two or three tiers, but the larger cells in a single layer. The distribution of the larger and smaller cells is in some instances very regular, as shewn in the surface view on Pl. 25, fig. 33. There can, it appears to me, be no doubt that Schultz's view of the smaller cells being lymph-cells which have migrated into the follicle cannot be maintained.
The thickness of the epithelium at this stage is about 0.04 mm. In the succeeding stages, during which the egg is rapidly growing to the colossal size which it eventually attains, the follicular epithelium does not to any great extent alter in constitution. It grows thicker on the whole, and as the vitelline membrane gradually atrophies, its lower surface becomes irregular, exhibiting somewhat flattened prominences, which project into the yolk. At the greatest height of the prominences the epithelium may reach a thickness of 0.06 mm., or even more. The arrangement of the tissues external to the follicular epithelium is the same in Raja as in Scyllium.
The most interesting point connected with the follicle, both in Scyllium and Raja and presumably in other Elasmobranchii is that its epithelium at the time when the egg is rapidly approaching maturity is composed with more or less of distinctness of two forms of cells. One of these is large flask-shaped and rich in protoplasm, the other is small, consisting of a mere film of protoplasm round a nucleus. Considering that the larger cells appear at the time of rapid growth, it is natural to interpret their presence as connected with the nutrition of the ovum. This view is supported by the observations of Eimer and Braun, on the development of Reptilian ova. In many Reptilian ova it appears from Eimer's[387] observations, that the follicular epithelium becomes several layers thick, and that a differentiation of the cells, similar to that in Elasmobranchii, takes place. The flask-shaped cells eventually undergo peculiar changes, becoming converted into a kind of beaker-cell, with prolongations through the egg membranes, which take the place of canals leading to the interior of the egg. Braun also expresses himself strongly in favour of the flask-shaped cells functioning in the nutrition of the egg[388]. That these cells in the Reptilian ova really correspond [Pg 578] with those in Elasmobranchii appears to me clear from Eimer's figures, but I have not myself studied any Reptilian ovum. My reasons for dissenting from both Semper's and Schultz's views on the nature of the two forms of follicular cells have already been stated.
The Vitellus and the development of the yolk spherules.—Leydig, Gegenbaur, and Schultz, have recorded important observations on this head. Leydig[389] chiefly describes the peculiar characters of the yolk spherules.
Gegenbaur[390] finds in the youngest eggs fine granules, which subsequently develop into vesicles, in the interior of which the solid oval spheres, so characteristic of Elasmobranchii, are developed.
Schultz describes in the youngest ova of Torpedo the minute yolk spherules arranged in a semilunar form around the eccentric germinal vesicle. In older ova they spread through the whole. He also gives a description of their arrangement in the ripe ovum. Dr Schultz further finds in the body of the ovum peculiar protoplastic striæ, arranged as a series of pyramids, with the bases directed outwards. In the periphery of the ovum a protoplastic network is also present, which is continuous with the above-mentioned pyramidal structures.
My observations do not very greatly extend those of Gegenbaur and Schultz with reference to the development of the yolk, and closely agree with what Gegenbaur has given in the paper above quoted more fully for Aves and Reptilia than for Elasmobranchii.
In very young ova the body of the ovum is simply granular, but when it has reached about 0.5 mm. the granules are seen to be arranged in a kind of network, or sponge-work (Pl. 25, fig. 21), already spoken of in my monograph on Elasmobranch Fishes.
This network becomes more distinct in succeeding stages, especially in chromic acid specimens (Pl. 25, fig. 22), probably in part owing to a granular precipitation of the protoplasm. In [Pg 579] the late stages, when the yolk spherules are fully developed, it is difficult to observe this network, but, as has been shewn in my monograph above quoted, it is still present after the commencement of embryonic development. An arrangement of the protoplasmic striæ like that described by Schultz has not come under my notice.
The development of the yolk appears to me to present special difficulties, owing to the fact pointed out by His[391] that the conditions of development vary greatly according to whether the ovary is in a state of repose or of active development. I do not feel satisfied with my results on this subject, but believe there is still much to be made out. Observations on the yolk spherules may be made either in living ova, in ova hardened in osmic acid, or in ova hardened in picric or chromic acids. The two latter reagents, as well as alcohol, are however unfavourable for the purpose of this study, since by their action the yolk spherules appear frequently to be broken up and otherwise altered. This has to some extent occurred in Pl. 25, fig. 21, and the peculiar appearance of the yolk of this ovum is in part due to the action of the reagent. On the whole I have found osmic acid the most suitable reagent for the study of the yolk, since without breaking up the developing spherules, it stains them of a deep black colour. The yolk spherules commence to be formed in ova, of not more than 0.06 mm. in the ovaries of moderately old females. In young females they are apparently not formed in such small ova. They arise as extremely minute, highly refracting particles, in a stratum of protoplasm some little way below the surface, and are always most numerous at the pole opposite the germinal vesicle. Their general arrangement is very much that figured and described by Allen Thomson in Gasterosteus[392], and by Gegenbaur and Eimer in young Reptilian ova. In section they naturally appear as a ring, their general mode of distribution being fairly typically represented on Pl. 25, fig. 27. The ovum represented in fig. 27 was 0.5 mm. in diameter, and the yolk spherules were already largely developed; in smaller ova they are far less numerous, though arranged in a similar fashion. The developing yolk spherules are not uniformly distributed [Pg 580] but are collected in peculiar little masses or aggregations (Pl. 25, fig. 21). These resemble the granular masses, figured by His (loc. cit. Pl. 4, fig. 33) in the Salmon, and may be compared with the aggregations figured by Götte in his monograph on Bombinator igneus (Pl. 1, fig. 9). It deserves to be especially noted, that when the yolk spherules are first formed, the peripheral layer of the ovum is entirely free from them, a feature which is however apt to be lost in ova hardened in picric acid (Pl. 25, fig. 21). Two points about the spherules appear clearly to point to their being developed in the protoplasm of the ovum, and not in the follicular epithelium. (1) That they do not make their appearance in the superficial stratum of the ovum. (2) That no yolk spherules are present in the cells of the follicular epithelium, in which they could not fail to be detected, owing to the deep colour they assume on being treated with osmic acid.
It need scarcely be said that the yolk spherules at this stage are not cells, and have indeed no resemblance to cells. They would probably be regarded by His as spherules of fatty material, unrelated to the true food yolk.
As the ova become larger the granules of the peripheral layer before mentioned gradually assume the character of the yolk spheres of the adult, and at the same time spread towards the centre of the egg. Not having worked at fresh specimens, I cannot give a full account of the growth of the spherules; but am of opinion that Gegenbaur's account is probably correct, according to which the spheres at first present gradually grow and develop into vesicles, in the interior of which solid bodies (nuclei of His?) appear and form the permanent yolk spheres. When the yolk spheres are still very small they have the typical oblong form[393] of the ripe ovum, and this form is acquired while the centre of the ovum is still free from them.
The growth of the yolk appears mainly due to the increase in size and number of the individual yolk spheres. Even when the ovum is quite filled with large yolk spheres, the granular [Pg 581] protoplastic network of the earlier stages is still present, and serves to hold together the constituents of the yolk. In the cortical layer of nearly ripe ova, the yolk has a somewhat different character to that which it exhibits in the deeper layers, chiefly owing to the presence of certain delicate granular (in hardened specimens) bodies, whose nature I do not understand, and to special yolk spheres rather larger than the ordinary, provided with numerous smaller spherules in their interior, which are probably destined in the course of time to become free and to form ordinary yolk spheres.
The mode of formation of the yolk spheres above described appears to me
to be the normal, and possibly the only one. Certain peculiar structures
have, however, come under my notice, which may perhaps be connected with
the formation of the yolk. One of these resembles the bodies described by
Eimer[394] as Dotterschorfe.
I have only met these bodies in a single instance
in ova of 0.6 mm., from the ovary (in
active growth) of a specimen of Scy.
canicula 23 inches in length. In this instance they consisted of
homogeneous clear bodies (not bounded by any membrane) of somewhat
irregular shape, though usually more or less oval, and rarely more than
0.02 mm. in their longest diameter. They
were very numerous in the peripheral layer of the ovum, but quite absent in
the centre, and also not found outside the ovum (as they appear to be in
Reptilia). Yolk granules formed in the normal way, and staining deeply by
osmic acid, were present, but the Dotterschorfe
presented a marked
contrast to the remainder of the ovum, in being absolutely unstained by
osmic acid, and indeed they appeared more like a modified form of vacuole
than any definite body. Their general appearance in Scyllium may be
gathered from Eimer's figure 8, Pl. 11, though
they were much more numerous than represented in that figure, and confined
to the periphery of the ovum.
Dr Eimer describes a much earlier condition of these structures, in which they form a clear shell enclosing a central dark nucleus. This stage I have not met with, nor can I see any grounds for connecting these bodies with the formation [Pg 582] of the yolk, and the fact of their not staining with osmic acid is strongly opposed to this view of their function. Dr Eimer does not appear to me to bring forward any satisfactory proof that they are in any way related to the formation of the yolk, but wishes to connect them with the peculiar body, well known as the yolk nucleus, which is found in the Amphibian ovum[395].
Another peculiar body found in the ova may be mentioned here, though it more probably belongs to the germinal vesicle than to the yolk. It has only been met with in the vitellus of some of the medium sized ova of a young female. Examples of this body are represented on Pl. 25, fig. 25A, x. As a rule there is only one in each of the ova in which they are present, but there may be as many as four. They consist of small vesicles with a very thick doubly contoured membrane, which are filled with numerous deeply staining spherical granules. At times they contain a vacuole. Some of the larger of them are not very much smaller than the germinal vesicle of their ovum, while the smallest of them present a striking resemblance to the nucleoli (fig. 25B), which makes me think that they may possibly be nucleoli which have made their way out of the germinal vesicle. I have not found them in the late stages or large ova.
The following measurements shew the size of some of these bodies in relation to the germinal vesicle and ovum:—
| Diameter of Ovum. |
Diameter of Germinal Vesicle. |
Diameter of Body in Vitellus. | |
|---|---|---|---|
| 0.096 mm. | 0.03 mm. | 0.009 mm. | |
| 0.064 mm. | 0.025 mm. | 0.012 mm. | |
| ⎧ | 0.019 mm. | ||
| 0.096 mm. | 0.03 mm. | ⎨ | |
| ⎩ | 0.003 mm. | ||
Germinal vesicle.—Gegenbaur[396] finds the germinal vesicle completely homogeneous and without the trace of a germinal spot. In Raja granules or vesicles may appear as artificial products, and in Acanthias even in the fresh condition isolated vesicles or masses of such may be present. To these structures he attributes no importance.
Alexander Schultz[397] states that there is nothing remarkable in the germinal vesicle of the Torpedo egg, but that till the egg [Pg 583] reaches 0.5 mm., a single germinal spot is always present (measuring about 0.01 mm.), which is absent in larger ova.
The bodies described by Gegenbaur are now generally recognised as germinal spots, and will be described as such in the sequel. I have very rarely met with the condition with the single nucleolus described by Schultz in Torpedo.
My own observations are confined to Scyllium. In very young females, with ova not larger than 0.09 mm., the germinal vesicle has the same characters as during the embryonic periods. The contents are clear but traversed by a very distinct and deeply staining reticulum of fibres connected with the several nucleoli which are usually present and situated close to the membrane.
In a somewhat older female in the largest ova of about 0.12 mm., the germinal vesicle measures about 0.06 mm., and usually occupies an eccentric position. It is provided with a distinct though delicate membrane. The network, so conspicuous during the embryonic period, is not so clear as it was, and has the appearance of being formed of lines of granules rather than of fibres. The fluid contents of the nucleus remain as a rule, even in the hardened specimens, perfectly clear, though they become in some instances slightly granular. There are usually two, three, or more nucleoli generally situated, as described by Eimer, close to the membrane of the vesicle, the largest of which may measure as much as 0.006 mm. They are highly refracting bodies, containing in most instances a vacuole, and very frequently a smaller spherical body of a similar nature to themselves[398]. Granules are sometimes also present in the germinal vesicle, but are probably only extremely minute nucleoli.
In ova of 0.5 mm. the germinal vesicle has a diameter of 0.12 mm. (Pl. 25, fig. 21). It is usually shrunk in hardened specimens though nearly spherical in the living ovum. Its contents are rendered granular by reagents though quite clear when fresh, and the reticulum of the earlier stages is sometimes with difficulty to be made out, though in other instances fairly clear. In all cases the fibres composing it are very granular. The membrane [Pg 584] is thick. Peculiar highly refracting nucleoli, usually enclosing a large vacuole, are present in considerable numbers, and are either arranged in a circle round the periphery, or sometimes aggregated towards one side of the vesicle; and in addition, numerous deeply staining smaller granular aggregations, probably belonging to the same category as the nucleoli (from which in the living ovum they can only be distinguished by their size), are scattered close to the inner side of the membrane over the whole or only a part of the surface of the germinal vesicle. In a fair number of instances bodies like that figured on Pl. 25, fig. 27, are to be found in the germinal vesicle. They appear to be nucleoli in which a number of smaller nucleoli are originating by a process of endogenous growth, analogous perhaps to endogenous cell-formation. The nucleoli thus formed are, no doubt, destined to become free. The above mode of increase for the nucleoli appears to be exceptional. The ordinary mode is, no doubt, that by simple division into two, as was long ago shewn by Auerbach.
Of the later stages of the germinal vesicle and its final fate, I can give no account beyond the very fragmentary statements which have already appeared in my monograph on Elasmobranch Fishes.
Formation of fresh ova and ovarian nests in the post-embryonic stages.—Ludwig[399] was the first to describe the formation of ova in the post-embryonic periods. His views will be best explained by quoting the following passage:—
The follicle of Skates and Dog-fish, with the ovum
it contains, is to be considered as an aggregation of the cells of the
single-layered ovarian epithelium which have grown into the stroma, and of
which one cell has become the ovum and the others the follicular
epithelium. The follicle, however, draws in with it into the stroma a
number of additional epithelial cells in the form of a stalk connecting the
follicle with the superficial epithelium. At a later period the lower part
of the stalk at its junction with the follicle becomes continuously
narrowed, and at the same time a rupture takes place in the cells which
form it. In this manner the follicle becomes at last constricted [Pg 585] off from
the stalk, and so from its place of origin in the superficial epithelium,
and subsequently lies freely in the stroma of the ovary.
He further explains that the separation of the follicles from the epithelium takes place much earlier in Acanthias than in Raja, and that the sinkings of the epithelium into the stroma may have two or three branches each with a follicle.
Semper gives very little information with reference to the post-embryonic formation of ova. He expresses his agreement on the whole with Ludwig, but, amongst points not mentioned by Ludwig, calls attention to peculiar aggregations of primitive ova in the superficial epithelium, which he regards as either rudimentary testicular follicles or as nests similar to those in the embryo.
My observations on this subject do not agree very closely with those either of Ludwig or Semper. The differences between us partly, though not entirely, depend upon the fundamentally different views we hold about the constitution of the ovary and the nature of the epithelium covering it (vide pp. 555 and 556).
In very young ovaries (Pl. 24, fig. 8) nests of ova (in my sense of the term) are very numerous, but though usually superficial in position are also found in the deeper layers of the ovary. They are especially concentrated in their old position, close to the dorsal edge of the organ. In some instances they do not present quite the same appearance as in the embryo, owing to the outlines of the ova composing them being distinct, and to the presence between the ova of numerous interstitial cells derived from the germinal epithelium, and destined to become follicular epithelium. These latter cells at first form a much flatter follicular epithelium than in the embryonic periods, so that the smaller adult ova have a much less columnar investment than ova of the same size in the embryo. A few primitive ova may still be found in a very superficial position, but occasionally also in the deeper layers. I am inclined to agree with Semper that some of these are freshly formed from the cells of the germinal epithelium.
In the young female with ova of about 0.5 mm. nests of ova are still fairly numerous. The nests are characteristic, and present the various remarkable peculiarities already described [Pg 586] in the embryo. In many instances they form polynuclear masses, not divided into separate cells, generally, however, the individual ova are distinct. The ova in these nests are on the average rather smaller than during the embryonic periods. The nests are frequently quite superficial and at times continuous with the pseudo-epithelium, and individual ova also occasionally occupy a position in the superficial epithelium. Some of the appearances presented by separate ova are not unlike the figures of Ludwig, but a growth such as he describes has, according to my observations, no existence. The columns which he believes to have grown into the stroma are merely trabeculæ connecting the deeper and more superficial parts of the germinal epithelium; and his whole view about the formation of the follicular epithelium round separate ova certainly does not apply, except in rare cases, to Scyllium. It is, indeed, very easy to see that most freshly formed ova are derived from nests, as in the embryo; and the formation of a follicular epithelium round these ova takes place as they become separated from the nests. A few solitary ova, which have never formed part of a nest, seem to be formed in this stage as in the embryo; but they do not grow into the stroma surrounded by the cells of the pseudo-epithelium, and only as they reach a not inconsiderable size is a definite follicular epithelium formed around them. The follicular epithelium, though not always formed from the pseudo-epithelium, is of course always composed of cells derived from the germinal epithelium.
In all the ova formed at this stage the nucleus would seem to pass through the same metamorphosis as in the embryo.
In the later stages, and even in the full-grown female of Scyllium, fresh ova seemed to be formed and nests also to be present. In Raja I have not found freshly formed ova or nests in the adult, and have had no opportunity of studying the young forms.
Summary of observations on the development of the ovary in Scyllium and Raja.
(1) The ovary in the embryo is a ridge, triangular in section, attached along the base. It is formed of a core of stroma and a covering of epithelium. A special thickening of the epithelium [Pg 587] on the outer side forms the true germinal epithelium, to which the ova are confined (Pl. 24, fig. 1). In the development of the ovary the stroma becomes differentiated into an external vascular layer, especially developed in the neighbourhood of the germinal epithelium, and an internal lymphatic portion, which forms the main mass of the ovarian ridge (Pl. 24, figs. 2, 3, and 6).
(2) At first the thickened germinal epithelium is sharply separated by a membrane from the subjacent stroma (Pl. 24, figs. 1, 2, and 3), but at about the time when the follicular epithelium commences to be formed round the ova, numerous strands of stroma grow into the epithelium, and form a regular network of vascular channels throughout it, and partially isolate individual ova (Pl. 24, figs. 7 and 8). At the same time the surface of the epithelium turned towards the stroma becomes irregular (Pl. 24, fig. 9), owing to the development of individual ova. In still later stages the stroma ingrowths form a more or less definite tunic close to the surface of the ovary. External to this tunic is the superficial layer of the germinal epithelium, which forms what has been spoken of as the pseudo-epithelium. In many instances the protoplasm of its cells is produced into peculiar fibrous tails which pass into the tunic below.
(3) Primitive ova.—Certain cells in the epithelium lining the dorsal angle of the body-cavity become distinguished as primitive ova by their abundant protoplasm and granular nuclei, at a very early period in development, even before the formation of the genital ridges. Subsequently on the formation of the genital ridges these ova become confined to the thickened germinal epithelium on the outer aspect of the ridges (Pl. 24, fig. 1).
(4) Conversion of primitive ova into permanent ova.—Primitive ova may in Scyllium become transformed into permanent ova in two ways—the difference between the two ways being, however, of secondary importance.
(a) A nest of primitive ova makes its appearance, either by continued division of a single primitive ovum or otherwise. The bodies of all the ova of the nest fuse together, and a polynuclear mass is formed, which increases in size concomitantly with the division of its nuclei. The nuclei, moreover, pass through a series of transformations. They increase in size and form delicate [Pg 588] vesicles filled with a clear fluid, but contain close to one side a granular mass which stains very deeply with colouring reagents. The granular mass becomes somewhat stellate, and finally assumes a reticulate form with one more highly refracting nucleoli at the nodal points of the reticulum. When a nucleus has reached this condition the protoplasm around it has become slightly granular, and with the enclosed nucleus is segmented off from the nest as a special cell—a permanent ovum (figs. 13, 14, 15, 16). Not all the nuclei in a nest undergo the whole of the above changes; certain of them, on the contrary, stop short in their development, atrophy, and become employed as a kind of pabulum for the remainder. Thus it happens that out of a large nest perhaps only two or three permanent ova become developed.
(b) In the second mode of development of ova the nuclei and protoplasm undergo the same changes as in the first mode; but the ova either remain isolated and never form part of a nest, or form part of a nest in which no fusion of the protoplasm takes place, and all the primitive ova develop into permanent ova. Both the above modes of the formation continue through a great part of life.
(5) The follicle.—The cells of the germinal epithelium arrange themselves as a layer around each ovum, almost immediately after its separation from a nest, and so constitute a follicle. They are at first flat, but soon become more columnar. In Scyllium they remain for a long time uniform, but in large eggs they become arranged in two or three layers, while at the same time some of them become large and flask-shaped, and others small and oval (fig. 29). The flask-shaped cells have probably an important function in the nutrition of the egg, and are arranged in a fairly regular order amongst the smaller cells. Before the egg is quite ripe both kinds of follicle cells undergo retrogressive changes (Pl. 25, fig. 23).
In Raja a great irregularity in the follicle cells is observable at an early stage, but as the ovum grows larger the cells gradually assume a regular arrangement more or less similar to that in Scyllium (Pl. 25, figs. 30-33).
(6) The egg membranes.—Two membranes are probably always present in Elasmobranchii during some period of their [Pg 589] growth. The first formed and outer of these arises in some instances before the formation of the follicular epithelium, and would seem to be of the nature of a vitelline membrane. The inner one is the zona radiata with a typical radiately striated structure. It is formed from the vitellus at a much later period than the proper vitelline membrane. It is more developed in Scyllium than in Raja, but atrophies early in both genera. By the time the ovum is nearly ripe both membranes are very much reduced, and when the egg (in Scyllium and Pristiurus) is laid, no trace of any membrane is visible.
(7) The vitellus.—The vitellus is at first faintly granular, but at a later period exhibits a very distinct (protoplasmic) network of fibres, which is still present after the ovum has been laid.
The yolk arises, in the manner described by Gegenbaur, in ova of about 0.06 mm. as a layer of fine granules, which stain deeply with osmic acid. They are at first confined to a stratum of protoplasm slightly below the surface of the ovum, and are most numerous at the pole furthest removed from the germinal vesicle. They are not regularly distributed, but are aggregated in small masses. They gradually grow into vesicles, in the interior of which oval solid bodies are developed, which form the permanent yolk-spheres. These oval bodies in the later stages exhibit a remarkable segmentation into plates, which gives them a peculiar appearance of transverse striation.
Certain bodies of unknown function are occasionally met with in the vitellus, of which the most remarkable are those figured at x on Pl. 25, fig. 25A.
(8) The germinal vesicle.—A reticulum is very conspicuous in the germinal vesicle in the freshly formed ova, but becomes much less so in older ova, and assumes, moreover, a granular appearance. At first one to three nucleoli are present, but they gradually increase in number as the germinal vesicle grows older, and are frequently situated in close proximity to the membrane.
[Pg 590] The Mammalian Ovary (Pl. 26).
The literature of the mammalian ovary has been so often dealt with that it may be passed over with only a few words. The papers which especially call for notice are those of Pflüger[400], Ed. van Beneden[401], and especially Waldeyer[402], as inaugurating the newer view on the nature of the ovary, and development of the ova; and of Foulis[403] and Kölliker[404], as representing the most recent utterances on the subject. There are, of course, many points in these papers which are touched on in the sequel, but I may more especially here call attention to the fact that I have been able to confirm van Beneden's statement as to the existence of polynuclear protoplasmic masses. I have found them, however, by no means universal or primitive; and I cannot agree in a general way with van Beneden's account of their occurrence. I have found no trace of a germogene (Keimfache) in the sense of Pflüger and Ed. van Beneden. My own results are most in accordance with those of Waldeyer, with whom I agree in the fundamental propositions that both ovum and follicular epithelium are derived from the germinal epithelium, but I cannot accept his views of the relation of the stroma to the germinal epithelium.
In the very interesting paper of Foulis, the conclusion is arrived at, that while the ova are derived from the germinal epithelium, the cells of the follicle originate from the ordinary connective tissue cells of the stroma. Foulis regards the zona pellucida as a product of the ovum and not of the follicle. To both of these views I shall return, and hope to be able to shew that Foulis has not traced back the formation of the follicle through a sufficient number of the earlier stages. It thus comes about that though I fully recognise the accuracy of his figures, I am unable to admit his conclusions. Kölliker's statements [Pg 591] are again very different from those of Foulis. He finds certain cords of cells in the hilus of the ovary, which he believes to be derived from the Wolffian body, and has satisfied himself that they are continuous with Pflüger's egg-tubes, and that they supply the follicular epithelium. To the general accuracy of Kölliker's statements with reference to the relations of these cords in the hilus of the ovary I can fully testify, but am of opinion that he is entirely mistaken as to their giving rise to the follicular epithelium, or having anything to do with the ova. I hope to be able to give a fuller account of their origin than he or other observers have done.
My investigations on the mammalian ovary have been made almost entirely on the rabbit—the type of which it is most easy to procure a continuous series of successive stages; but in a general way my conclusions have been controlled and confirmed by observations on the cat, the dog, and the sheep. My observations commence with an embryo of eighteen days. A transverse section, slightly magnified, through the ovary at this stage, is represented on Pl. 26, fig. 35, and a more highly magnified portion of the same in fig. 35A. The ovary is a cylindrical ridge on the inner side of the Wolffian body, composed of a superficial epithelium, the germinal epithelium (g.e.), and of a tissue internal to this, which forms the main mass of it. In the latter two constituents have to be distinguished—(1) an epithelial-like tissue (t), coloured brown, which forms the most important element, and (2) vascular and stroma elements in this.
The germinal epithelium is a layer about 0.03 - 0.04 mm. in thickness. It is (vide fig. 35A, g.e.) composed of two or three layers of cells, with granular nuclei, of which the outermost layer is more columnar than the remainder, and has elongated rather than rounded nuclei. Its cells, though they vary slightly in size, are all provided with a fair amount of protoplasm, and cannot be divided (as in the case of the germinal epithelium of Birds, Elasmobranchii, &c.), into primitive ova, and normal epithelial cells. Very occasionally, however, a specially large cell, which, perhaps, deserves the appellation primitive ovum, may be seen. From the subjacent tissue the germinal epithelium is in most parts separated by a membrane-like structure [Pg 592] (fluid coagulum); but this is sometimes absent, and it is then very difficult to determine with exactness the inner border of the epithelium. The tissue (t), which forms the greater mass of the ovary at this stage, is formed of solid columns or trabeculæ of epithelial-like cells, which present a very striking resemblance in size and character to the cells of the germinal epithelium. The protoplasm of these cells stains slightly more deeply with osmic acid than does that of the cells of the germinal epithelium, so that it is rather easier to note a difference between the two tissues in osmic acid than in picric acid specimens. This tissue approaches very closely, and is in many parts in actual contact with the germinal epithelium. Between the columns of it are numerous vascular channels (shewn diagrammatically in my figures) and a few normal stroma cells. This remarkable tissue continues visible through the whole course of the development of the ovary, till comparatively late in life, and during all the earlier stages might easily be supposed to be about to play some part in the development of the ova, or even to be part of the germinal epithelium. It really, however, has nothing to do with the development of the ova, as is easily demonstrated when the true ova begin to be formed. In the later stages, as will be mentioned in the description of those stages, it is separated from the germinal epithelium by a layer of stroma; though at the two sides of the ovary it is, even in later stages, sometimes in contact with the germinal epithelium.
In most parts this tissue is definitely confined within the limits of the ovary, and does not extend into the mesentery by which the ovary is attached. It may, however, be traced at the anterior end of the ovary into connection with the walls of the Malpighian bodies, which lie on the inner side of the Wolffian body (vide fig. 35B), and I have no doubt that it grows out from the walls of these bodies into the ovary. In the male it appears to me to assist in forming, together with cells derived from the germinal epithelium, the seminiferous tubules, the development of which is already fairly advanced by this stage. I shall speak of it in the sequel as tubuliferous tissue. The points of interest in connection with it concern the male sex, which I hope to deal with in a future paper, but I have no [Pg 593] hesitation in identifying it with the segmental cords (segmentalstränge) discovered by Braun in Reptilia, and described at length in his valuable memoir on their urogenital system[405]. According to Braun the segmental cords in Reptilia are buds from the outer walls of the Malpighian bodies. The bud from each Malpighian body grows into the genital ridge before the period of sexual differentiation, and sends out processes backwards and forwards, which unite with the buds from the other Malpighian bodies. There is thus formed a kind of trabecular work of tissue in the stroma of the ovary, which in the Lacertilia comes into connection with the germinal epithelium in both sexes, but in Ophidia in the male only. In the female, in all cases, it gradually atrophies and finally vanishes, but in the male there pass into it the primitive ova, and it eventually forms, with the enclosed primitive ova, the tubuli seminiferi. From my own observations in Reptilia I can fully confirm Braun's statements as to the entrance of the primitive ova into this tissue in the male, and the conversion of it into the tubuli seminiferi. The chief difference between Reptilia and Mammalia, in reference to this tissue, appears to be that in Mammalia it arises only from a few of the Malpighian bodies at the anterior extremity of the ovary, but in Reptilia from all the Malpighian bodies adjoining the genital ridge. More extended observations on Mammalia will perhaps shew that even this difference does not hold good.
It is hardly to be supposed that this tissue, which is so conspicuous in all young ovaries, has not been noticed before; but the notices of it are not so numerous as I should have anticipated. His[406] states that the parenchyma of the sexual glands undoubtedly arises from the Wolffian canals, and adds that while the cortical layer (Hulle) represents the earlier covering of a part of the Wolffian body, the stroma of the hilus, with its vessels, arises from a Malpighian body. In spite of these statements of His, I still doubt very much whether he has really observed either the tissue I allude to or its mode of development. In any case he gives no recognisable description or figure of it.
[Pg
594] Waldeyer[407] notices this tissue in the dog,
cat, and calf. The following is a free translation of what he says,
(p. 141):—In a full grown but young dog, with numerous ripe
follicles, there were present in the vascular zone of the ovary numerous
branched elongated small columns (Schläuche) of epithelial cells, between
which ran blood-vessels. They were only separated from the egg columns of
the cortical layer by a row of large follicles. There can be no doubt that
we have here remains of the sexual part of the Wolffian body—the
canals of the parovarium—which in the female sex have developed
themselves to an extraordinary extent into the stroma of the sexual gland,
and perhaps are even to be regarded as homologues of the seminiferous
tubules (the italics are my own). I have almost always found the above
condition in the dog, only in old animals these seminiferous canals seem
gradually to atrophy. Similar columns are present in the cat, only they do
not appear to grow so far into the stroma.
Identical structures are also
described in the calf.
Romiti gives a very similar description to Waldeyer of these bodies in the dog[408]. Born also describes this tissue in young and embryonic ovaries of the horse as the Keimlager[409]. The columns described by Kölliker[410] and believed by him to furnish the follicular epithelium, are undoubtedly my tubuliferous tissue, and, as Kölliker himself points out, are formed of the same tissue as that described by Waldeyer.
Egli gives a very clear and accurate description of this tissue, though he apparently denies its relation with the Wolffian body.
My own interpretation of the tissue accords with that of Waldeyer. In addition to the rabbit, I have observed it in the dog, cat, and sheep. In all these forms I find that close to the attachment of the ovary, and sometimes well within it, a fair number of distinct canals with a large lumen are present, which are probably to be distinguished from the solid epithelial columns. Such large canals are not as a rule present in the rabbit. In the [Pg 595] dog solid columns are present in the embryo, but later they appear frequently to acquire a tubular form, and a lumen. Probably there are great variations in the development of the tissue, since in the cat (not as Waldeyer did in the dog) I have found it most developed.
In the very young embryonic ovary of the cat the columns are very small and much branched. In later embryonic stages they are frequently elongated, sometimes convoluted, and are very similar to the embryonic tubuli seminiferi. In the young stages these columns are so similar to the egg tubes (which agree more closely with Pflüger's type in the cat than in other forms I have worked at) that to any one who had not studied the development of the tissue an embryo cat's ovary at certain stages would be a very puzzling object. I have, however, met with nothing in the cat or any other form which supports Kölliker's views.
My next stage is that of a twenty-two days' embryo. Of this stage I have given two figures corresponding to those of the earlier stage (figs. 36 and 36A).
From these figures it is at once obvious that the germinal epithelium has very much increased in bulk. It has a thickness 0.1 - 0.09 mm. as compared to 0.03 mm. in the earlier stage. Its inner outline is somewhat irregular, and it is imperfectly divided into lobes, which form the commencement of structures nearly equivalent to the nests of the Elasmobranch ovary. The lobes are not separated from each other by connective tissue prolongations; the epithelium being at this stage perfectly free from any ingrowths of stroma. The cells constituting the germinal epithelium have much the same character as in the previous stage. They form an outer row of columnar cells internal to which the cells are more rounded. Amongst them a few large cells with granular nuclei, which are clearly primitive ova, may now be seen, but by far the majority of the cells are fairly uniform in size, and measure from 0.01 - 0.02 mm. in diameter, and their nuclei from 0.004 - 0.006 mm. The nuclei of the columnar outer cells measure about 0.008 mm. They are what would ordinarily be called granular, though high powers shew that they have the usual nuclear network. There is no special nucleolus. The rapid growth of the germinal epithelium is due [Pg 596] to the division of its cells, and great masses of these may frequently be seen to be undergoing division at the same time. Of the tissue of the ovary internal to the germinal epithelium, it may be noticed that the tubuliferous tissue derived from the Malpighian bodies is no longer in contact with the germinal epithelium, but that a layer of vascular stroma is to a great extent interposed between the two. The vascular stroma of the hilus has, moreover, greatly increased in quantity.
My next stage is that of a twenty-six days' embryo, but the characters of the ovary at this stage so closely correspond with those of the succeeding one at twenty-eight days that, for the sake of brevity, I pass over this stage in silence.
Figs. 37 and 37A are representative sections of the ovary of the twenty-eighth day corresponding with those of the earlier stages.
Great changes have become apparent in the constitution of the germinal epithelium. The vascular stroma of the ovary has grown into the germinal epithelium precisely as in Elasmobranchii. It appears to me clear that the change in the relations between the stroma and epithelium is not due to a mutual growth, but entirely to the stroma, so that, as in the case of Elasmobranchii, the result of the ingrowth is that the germinal epithelium is honeycombed by vascular stroma. The vascular growths generally take the paths of the lines which separated the nests in an earlier condition, and cause these nests to become the egg tubes of Pflüger. It is obvious in figure 37 that the vascular ingrowths are so arranged as imperfectly to divide the germinal epithelium into two layers separated by a space with connective tissue and blood-vessels. The outer part is relatively thin, and formed of a superficial row of columnar cells, and one or two rows of more rounded cells; the inner layer is much thicker, and formed of large masses of rounded cells. The two layers are connected together by numerous trabeculæ, the stroma between which eventually gives rise to the connective tissue capsule, or tunica albuginea, of the adult ovary.
The germinal epithelium is now about 0.19 to 0.22 mm. in thickness. Its cells have undergone considerable changes. A fair number of them (fig. 37A, p.o.), especially in the outer layer of the epithelium, have become larger than the cells around [Pg 597] them, from which they are distinguished, not only by their size, but by their granular nucleus and abundant protoplasm. They are in fact undoubted primitive ova with all the characters which primitive ova present in Elasmobranchii, Aves, &c. In a fairly typical primitive ovum of this stage the body measures 0.02 mm. and the nucleus 0.014 mm. In the inner part of the germinal epithelium there are very few or no cells which can be distinguished by their size as primitive ova, and the cells themselves are of a fairly uniform size, though in this respect there is perhaps a greater variation than might be gathered from fig. 37A. The cells are on the average about 0.016 mm. in diameter, and their nuclei about 0.008 to 0.001 mm., considerably larger, in fact, than in the earlier stage. The nuclei are moreover more granular, and make in this respect an approach to the character of the nuclei of primitive ova.
The germinal epithelium is still rapidly increasing by the division of its cells, and in fig. 37A there are shewn two or three nuclei in the act of dividing. I have represented fairly accurately the appearance they present when examined with a moderately high magnifying power. With reference to the stroma of the ovary, internal to the germinal epithelium, it is only necessary to refer to fig. 37 to observe that the tubuliferous tissue (t) forms a relatively smaller part of the stroma than in the previous stage, and is also further removed from the germinal epithelium.
My next stage is that of a young rabbit two days after birth, but to economise space I pass on at once to the following stage five days after birth. This stage is in many respects a critical one for the ovary, and therefore of great interest. Figure 38 represents a transverse section through the ovary (on rather a smaller scale than the previous figures) and shews the general relations of the tissues.
The germinal epithelium is very much thicker than before—about 0.38 mm. as compared with 0.22 mm. It is divided into three obvious layers: (1) an outer epithelial layer which corresponds with the pseudo-epithelial layer of the Elasmobranch ovary, average thickness 0.03 mm. (2) A middle layer of small nests, which corresponds with the middle vascular layer of the previous stage; average thickness 0.1 mm. (3) An inner layer of larger nests; average thickness 0.23 mm.
[Pg 598] The general appearance of the germinal epithelium at this stage certainly appears to me to lend support to my view that the whole of it simply constitutes a thickened epithelium interpenetrated with ingrowths of stroma.
The cells of the germinal epithelium, which form the various layers, have undergone important modifications. In the first place a large number of the nuclei—at any rate of those cells which are about to become ova—have undergone a change identical with that which takes place in the conversion of the primitive into the permanent ova in Elasmobranchii. The greater part of the contents of the nucleus becomes clear. The remaining contents arrange themselves as a deeply staining granular mass on one side of the membrane, and later on as a somewhat stellate figure: the two stages forming what were spoken of as the granular and stellate varieties of nucleus. To avoid further circumlocution I shall speak of the nucleus undergoing the granular and the stellate modifications. At a still later period the granular contents form a beautiful network in the nucleus.
The pseudo-epithelium (fig. 38A) is formed of several tiers of cells, the outermost of which are very columnar and have less protoplasm than in an earlier stage. In the lower tiers of cells there are many primitive ova with granular nuclei, and others in which the nuclei have undergone the granular modification. The primitive ova are almost all of the same size as in the earlier stage. The pseudo-epithelium is separated from the middle layer by a more or less complete stratum of connective tissue, which, however, is traversed by trabeculæ connecting the two layers of the epithelium. In the middle layer there are comparatively few modified nuclei, and the cells still retain for the most part their earlier characters. The diameter of the cells is about 0.012 mm., and that of the nucleus about 0.008 mm. In the innermost layer (fig. 38B), which is not sharply separated from the middle layer, the majority of the cells, which in the previous stage were ordinary cells of the epithelium, have commenced to acquire modified nuclei. This change, which first became apparent to a small extent in the young two days after birth, is very conspicuous at this stage. In some of the cells the nucleus is modified in the granular manner, in others in the [Pg 599] stellate, and in a certain number the nucleus has assumed a reticular structure characteristic of the young permanent ovum.
In addition, however, to the cells which are becoming converted into ova, a not inconsiderable number may be observed, if carefully looked for, which are for the most part smaller than the others, generally somewhat oval, and in which the nucleus retains its primitive characters. A fair number of such cells are represented in fig. 38B. In the larger ones the nucleus will perhaps eventually become modified; but the smaller cells clearly correspond with the interstitial cells of the Elasmobranch germinal epithelium, and are destined to become converted into the epithelium of the Graafian follicle. In some few instances indeed (at this stage very few), in the deeper part of the germinal epithelium, these cells commence to arrange themselves round the just formed permanent ova as a follicular epithelium. An instance of this kind is shewn in fig. 38B, o. The cells with modified nuclei, which are becoming permanent ova, usually present one point of contrast to the homologous cells in Elasmobranchii, in that they are quite distinct from each other, and not fused into a polynuclear mass. They have around them a dark contour line, which I can only interpret as the commencement of the membrane (zona radiata?), which afterwards becomes distinct, and which would thus seem, as Foulis has already insisted, to be of the nature of a vitelline membrane.
In a certain number of instances the protoplasm of the cells which are becoming permanent ova appears, however, actually to fuse, and polynuclear masses identical with those in Elasmobranchii are thus formed (cf. E. van Beneden[411]). These masses become slightly more numerous in the succeeding stages. Indications of a fusion of this kind are shewn in fig. 38B. That the polynuclear masses really arise from a fusion of primitively distinct cells is clear from the description of the previous stages. The ova in the deeper layers, with modified granular nuclei, measure about 0.016 - 0.02 mm., and their nuclei from 0.01 - 0.012 mm.
With reference to the tissue of the hilus of the ovary, it may be noticed that the tubuliferous tissue (t) is relatively [Pg 600] reduced in quantity. Its cells retain precisely their previous characters.
The chief difference between the stage of five days and that of two days after birth consists in the fact that during the earlier stage comparatively few modified nuclei were present, but the nuclei then presented the character of the nuclei of primitive ova.
I have ovaries both of the dog and cat of an equivalent stage, and in both of these the cells of the nests or egg tubes may be divided into two categories, destined respectively to become ova and follicle cells. Nothing which has come under my notice tends to shew that the tubuliferous tissue is in any way concerned in supplying the latter form of cell.
In a stage, seven days after birth, the same layers in the germinal epithelium may be noticed as in the last described stage. The outermost layer or pseudo-epithelium contains numerous developing ova, for the most part with modified nuclei. It is separated by a well marked layer of connective tissue from the middle layer of the germinal epithelium. The outer part of the middle layer contains more connective tissue and smaller nests than in the earlier stage, and most of the cells of this layer contain modified nuclei. In a few nests the protoplasm of the developing ova forms a continuous mass, not divided into distinct cells, but in the majority of instances the outline of each ovum can be distinctly traced. In addition to the cells destined to become ova, there are present in these nests other cells, which will clearly form the follicular epithelium. A typical nest from the middle layer is represented on Pl. 26, fig. 39A.
The nests or masses of ova in the innermost layer are for the most part still very large, but, in addition to the nests, a few isolated ova, enclosed in follicles, are to be seen.
A fairly typical nest, selected to shew the formation of the follicle, is represented on Pl. 26, fig. 39B.
The nest contains (1) fully formed permanent ova, completely or wholly enclosed in a follicle. (2) Smaller ova, not enclosed in a follicle. (3) Smallish cells with modified nuclei of doubtful destination. (4) Small cells obviously about to form follicular epithelium.
The inspection of a single such nest is to my mind a satisfactory [Pg 601] proof that the follicular epithelium takes its origin from the germinal epithelium and not from the stroma or tubuliferous tissue. The several categories of elements observable in such a nest deserve a careful description.
(1) The large ova in their follicles.—These ova have precisely the character of the young ova in Elasmobranchii. They are provided with a granular body invested by a delicate, though distinct membrane. Their nucleus is large and clear, but traversed by the network so fully described for Elasmobranchii. The cells of their follicular epithelium have obviously the same character as many other small cells of the nest. Two points about them deserve notice—(a) that many of them are fairly columnar. This is characteristic only of the first formed follicles. In the later formed follicles the cells are always flat and spindle-shaped in section. In this difference between the early and late formed follicles Mammals agree with Elasmobranchii. (b) The cells of the follicle are much more columnar towards the inner side than towards the outer. This point also is common to Mammals and Elasmobranchii.
Round the completed follicle a very delicate membrana propria folliculi appears to be present[412].
The larger ova, with follicular epithelium, measure about 0.04 mm., and their nucleus about 0.02 mm., the smaller ones about 0.022 mm., and their nucleus about 0.014 mm.
(2) Medium sized ova.—They are still without a trace of a follicular epithelium, and present no special peculiarities.
(3) The smaller cells with modified nuclei.—I have great doubt as to what is the eventual fate of these cells. There appear to be three possibilities.
(a) That they become cells of the follicular epithelium; (b) that they develop into ova; (c) that they are absorbed as a kind of food by the developing ova. I am inclined to think that some of these cells may have each of the above-mentioned destinations.
(4) The cells which form the follicle.—The only point to be noticed about these is that they are smaller than the indifferent [Pg 602] cells of the germinal epithelium, from which they no doubt originate by division. This fact has already been noticed by Waldeyer.
The isolated follicles at this stage are formed by ingrowths of connective tissue cutting off fully formed follicles from a nest. They only occur at the very innermost border of the germinal epithelium. This is in accordance with what has so often been noticed about the mammalian ovary, viz. that the more advanced ova are to be met with in passing from without inwards.
By the stage seven days after birth the ovary has reached a sufficiently advanced stage to answer the more important question I set myself to solve, nevertheless, partly to reconcile the apparent discrepancy between my account and that of Dr Foulis, and partly to bring my description up to a better known condition of the ovary, I shall make a few remarks about some of the succeeding stages.
In a young rabbit about four weeks old the ovary is a very beautiful object for the study of the nuclei, &c.
The pseudo-epithelium is now formed of a single layer of columnar cells, with comparatively scanty protoplasm. In it there are present a not inconsiderable number of developing ova.
A layer of connective tissue—the albuginea—is now present below the pseudo-epithelium, which contains a few small nests with very young permanent ova. The layer of medium sized nests internal to the albuginea forms a very pretty object in well stained sections, hardened in Kleinenberg's picric acid. The ova in it have all assumed the permanent form, and are provided with beautiful reticulate nuclei, with, as a rule, one more especially developed nucleolus, and smaller granular bodies. Their diameter varies from about 0.028 to 0.04 mm. and that of their nucleus from 0.016 to 0.02 mm. The majority of these ova are not provided with a follicular investment, but amongst them are numerous small cells, clearly derived from the germinal epithelium, which are destined to form the follicle (vide fig. 40Aand B). In a few cases the follicles are completed, and are then formed of very flattened spindle-shaped (in section) cells. In the majority of cases all the ova of each nest are quite distinct, and each provided with a delicate vitelline membrane (fig. 40A) [Pg 603] In other instances, which, so far as I can judge, are more common than in the previous stages, the protoplasm of two or more ova is fused together.
Examples of this are represented in Pl. 26, fig. 40A. In some of these the nuclei in the undivided protoplasm are all of about the same size and distinctness, and probably the protoplasm eventually becomes divided up into as many ova as nuclei; in other cases, however, one or two nuclei clearly preponderate over the others, and the smaller nuclei are indistinct and hazy in outline. In these latter cases I have satisfied myself as completely as in the case of Elasmobranchii, that only one or two ova (according to the number of distinct nuclei) will develop out of the polynuclear mass, and that the other nuclei atrophy, and the material of which they were composed serves as the nutriment for the ova which complete their development. This does not, of course, imply that the ova so formed have a value other than that of a single cell, any more than the development of a single embryo out of the many in one egg capsule implies that the embryo so developing is a compound organism.
In the innermost layer of the germinal epithelium the outlines of the original large nests are still visible, but many of the follicles have been cut off by ingrowths of stroma. In the still intact nests the formation of the follicles out of the cells of the germinal epithelium may be followed with great advantage. The cells of the follicle, though less columnar than was the case at an earlier period, are more so than in the case of follicles formed in the succeeding stages. The previous inequality in the cells of the follicles is no longer present.
The tubuliferous tissue in the zona vasculosa appears to me to have rather increased in quantity than the reverse; and is formed of numerous solid columns or oval masses of cells, separated by strands of connective tissue, with typical spindle nuclei.
It is partially intelligible to me how Dr Foulis might from an examination of the stages similar to this, conclude that the follicle cells were derived from the stroma; but even at this stage the position of the cells which will form the follicular epithelium, their passage by a series of gradations into obvious [Pg 604] cells of the germinal epithelium and the peculiarities of their nuclei, so different from those of the stroma cells, supply a sufficient series of characters to remove all doubt as to the derivation of the follicle cells. Apart from these more obvious points, an examination of the follicle cells from the surface, and not in section, demonstrates that the general resemblance in shape of follicle cells to the stroma cells is quite delusory. They are in fact flat, circular, or oval, plates not really spindle-shaped, but only apparently so in section. While I thus fundamentally differ from Foulis as to the nature of the follicle cells, I am on this point in complete accordance with Waldeyer, and my own results with reference to the follicle cannot be better stated than in his own words (pp. 43, 44).
At six weeks after birth the ovary of the rabbit corresponds very much more with the stages in the development of the ovary, which Foulis has more especially studied, for the formation of the follicular epithelium, than during the earlier stages. His figure (Quart. Journ. Mic. Sci., Vol. XVI., Pl. 17, fig. 6) of the ovary of a seven and a half months' human fœtus is about the corresponding age. Different animals vary greatly in respect to the relative development of the ovary. For example, the ovary of a lamb at birth about corresponds with that of a rabbit six weeks after birth. The points which may be noticed about the ovary at this age are first that the surface of the ovary begins to be somewhat folded. The appearances of these folds in section have given rise, as has already been pointed out by Foulis, to the erroneous view that the germinal epithelium (pseudo-epithelium) became involuted in the form of tubular open pits. The folds appear to me to have no connection with the formation of ova, but to be of the same nature as the somewhat similar folds in Elasmobranchii. A follicular epithelium is present around the majority of the ova of the middle layer, and around all those of the inner layer of the germinal epithelium. The nests are, moreover, much more cut up by connective tissue ingrowths than in the previous stages.
The follicle cells of the middle layers are very flat, and spindle-shaped in section, and though they stain more deeply than the stroma cells, and have other not easily characterised peculiarities, they nevertheless do undoubtedly closely resemble [Pg 605] the stroma cells when viewed (as is ordinarily the case) in optical section.
In the innermost layer many of the follicles with the enclosed ova have
advanced considerably in development and are formed of columnar cells. The
somewhat heterodox view of these cells propounded by Foulis I cannot quite
agree to. He says (Quart. J. Mic. Sci., Vol. XVI., p. 210): The protoplasm which surrounds
the vesicular nuclei acts as a sort of cement substance, holding them
together in the form of a capsular membrane round the young ovum. This
capsular membrane is the first appearance of the membrana granulosa.
I
must admit that I find nothing similar to this, nor have I met with any
special peculiarities (as Foulis would seem to indicate) in the cells of
the germinal epithelium or other cells of the ovary.
Figure 41 is a representation of an advanced follicle of a six weeks' rabbit, containing two ova, which is obviously in the act of dividing into two. Follicles of this kind with more than one ovum are not very uncommon. It appears to me probable that follicles, such as that I have figured, were originally formed of a single mass of protoplasm with two nuclei; but that instead of one of the nuclei atrophying, both of them eventually developed and the protoplasm subsequently divided into two masses. In other cases it is quite possible that follicles with two ova should rather be regarded as two follicles not separated by a septum of stroma.
On the later stages of development of the ovary I have no complete series of observations. The yolk spherules I find to be first developed in a peripheral layer of the vitellus. I have not been able definitely to decide the relation of the zona radiata to the first formed vitelline membrane. Externally to the zona radiata there may generally be observed a somewhat granular structure, against which the follicle cells abut, and I cannot agree with Waldeyer (loc cit., p. 40) that this structure is continuous with the cells of the discus, or with the zona radiata. Is it the remains of the first formed vitelline membrane? I have obtained some evidence in favour of this view, but have not been successful in making observations to satisfy me on the point, and must leave open the question whether my vitelline membrane becomes the zona radiata or whether the zona is not a [Pg 606] later and independent formation, but am inclined myself to adopt the latter view. The first formed membrane, whether or no it becomes the zona radiata, is very similar to the vitelline membrane of Elasmobranchii and arises at a corresponding stage.
Summary of observations on the mammalian ovary.—The general results of my observations on the mammalian ovary are the following:—
(1) The ovary in an eighteen days' embryo consists of a cylindrical ridge attached along the inner side of the Wolffian body, which is formed of two parts; (a) an external epithelium—two or three cells deep (the germinal epithelium); (b) a hilus or part forming in the adult the vascular zone, at this stage composed of branched masses of epithelial tissue (tubuliferous tissue) derived from the walls of the anterior Malpighian bodies, and numerous blood-vessels, and some stroma cells.
(2) The germinal epithelium gradually becomes thicker, and after a certain stage (twenty-three days) there grow into it numerous stroma ingrowths, accompanied by blood-vessels. The germinal epithelium thus becomes honeycombed by strands of stroma. Part of the stroma eventually forms a layer close below the surface, which becomes in the adult the tunica albuginea. The part of the germinal epithelium external to this layer becomes reduced to a single row of cells, and forms what has been spoken of in this paper as the pseudo-epithelium of the ovary. The greater part of the germinal epithelium is situated internal to the tunica albuginea, and this part is at first divided up by strands of stroma into smaller divisions externally, and larger ones internally. These masses of germinal epithelium (probably sections of branched trabeculæ) may be spoken of as nests. In the course of the development of the ova they are broken up by stroma ingrowths, and each follicle with its enclosed ovum is eventually isolated by a layer of stroma.
(3) The cells of the germinal epithelium give rise both to the permanent ova and to the cells of the follicular epithelium. For a long time, however, the cells remain indifferent, so that the stages, like those in Elasmobranchii, Osseous Fish, Birds, Reptiles, &c., with numerous primitive ova embedded amongst the small cells of the germinal epithelium, are not found.
[Pg 607](4) The conversion of the cells of the germinal epithelium into permanent ova commences in an embryo of about twenty-two days. All the cells of the germinal epithelium appear to be capable of becoming ova: the following are the stages in the process, which are almost identical with those in Elasmobranchii:—
(a) The nucleus of the cells loses its more or less distinct network, and becomes very granular, with a few specially large granules (nucleoli). The protoplasm around it becomes clear and abundant—primitive ovum stage. It may be noted that the largest primitive ova are very often situated in the pseudo-epithelium. (b) A segregation takes place in the contents of the nucleus within the membrane, and the granular contents pass to one side, where they form an irregular mass, while the remaining space within the membrane is perfectly clear. The granular mass gradually develops itself into a beautiful reticulum, with two or three highly refracting nucleoli, one of which eventually becomes the largest and forms the germinal spot par excellence. At the same time the body of the ovum becomes slightly granular. While the above changes, more especially those in the nucleus, have been taking place, the protoplasm of two or more ova may fuse together, and polynuclear masses be so formed. In some cases the whole of such a polynuclear mass gives rise to only a single ovum, owing to the atrophy of all the nuclei but one, in others it gives rise by subsequent division to two or more ova, each with a single germinal vesicle.
(5) All the cells of a nest do not undergo the above changes, but some of them become smaller (by division) than the indifferent cells of the germinal epithelium, arrange themselves round the ova, and form the follicular epithelium.
(6) The first membrane formed round the ovum arises in some cases even before the appearance of the follicular epithelium, and is of the nature of a vitelline membrane. It seems probable, although not definitely established by observation, that the zona radiata is formed internally to the vitelline membrane, and that the latter remains as a membrane, somewhat irregular on its outer border, against which the ends of the follicle cells abut.
[Pg 608]General Observations on the Structure and Development of the Ovary.
In selecting Mammalia and Elasmobranchii as my two types for investigation, I had in view the consideration that what held good for such dissimilar forms might probably be accepted as true for all Vertebrata with the exception of Amphioxus.
The structure of the ovary.—From my study of these two types, I have been led to a view of the structure of the ovary, which differs to a not inconsiderable extent from that usually entertained. For both types the conclusion has been arrived at that the whole egg-containing part of the ovary is really the thickened germinal epithelium, and that it differs from the original thickened patch or layer of germinal epithelium, mainly in the fact that it is broken up into a kind of meshwork by growths of vascular stroma. If the above view be accepted for Elasmobranchii and Mammalia, it will hardly be disputed for the ovaries of Reptilia and Aves. In the case also of Osseous Fish and Amphibia, this view of the ovary appears to be very tenable, but the central core of stroma present in the other types is nearly or quite absent, and the ovary is entirely formed of the germinal epithelium with the usual strands of vascular stroma[413]. It is obvious that according to the above view Pflüger's egg-tubes are merely trabeculæ of germinal epithelium, and have no such importance as has been attributed to them. They are present in a more or less modified form in all types of ovaries. Even in the adult Amphibian ovary, columns of cells of the germinal epithelium, some indifferent, others already converted into ova, are present, and, as has been pointed out by Hertwig[414], represent Pflüger's egg-tubes.
The formation of the permanent ova.—The passage of primitive ova into permanent ova is the part of my investigation to which the greatest attention was paid, and the results arrived at for Mammalia and Elasmobranchii are almost identical. Although [Pg 609] there are no investigations as to the changes undergone by the nucleus in other types, still it appears to me safe to conclude that the results arrived at hold good for Vertebrates generally[415]. As has already been pointed out the transformation which the so-called primitive ova undergo is sufficient to shew that they are not to be regarded as ova but merely as embryonic sexual cells. A feature in the transformation, which appears to be fairly constant in Scyllium, and not uncommon in the rabbit, is the fusion of the protoplasm of several ova into a syncytium, the subsequent increase in the number of nuclei in the syncytium, the atrophy and absorption of a portion of the nuclei, and the development of the remainder into the germinal vesicles of ova; the vitellus of each ovum being formed by a portion of the protoplasm of the syncytium.
As to the occurrence of similar phenomena in the Vertebrata generally, it has already been pointed out that Ed. van Beneden has described the polynuclear masses in Mammalia, though he does not appear to me to have given a complete account of their history. Götte[416] describes a fusion of primitive ova in Amphibia, but he believes that the nuclei fuse as well as the bodies of the ova, so that one ovum (according to his view no longer a cell) is formed by the fusion of several primitive ova with their nuclei. I have observed nothing which tends to support Götte's view about the fusion of the nuclei, and regard it as very improbable. As regards the interpretation to be placed upon the nests formed of fused primitive ova, Ed. van Beneden maintains that they are to be compared with the upper ends of the egg tubes of Insects, Nematodes, Trematodes, &c. There is no doubt a certain analogy between the two, in that in both cases certain nuclei of a polynuclear mass increase in size, and with the protoplasm around them become segmented off from the remainder of the mass as ova, but the analogy cannot be pressed. The primitive ova, or even the general germinal epithelium, rather than these nests, must be regarded as giving origin to the ova, and the nests should be looked on, in my opinion, as connected [Pg 610] more with the nutrition than with the origin of the ova. In favour of this view is the fact that as a rule comparatively few ova are developed from the many nuclei of a nest; while against the comparison with the egg tubes of the Invertebrata it is to be borne in mind that many ova appear to develop independently of the nests.
In support of my view about the nests there may be cited many analogous instances from the Invertebrata. In none of them, however, are the phenomena exactly identical with those in Vertebrata. In the ovary of many Hydrozoa (e.g. Tubularia mesembryanthemum), out of a large number of ova which develop up to a certain point, a comparatively very small number survive, and these regularly feed upon the other ova. During this process the boundary between a large ovum and the smaller ova is indistinct: in the outermost layer of a large ovum a number of small ova are embedded, the outlines of the majority of which have become obscure, although they can still be distinguished. Just beyond the edge of a large ovum the small ova have begun to undergo retrogressive changes; while at a little distance from the ovum they are quite normal. An analogous phenomenon has been very fully described by Weismann[417] in the case of Leptodera, the ovary of which consists of a germogene, in which the ova develop in groups of four. Each group of four occupies a separate chamber of the ovary, but in summer only one of the four eggs (the third from the germogene) develops into an ovum, the other three are used as pabulum. In the case of the winter eggs the process is carried still further, in that the contents of the alternate chambers, instead of developing into ova, are entirely converted, by a series of remarkable changes, into nutritive reservoirs. Fundamentally similar occurrences to the above are also well known in Insects. Phenomena of this nature are obviously in no way opposed to the view of the ovum being a single cell.
With reference to the origin of the primitive ova, it appears to me that their mode of development in Mammals proves beyond a doubt that they are modified cells of the germinal epithelium. In Elasmobranchii their very early appearance, and the difficulty [Pg 611] of finding transitional forms between them and ordinary cells of the germinal epithelium, caused me at one time to seek (unsuccessfully) for a different origin for them. Any such attempts appear to me, however, out of the question in the case of Mammals.
The egg membranes.—The homologies of the egg membranes in the Vertebrata are still involved in some obscurity. In Elasmobranchii there are undoubtedly two membranes present. (1) An outer and first formed membrane—the albuminous membrane of Gegenbaur—which, in opposition to previous observers, I have been led to regard as a vitelline membrane. (2) An inner radiately striated membrane, formed as a differentiation of the surface of the yolk at a later period. Both these membranes usually atrophy before the ovum leaves the follicle. In Reptilia[418] precisely the same arrangement is found as in Elasmobranchii, except that as a rule the zona radiata is relatively more important. The vitelline membrane external to this (or as it is usually named the chorion) is, as a rule, thin in Reptilia; but in Crocodilia is thick (Gegenbaur), and approaches the condition found in Scyllium and other Squalidæ. It appears, as in Elasmobranchii, to be formed before the zona radiata. A special internal differentiation of the zona radiata is apparently found (Eimer) in many Reptilia. No satisfactory observations appear to be recorded with reference to the behaviour of the two reptilian membranes as the egg approaches maturity. In Birds[419] the same two membranes are again found. The first formed and outer one is, according to Gegenbaur and E. van Beneden, a vitelline membrane; and from the analogy of Elasmobranchii I feel inclined to accept their view. The inner one is the zona radiata, which disappears comparatively early, leaving the ovum enclosed only by the vitelline membrane, when it leaves the follicle. All the large-yolked vertebrate ova appear then to agree very well with Elasmobranchii in presenting during some period of their development the two membranes above mentioned.
Osseous fish have almost always a zona radiata, which it seems best to assume to be equivalent to that in Elasmobranchii. [Pg 612] Internal to this is a thin membrane, the equivalent, according to Eimer, of the membrane found by the same author within the zona in Reptilia. A membrane equivalent to the thick vitelline membrane of Elasmobranchii would seem to be absent in most instances, though a delicate membrane, external to the zona, has not infrequently been described; Eimer more especially asserts that such a membrane exists in the perch within the peculiar mucous covering of the egg of that fish.
In Petromyzon, a zona radiata appears to be present[420], which is divided in the adult into two layers, both of them perforated. The inner of the two perhaps corresponds with the membrane internal to the zona radiata in other types. In Amphibia the single late formed and radiately striated (Waldeyer) membrane would appear to be a zona radiata. If the suggestion on page 605 turns out to be correct the ova of Mammalia possess both a vitelline membrane and zona radiata. E. van Beneden[421] has, moreover, shewn that they are also provided at a certain period with a delicate membrane within the zona.
The reticulum of the germinal vesicle.—In the course of
description of the ovary it has been necessary for me to enter with some
detail into the structure of the nucleus, and I have had occasion to figure
and describe a reticulum identical with that recently described by so many
observers. The very interesting observations of Dr Klein in the last number
of this Journal[422] have induced me to say one or
two words in defence of some points in my description of the reticulum. Dr
Klein says, on page 323, I have distinctly seen that when nucleoli are
present—the instances are fewer than is generally supposed; they are
accumulations of the fibrils of the network.
I have no doubt that Klein
is correct in asserting that nucleoli are fewer than is generally supposed;
and that in many of these instances what are called nucleoli are
accumulations, natural or artificial,
of the fibrils of the network;
but I cannot accept the universality of the latter statement, which appears
to me most certainly not to hold good in the case of ova, in which nucleoli
frequently exist in the absence of the network.
Again, I find that at the point of intersection of two or more [Pg 613] fibrils there is, as a rule, a distinct thickening of the matter of the fibrils, and that many of the dots seen are not merely, as Dr Klein would maintain, optical sections of fibrils.
It appears to me probable that both the network and the nucleoli are composed of the same material—what Hertwig calls nuclear substance—and if Dr Klein merely wishes to assert this identity in the passage above quoted, I am at one with him.
Although a more or less distinct network is present in most nuclei (I have found it in almost all embryonic nuclei) it is not universally so. In the nuclei of primitive ova I have no doubt that it is absent, though present in the unmodified nuclei of the germinal epithelium; and it is present only in a very modified form in the nuclei of primitive ova undergoing a transformation into permanent ova. The absence of the reticulum does not, of course, mean that the substance capable of forming a reticulum is absent, but merely that it does not assume a particular arrangement.
One of the most interesting points in Klein's paper, as well as in those
of Heitzmann and Eimer, is the demonstration of a connection between the
reticulum of the nucleus and fibres in the body of the cell. Such a
connection I have not found in ova, but may point out that it appears to
exist between the sub-germinal nuclei in Elasmobranchii and the protoplasmic
network in the yolk in which they lie. This point is called attention to in
my Monograph on Elasmobranch Fishes, page 39[423], where it is stated that the network in favourable
cases may be observed to be in connection with the nuclei just described.
Its meshes are finer in the vicinity of the nuclei, and the fibres in some
cases appear almost to start from them.
The nuclei in the yolk are
knobbed bodies divided by a sponge work of septa into a number of areas
each with a nucleolar body.
[Pg 614] EXPLANATION OF PLATES 24, 25, 26.
Plate 24.
List of Reference Letters.
dn. Modified nucleus of primitive ovum. do. Permanent ovum in the act of being formed. dv. Developing blood-vessels. dyk. Developing yolk. ep. Non-ovarian epithelium of ovarian ridge. fe. Follicular epithelium. gv. Germinal vesicle. lstr. Lymphatic region of stroma. nn. Nests of nuclei of ovarian region. o. Permanent ovum. ovr. Ovarian portion of ovarian ridge. po. Primitive ovum. pse. Pseudo-epithelium of ovarian ridge. str. Stroma ingrowths into ovarian epithelium. v. Blood-vessel. vstr. Vascular region of stroma adjoining ovarian ridge. vt. Vitelline membrane. x. Modified nucleus. yk. Yolk. zn. Zona radiata.
Fig. 1. Transverse section of the ovarian ridge of an embryo of Scy. canicula, belonging to stage P, shewing the ovarian region with thickened epithelium and numerous primitive ova. Zeiss C, ocul. 2. Picric acid.
Fig. 2. Transverse section of the ovarian ridge of an embryo of Scyllium canicula, considerably older than stage Q. Zeiss C, ocul. 2. Picric acid. Several nests, some with distinct ova, and others with the ova fused together, are present in the section (n.n.), and several examples of modified nuclei in still distinct ova are also represented. One of these is marked x. The stroma of the ovarian ridge is exceptionally scanty.
Fig. 3. Transverse section through part of the ovarian ridge, including the ovarian region of an almost ripe embryo of Scyllium canicula. Zeiss C, ocul. 2. Picric acid. Nuclear nests (n.n.), developing ova (d.o.), and ova (o.), with completely formed follicular epithelium, are now present. The ovarian region is still well separated from the subjacent stroma, and does not appear to contain any cells except those of the original germinal epithelium.
Fig. 4. Section through ovarian ridge of the same embryo as fig. 3, to illustrate the relation of the stroma (str.) and ovarian region. Zeiss a a, ocul. 2. Picric acid.
Fig. 5. Section through the ovarian ridge of an embryo of Scyllium canicula, 10 cm. long, in which the ovary was slightly less advanced than in fig. 3. To illustrate the relation of the ovarian epithelium to the subjacent vascular stroma. Zeiss A, ocul. 2. Osmic acid. y. points to a small separated portion of the germinal epithelium.
Fig. 6. Section through the ovarian ridge of an embryo of Scyllium canicula, slightly older than fig. 5. To illustrate the relation of the ovarian epithelium to the subjacent vascular[TN12] stroma. Zeiss A, ocul. 2. Osmic acid.
Fig. 7. More highly magnified portion of the same ovary as fig. 6. To illustrate the same points. Zeiss C, ocul. 2. Osmic acid.
[Pg 615]Fig. 8. Section through the ovarian region (close to one extremity, where it is very small) from a young female of Scy. canicula. Zeiss C, ocul. 2. Picric acid. It shews the vascular ingrowths amongst the original epithelial cells of the ovarian region.
Fig. 9. Section through the ovarian region of the same embryo as fig. 8, at its point of maximum development. Zeiss A, ocul. 2. Picric acid.
Fig. 10. Section through superficial part of the ovary of an embryo, shewing the pseudo-epithelium; the cells of which are provided with tails prolonged into the general tissue of the ovary. At f.e. is seen a surface view of the follicular epithelium of an ovum. Zeiss C, ocul. 2. Picric acid.
Fig. 11. Section through part of an ovary of Scyllium canicula of stage Q, with three primitive ova, the most superficial one containing a modified nucleus.
Fig. 12. Section through part of an ovary of an example of Scyllium canicula, 8 cm. long. The section passes through a nest of ova with modified nuclei, in which the outlines of the individual ova are quite distinct. Zeiss E, ocul. 2. Picric acid.
Fig. 13. Section through part of ovary of the same embryo as in fig. 5. The section passes through a nest of nuclei, with at the least two developing ova, and also through one already formed permanent ovum. Zeiss E, ocul. 2. Osmic acid.
Figs. 14, 15, 16, 17, 18 [Figs. 17 and 18 are on Pl. 25]. Sections through parts of the ovary of the same embryo as fig. 3, with nests of nuclei and a permanent ova in the act of formation. Fig. 14 is drawn with Zeiss D D, ocul. 2. Figs. 15, 16, 17, with Zeiss E, ocul. 2. Picric acid.
Plate 25.
List of Reference Letters.
do. Permanent ovum in the act of being formed. dyk. Developing yolk. fe. Follicular epithelium. fe´. Secondary follicular epithelium. gv. Germinal vesicle. nn. Nests of nuclei of ovarian region. o. Permanent ovum. pse. Pseudo-epithelium. str. Stroma ingrowths into ovarian epithelium. vt. Vitelline membrane. x. Modified nucleus. yk. Yolk (vitellus). zn. Zona radiata.
[Figs. 17 and 18. Vide description of Plate 24.].
Fig. 19. Two nuclei from a nest which appear to be in the act of division. From ovary of the same embryo as fig. 3.
Fig. 20. Section through part of an ovary of the same embryo as fig. 6, containing a nest of nuclei. Zeiss F, ocul. 2. Osmic acid.
Fig. 21. Ovum from the ovary of a half-grown female, containing isolated deeply stained patches of developing yolk granules. Zeiss B, ocul. 2. Picric acid.
Fig. 22. Section through a small part of the ovum of an immature female of Scyllium canicula, to shew the constitution of the yolk, the follicular epithelium, and the egg membranes. Zeiss E, ocul. 2. Chromic acid.
Fig. 23. Section through part of the periphery of a nearly ripe ovum of Scy. canicula. Zeiss C, ocul. 2. It shews the remnant of the vitelline membrane (v.t.) separating the columnar but delicate cells of the follicular epithelium (f.e.) from the yolk (yk.). In the yolk are seen yolk-spherules in a protoplasmic network. The transverse markings in the yolk-spherules have been made oblique by the artist.
[Pg 616] Fig. 24. Fully formed ovum containing a second nucleus (x), probably about to be employed as pabulum; from the same ovary as fig. 5. The follicular epithelium is much thicker on the side adjoining the stroma than on the upper side of the ovum. Zeiss F, ocul. 2. Osmic acid.
Fig. 25. A. Ovum from the same ovary as fig. 21, containing in the yolk three peculiar bodies, similar in appearance to the two small bodies in the germinal vesicle. B. Germinal vesicle of a large ovum from the same ovary, containing a body of a strikingly similar appearance to those in the body of the ovum in A. Zeiss E, ocul. 2. Picric acid.
Fig. 26. Section of the ovary of a young female of Scyllium stellare 16½ centimetres in length. The ovary is exceptional, on account of the large size of the stroma ingrowths into the epithelium. Zeiss C, ocul. 2. Osmic acid.
Fig. 27. Ovum of Scyllium canicula, 5 mm. in diameter, treated with osmic acid. The figure illustrates the development of the yolk and a peculiar mode of proliferation of the germinal spots. Zeiss A, ocul. 2.
Fig. 28. Small part of the follicular epithelium and egg membranes of a somewhat larger ovum of Scyllium canicula than fig. 22. Zeiss D D, ocul. 2.
Fig. 29. The same parts as in fig. 28, from a still larger ovum. Zeiss D D, ocul. 2.
Fig. 30. Ovum of Raja with follicular epithelium. Zeiss C, ocul. 2.
Fig. 31. Small portion of a larger ovum of Raja than fig. 30. Zeiss D D, ocul. 2.
Fig. 32. Follicular epithelium, &c., from an ovum of Raja still larger than fig. 31. Zeiss D D, ocul. 2.
Fig. 33. Surface view of follicular epithelium from an ovum of Raja of about the same age as fig. 33.
Fig. 34. Vertical section through the superficial part of an ovary of an adult Raja to shew the relation of the pseudo-epithelium to the subjacent stroma. Zeiss D D, ocul. 2.
Plate 26.
Complete List of Reference Letters.
do. Developing ovum. fc. Cells which will form the follicular epithelium, fe. Follicular epithelium. ge. Germinal epithelium. mg. Malpighian body. n. Nest of cells of the germinal epithelium. nd. Nuclei in the act of dividing. o. Permanent ovum. ov. Ovary. po. Primitive ovum. t. Tubuliferous tissue, derived from Malpighian bodies.
Fig. 35. Transverse section through the ovary of an embryo rabbit of eighteen days, hardened in osmic acid. The colours employed are intended to render clear the distinction between the germinal epithelium (ge.) and the tubuliferous tissue (t.), which has grown in from the Wolffian body, and which gives rise in the male to parts of the tubuli seminiferi. Zeiss A, ocul. 2.
[Pg 617]Fig. 35A. Transverse section through a small part of the ovary of an embryo from the same female as fig. 35, hardened in picric acid, shewing the relation of the germinal epithelium to the subjacent tissue. Zeiss D D, ocul. 2.
Fig. 35B. Longitudinal section through part of the Wolffian body and the anterior end of the ovary of an eighteen days' embryo, to shew the derivation of tubuliferous tissue (t.) from the Malpighian bodies, close to the anterior extremity of the ovary. Zeiss A, ocul. 1.
Fig. 36. Transverse section through the ovary of an embryo rabbit of twenty-two days, hardened in osmic acid. It is coloured in the same manner as fig. 35. Zeiss A, ocul. 2.
Fig. 36A. Transverse section through a small part of the ovary of an embryo, from the same female as fig. 36, hardened in picric acid, shewing the relation of the germinal epithelium to the stroma of the ovary. Zeiss D D, ocul. 2.
Figs. 37 and 37A. The same parts of an ovary of a twenty-eight days' embryo as figs. 36 and 36A of a twenty-two days' embryo.
Fig. 38. Ovary of a rabbit five days after birth, coloured in the same manner as figs. 35, 36 and 37, but represented on a somewhat smaller scale. Picric acid.
Fig. 38A. Vertical section through a small part of the surface of the same ovary as fig. 38. Zeiss D D, ocul. 2.
Fig. 38B. Small portion of the deeper layer of the germinal epithelium of the same ovary as fig. 38. The figure shews the commencing differentiation of the cells of the germinal epithelium into true ova and follicle cells. Zeiss D D, ocul. 2.
Fig. 39A. Section through a small part of the middle region of the germinal epithelium of a rabbit seven days after birth. Zeiss D D, ocul. 2.
Fig. 39B. Section through a small part of the innermost layer of the germinal epithelium of a rabbit seven days after birth, shewing the formation of Graafian follicles. Zeiss D D, ocul. 2.
Figs. 40A and 40B. Small portions of the middle region of the germinal epithelium of a rabbit four weeks after birth. Zeiss D D, ocul. 2.
Fig. 41. Graafian follicle with two ova, about to divide into two follicles, from a rabbit six weeks after birth. Zeiss D D, ocul. 2.
[370] From the Quarterly Journal of Microscopical Science, Vol. 18, 1878.
[371] Arbeiten a. d. zool.-zoot. Institut Würzburg, Bd. I.
[372] Archiv f. micr. Anat. Vol. XI.
[373] Arbeiten a. d. zool.-zoot. Institut Würzburg, Bd. II.
[374] Loc. cit. p. 361.
[375] Archiv f. micr. Anat. Vol. XIV.
[376] Vide especially Klein, Quart. Journ. of Mic. Sci. July 1878.
[377] Rochen u. Haie.
[378] By chorion I mean, following E. van Beneden's nomenclature, a membrane formed by the follicular epithelium, and, by vitelline membrane, one formed by the vitellus or body of the ovum.
[379]
Bau und Entwicklung d. Wirbelthiereier,
&c., Müll. Archiv, 1861.
[380]
Zur Entwicklungsgeschichte d. Selachier,
Arch. f. mikr. Anat. Vol.
XI.
[381] The apparent structure in the vitelline membrane in my figure is merely intended to represent the dark colour assumed by it on being stained. The zona radiata has been made rather too thick by the artist.
[382] Loc. cit.
[383] Loc. cit.
[384] Loc. cit.
[385] Das Ei bei Knochenfischen.
[386] Arch. f. Anat. Phys. 1877.
[387] Archiv f. mikr. Anat. Vol. VIII.
[388]
Braun, Urogenitalsystem d. Amphibien,
Arbeiten a. d. zool.-zoot. Institut Würzburg, Bd.
IV. He says,
in reference to the flask-shaped cell, p. 166, Höchstens
würde ich die Funktion der grossen Follikelzellen als einzellige
Drüsen mehr betonen.
[389] Loc. cit.
[390] Loc. cit.
[391] Das Ei bei Knochenfischen.
[392]
Ovum
in Todd's Encyclopædia, fig. 69.
[393] The peculiar oval, or at times slightly rectangular and striated yolk spherules of Elasmobranchii are mentioned by Leydig and Gegenbaur (Pl. 11, fig. 20), and myself, Preliminary Account of Development of Elasmobranch Fishes, and by Filippi and His in Osseous Fishes.
[394]
Untersuchung über die Eier d. Reptilien,
Archiv f. mikros. Anat. Vol.
VIII.
[395]
Vide Allen Thomson, article Ovum,
Todd's
Encyclopædia, p. 95.
[396] Loc. cit.
[397] Loc. cit.
[398] Compare, with reference to several points, the germinal vesicle at this stage with the germinal vesicle of the frog's ovum figured by O. Hertwig, Morphologisches Jahrbuch, Vol. III. pl. 4, fig. 1.
[399] Loc. cit.
[400] Die Eierstöcke d. Säugethiere u. d. Menschen, Leipzig, 1863.]
[401]
Composition et Signification de l'œuf,
Acad. r. de Belgique, 1868.
[402] Eierstock u. Ei. Leipzig, 1870.
[403] Trans. of Royal Society, Edinburgh, Vol. XXVII. 1875, and Quarterly Journal of Microscopical Science, Vol. XVI.
[404] Verhandlung d. Phys. Med. Gesellschaft, Würzburg, 1875, N. F. Bd. VIII.
[405] Arbeiten a. d. Zool.-zoot. Institut Würzburg, Bd. IV.
[406] Archiv f. mikros. Anat. Vol. I. p. 160.
[407] Loc. cit.
[408] Archiv f. mikr. Anat. Vol. X.
[409] Archiv f. Anatomie, Physiologie, u. Wiss. Medicin. 1874.
[410] Loc. cit.
[411] Loc. cit.
[412] Loc. cit., Waldeyer, p. 23, denies the existence of this membrane for Mammalia. It certainly is not so conspicuous as in some other types, but appears to me nevertheless to be always present.
[413] My view of the structure of the ovary would seem to be that held by Götte, Entwicklungsgeschichte d. Unke, pp. 14 and 15.
[414] Loc. cit. 36.
[415] Since writing the above I have made out that in the Reptilia the formation of the permanent ova takes place in the same fashion as in Elasmobranchii and Mammalia.
[416] Entwicklungsgeschichte d. Unke.
[417] Zeit. für wiss. Zool. Bd. XXVII.
[418] Gegenbaur, loc. cit.; Waldeyer, loc. cit.; Eimer, loc. cit.; and Ludwig, loc. cit.
[419] Gegenbaur, Waldeyer, E. van Beneden, Eimer.
[420] Carlberla, Zeit. f. wiss. Zool. Bd. XXX.
[421] Loc. cit.
[422] [Quarterly Journal Microscopical Science, July 1878.]
(With Plates 27 and 28.)
The following paper is divided into three sections. The first
of these records the existence of certain structures in the embryo chick,
which eventually become in part the abdominal opening of the Müllerian
duct, and which, we believe, correspond with the head-kidney, or Vorniere
of German authors. The second deals with the growth
and development of the Müllerian duct. With reference to this we have come
to the conclusion that the Müllerian duct does not develop entirely
independently of the Wolffian duct. The third section of our paper is of a
more general character, and contains a discussion of the rectifications in
the views of the homologies of the parts of the excretory system in Aves,
necessitated by the results of our investigations.
We have, as far as possible, avoided entering into the extended literature of the excretory system, since this has been very fully given in three general papers which have recently appeared by Semper[425], Fürbinger[426], and by one of us[427].
All recent observers, including Braun[428] for Reptilia, and Egli[429] for Mammalia, have stated that the Müllerian duct develops as [Pg 619] a groove in the peritoneal epithelium, which is continued backward as a primitively solid rod in the space between the Wolffian duct and peritoneal epithelium.
In our preliminary account we stated[430], in accordance with the general view, that the Müllerian duct was formed as a groove, or elongated involution of the peritoneal epithelium adjoining the Wolffian duct. We have now reason to believe that this is not the case. In the earliest condition of the Müllerian duct which we have been able to observe, it consists of three successive open involutions of the peritoneal epithelium, connected together by more or less well-defined ridge-like thickenings of the epithelium. We believe, on grounds hereafter to be stated, that the whole of this formation is equivalent to the head-kidney of the Ichthyopsida. The head-kidney, as we shall continue to call it, takes its origin from the layer of thickened epithelium situated near the dorsal angle of the body-cavity, close to the Wolffian duct, which has been known since the publication of Waldeyer's important researches as the germinal epithelium. The anterior of the three open involutions or grooves is situated some little distance behind the front end of the Wolffian duct. It is simply a shallow groove in the thickest part of the germinal epithelium, and forms a corresponding projection into the adjacent stroma. In front the projection is separated by a considerable interval from the Wolffian duct; but near its hindermost part it almost comes into contact with the Wolffian duct. The groove extends in all for about five of our sections, and then terminates by its walls becoming gradually continued into a slight ridge-like thickening of the germinal epithelium. The groove arises as a simple depression in a linear area of thickened germinal epithelium. The linear area is, however, continued very considerably further forward than the groove, and sometimes exhibits a slight central depression, which might be regarded as a forward continuation of the groove. The passage from the groove to the ridge may best be conceived by supposing the groove to be suddenly filled up, so as to form a solid ridge pointing inwards towards the Wolffian duct.
The ridge succeeding the first groove is continued for about six sections, and is considerably more prominent at its posterior [Pg 620] extremity than in front. It is replaced by groove number two, which appears as if formed by the reverse process to that by which the ridge arose, viz., by a hollowing out of the ridge on the side towards the body-cavity. The wall of the second groove is, after a few sections, continued into a second ridge or thickening of the germinal epithelium, which, however, is so faintly marked as to be hardly visible in its middle part. In its turn this ridge is replaced by the third and last groove. This vanishes after one or two sections, and behind the point of its disappearance we have failed to find any further traces of the head-kidney. The whole formation extends through about twenty-four of our sections and one and a half segments (muscle-plates).
We have represented (Plate 27, Series A, Nos. 1-10) a fairly complete series of sections through part of the head-kidney of an embryo slightly older than that last described, containing the second and third grooves and accessory parts. The connection between the grooves and the ridges is very well illustrated in Nos. 3, 4, and 5 of this series. In No. 3 we have a prominent ridge, in the interior of which there appears in No. 4 a groove, which becomes gradually wider in Nos. 5 and 6. Both the grooves and ridges are better marked in this than in the younger stage; but the chief difference between the two stages consists in the third groove no longer forming the hindermost limit of the head-kidney. Instead of this, the last groove (No. 7) terminates by the upper part of its walls becoming constricted off as a separate rod, which appears at first to contain a lumen continuous with the open groove. This rod (Nos. 7, 8, 9, 10) situated between the germinal epithelium and Wolffian duct is continued backward for some sections. It finally terminates by a pointed extremity, composed of not more than two cells abreast (Nos. 8-10).
Our third stage, sections of which are represented in series B (Plate 27), is considerably advanced beyond that last described. The most important change which has been effected concerns the ridges connecting the successive grooves. A lumen has appeared in each of these, which seems to open at both ends into the adjacent grooves. At the same time the cells, which previously constituted the ridge, have become (except where [Pg 621] they are continuous with the walls of the grooves) partially constricted off from the germinal epithelium. The ridges, in fact, now form ducts situated in the stroma of the ovarian ridge, in the space between the Wolffian duct and the germinal epithelium. The duct continuous with the last groove is somewhat longer than before. In a general way, the head-kidney may now be described as a duct opening into the body-cavity by three groove-like apertures, and continuous behind with the rudiment of the true Müllerian duct. Although the general constitution of the head-kidney at this stage is fairly simple, there are a few features in our sections which we do not fully understand, and a few points about the organ which deserve a rather fuller description than we have given in this general sketch.
The anterior groove (Nos. 1-3, series B, Pl. 27) is at first somewhat separated from the Wolffian duct, but approaches close to it in No. 3. In Nos. 2 and 3 there appears a rod-like body on the outer side of the walls of the groove. In No. 2 this body is disconnected with the walls of the groove, and even appears as if formed by a second invagination of the germinal epithelium. In No. 3 this body becomes partially continuous with the walls of the groove, and finally in No. 4 it becomes completely continuous with the walls of the groove, and its lumen communicates freely with the groove[431].
The last trace of this body is seen on the upper wall of the groove in No. 5. We believe that the body (r1) represents the ridge between the first and second grooves of the earlier stage; so that in passing from No. 3 to No. 5 we pass from the first to the second groove. The meaning of the features of the body r1 in No. 2 we do not fully understand, but cannot regard them as purely accidental, since we have met with more or less similar features in other series of sections. The second groove becomes gradually narrower, and finally is continued into the second ridge (No. 8). The ridge contains a lumen, and is only connected with the germinal epithelium by a narrow wall of cells. A narrow passage from the body-cavity leads into that wall for a short distance in No. 8, but it is probably merely the hinder end of the groove of No. 7. The third groove appears in No. 11, [Pg 622] and opens into the lumen of the second ridge (r2) in No. 12. In No. 13 the groove is closed, and there is present in its place a duct (r3) connected with the germinal epithelium by a wall of cells. This duct is the further development of the third ridge of the last stage; its lumen opens into the body-cavity through the third and last groove (gr3). In the next section this duct (r3) is entirely separated from the germinal epithelium, and it may be traced backwards through several sections until it terminates by a solid point, very much as in the last stage.
In the figures of this series (B) there may be noticed on the outer side of the Müllerian duct a fold of the germinal epithelium (x) forming a second groove. It is especially conspicuous in the first six sections of the series. This fold sometimes becomes much deeper, and then forms a groove, the upper end of which is close to the grooves of the head-kidney. It is very often much deeper than these are, and without careful study might easily be mistaken for one of these grooves. Fig. C, taken from a series slightly younger than B, shews this groove (x) in its most exaggerated form.
The stage we have just described is that of the fullest development of the head-kidney. In it, as in all the previous stages, there appear to be only three main openings into the body-cavity; but we have met in some of our sections with indications of the possible presence of one or two extra rudimentary grooves.
In an embryo not very much older than the one last described the atrophy of the head-kidney is nearly completed, and there is present but a single groove opening into the body-cavity.
In series D (Pl. 28) are represented a number of sections from an embryo at this stage. Nos. 1 and 2 are sections through the hind end of the single groove now present. Its walls are widely separated from the Wolffian duct in front, but approach close to it at the hinder termination of the groove (No. 2). The features of the single groove present at this stage agree closely with those of the anterior groove of the previous stages. The groove is continued into a duct—the Müllerian duct (as it may now be called, but in a previous stage the hollow ridge connecting the first and second grooves of the head-kidney)—which, [Pg 623] after becoming nearly separated from the germinal epithelium, is again connected to it by a mass of cells at two points (Nos. 5, 6, and 8). The germinal epithelium is slightly grooved and is much reduced in thickness at these points of contact (gr2 and gr3), and we believe that they are the remnants of the posterior grooves of the head-kidney present at an earlier stage.
The Müllerian duct has by this stage grown much further backwards, but the peculiarities of this part of it are treated in a subsequent section.
We consider that, taking into account the rudiments we have just described, as well as the fact that the features of the single groove at this stage correspond with those of the anterior groove at an earlier stage, we are fully justified in concluding that the permanent abdominal opening of the Müllerian duct corresponds with the anterior of our three grooves.
Although we have, on account of their indefiniteness, avoided giving the ages of the chicks in which the successive changes of the head-kidney may be observed, we may, perhaps, state that all the changes we have described are usually completed between the 90th and 120th hour of incubation.
The Glomerulus of the Head-Kidney.
In connection with the head-kidney in Amphibians there is present, as is well known, a peculiar vascular body usually described as the glomerulus of the head-kidney. We have found in the chick a body so completely answering to this glomerulus that we have hardly any hesitation in identifying it as such.
In the chick the glomerulus is paired, and consists of a vascular outgrowth or ridge projecting into the body-cavity on each side at the root of the mesentery. It extends from the anterior end of the Wolffian body to the point where the foremost opening of the head-kidney commences. We have found it at a period slightly earlier than that of the first development of the head-kidney. It is represented in figs. E and F, Pl. 28, gl, and is seen to form a somewhat irregular projection into the body-cavity, covered by a continuation of the peritoneal epithelium, [Pg 624] and attached by a narrow stalk to the insertion of the embryonic mesentery (me).
In the interior of this body is seen a stroma with numerous vascular channels and blood corpuscles, and a vascular connection is apparently becoming established, if it is not so already, between the glomerulus and the aorta. We have reason to think that the corpuscles and vascular channels in the glomerulus are developed in situ. The stalk connecting the glomerulus with the attachment of the mesentery varies in thickness in different sections, but we believe that the glomerulus is continued unbroken throughout the very considerable region through which it extends. This point is, however, difficult to make sure of owing to the facility with which the glomerulus breaks away.
At the stage we are describing, no true Malpighian bodies are present in
the part of the Wolffian body on the same level with the anterior end of
the glomerulus, but the Wolffian body merely consists of the Wolffian duct.
At the level of the posterior part of the glomerulus this is no longer the
case, but here a regular series of primary Malpighian bodies is present
(using the term primary
to denote the Malpighian bodies developed
directly out of part of the primary segmental tubes), and the glomerulus of
the head-kidney may frequently be seen in the same section as a Malpighian
body. In most sections the two bodies appear quite disconnected, but in
those sections in which the glomerulus of the Malpighian body
comes into view it is seen to be derived from the same formation as the
glomerulus of the head-kidney (Pl. 28, fig.
F). It would seem, in fact, that the
vascular tissue of the glomerulus of the head-kidney grows into the
concavity of the Malpighian bodies. Owing to the stage we are now
describing, in which we have found the glomerulus most fully developed,
being prior to that in which the head-kidney appears, it is not possible to
determine with certainty the position of the glomerulus in relation to the
head-kidney. After the development of the head-kidney it is found, however,
as we have already stated, that the glomerulus terminates at a point just
in front of the anterior opening of the head-kidney. It is less developed
than before, but is still present up to the period of the atrophy of the
head-kidney. It [Pg 625] does not apparently alter in constitution,
and we have not thought it worth while giving any further representations
of it during the later stages of its existence.
Summary of the development of the head-kidney and glomerulus.—The first rudiment of the head-kidney arises as three successive grooves in the thickened germinal epithelium, connected by ridges, and situated some way behind the front end of the Wolffian duct. In the next stage the three ridges connecting the grooves have become more marked, and in each of them a lumen has appeared, opening at both extremities into the adjoining grooves. Still later the ridges become more or less completely detached from the peritoneal epithelium, and the whole head-kidney then consists of a slightly convoluted duct, with, at the least, three peritoneal openings, which is posteriorly continued into the Müllerian duct. Still later the head-kidney atrophies, its two posterior openings vanishing, and its anterior opening remaining as the permanent opening of the Müllerian duct. The glomerulus arises as a vascular prominence at the root of the mesentery, slightly prior in point of time to the head-kidney, and slightly more forward than it in position. We have not traced its atrophy.
We stated in our preliminary paper that the peculiar structures we had
interpreted as the head-kidney had completely escaped the attention of
previous observers, though we called attention to a well-known figure of
Waldeyer's (copied in the Elements of Embryology, fig. 51). In
this figure a connection between the germinal epithelium and the Müllerian
duct is drawn, which is probably part of the head-kidney, and may be
compared with our figures (Series B, No. 8, and Series D, No. 4). Since we made
the above statement, Dr Gasser has called our attention to a passage in his
valuable memoir on The Development of the Allantois[432],
in which certain structures are described which
are, perhaps, identical with our head-kidney. The following is a
translation of the passage:—
In the upper region of Müller's duct I have often
observed small canals, especially in the later stages of development, which
appear as a kind of doubling of the duct, and run for a short [Pg 626] distance
close to Müller's duct and in the same direction, opening, however, into
the body-cavity posterior to the main duct. Further, one may often observe
diverticula from the extreme anterior end of the oviduct of the bird, which
form blind pouches and give one the impression of being receptacula
seminis. Both these appearances can quite well be accounted for on the
supposition that an abnormal communication is effected between the germinal
epithelium and Müller's duct at unusual places; or else that an attempt at
such a communication is made, resulting, however, only in the formation of
a diverticulum of the wall of the oviduct.
The statement that these accessory canals are late in developing, prevents us from feeling quite confident that they really correspond with our head-kidney.
Before passing on to the other parts of this paper it is necessary to say a few words in justification of the comparison we have made between the modified abdominal extremity of the Müllerian duct in the chick and the head-kidney of the Ichthyopsida.
For the fullest statement of what is known with reference to the anatomy and development of the head-kidney in the lower types we may refer to Spengel and Fürbringer[433]. We propose ourselves merely giving a sufficient account of the head-kidney in Amphibia (which appears to be the type in which the head-kidney can be most advantageously compared with that in the bird) to bring out the grounds for our determination of the homologies.
The development of the head-kidney in Amphibia has been fully elucidated by the researches of W. Müller[434], Götte[435], and Fürbringer[436], while to the latter we are indebted for a knowledge of the development of the Müllerian duct in Amphibians. The first part of the urinogenital system to develop is the segmental duct (Vornieregang of Fürbringer), which is formed by a groove-like invagination of the peritoneal epithelium. It becomes constricted into a duct first of all in the middle, but soon in the [Pg 627] posterior part also. It then forms a duct, ending in front by a groove in free communication with the body-cavity, and terminating blindly behind. The open groove in front at first deepens, and then becomes partially constricted into a duct, which elongates and becomes convoluted, but remains in communication with the body-cavity by from two to four (according to the species) separate openings. The manner in which the primitive single opening is related to the secondary openings is not fully understood. By these changes there is formed out of the primitive groove an anterior glandular body, communicating with the body-cavity by several apertures, and a posterior duct, which carries off the secretion of the gland, and which, though blind at first, eventually opens into the cloaca. In addition to these parts there is also formed on each side of the mesentery, opposite the peritoneal openings, a very vascular projection into this part of the body-cavity, which is known as the glomerulus of the head-kidney, and which very closely resembles in structure and position the body to which we have assigned the same name in the chick.
The primitive segmental duct is at first only the duct for the head-kidney, but on the formation of the posterior parts of the kidney (Wolffian body, &c.) it becomes the duct for these also.
After the Wolffian bodies have attained to a considerable development, the head-kidney undergoes atrophy, and its peritoneal openings become successively closed from before backwards. At this period the formation of the Müllerian duct takes place. It is a solid constriction of the ventral or lateral wall of the segmental duct, which subsequently becomes hollow, and acquires an opening into the body-cavity quite independent of the openings of the head-kidney.
The similarity in development and structure between the head-kidney in Amphibia and the body we have identified as such in Aves, is to our minds too striking to be denied. Both consist of two parts—(1) a somewhat convoluted longitudinal canal, with a certain number of peritoneal openings; (2) a vascular prominence at the root of the mesentery, which forms a glomerulus. As to the identity in position of the two organs we hope to deal with that more fully in speaking of the general [Pg 628] structure of the excretory system, but may say that one of us[437] has already, on other grounds, attempted to shew that the abdominal opening of the Müllerian duct in the bird is the homologue of the abdominal opening of the segmental duct in Amphibia, Elasmobranchii, &c., and that we believe that this homology will be admitted by most anatomists. If this homology is admitted, the identity in position of this organ in Aves and Amphibia necessarily follows. The most striking difference between Aves and Amphibia in relation to these structures is the fact that in Aves the anterior pore of the head-kidney remains as the permanent opening of the Müllerian duct, while in Amphibia, the pores of the head-kidney atrophy, and an entirely fresh abdominal opening is formed for the Müllerian duct.
II.
The Growth of the Müllerian Duct.
Although a great variety of views have been expressed by different observers on the growth of the Müllerian duct, it is now fairly generally admitted that it grows in the space between a portion of the thickened germinal epithelium and the Wolffian duct, but quite independently of both of them. Both Braun and Egli, who have specially directed their attention to this point, have for Reptilia and Mammalia fully confirmed the views of previous observers. We were, nevertheless, induced, partly on account of the à priori difficulties of this view, and partly by certain peculiar appearances which we observed, to undertake the re-examination of this point, and have found ourselves unable altogether to accept the general account. We propose first describing, in as matter-of-fact a way as possible, the actual observations we have made, and then stating what conclusions we think may be drawn from these observations.
We have found it necessary to distinguish three stages in the growth of the Müllerian duct. Our first stage embraces the [Pg 629] period prior to the disappearance of the head-kidney. At this stage the structure we have already spoken of as the rudiment of the Müllerian duct consists of a solid rod of cells, continuous with the third groove of the head-kidney. It extends through a very few sections, and terminates by a fine point of about two cells, wedged in between the Wolffian duct and germinal epithelium (described above, Nos. 7-10, series A, Plate 27).
In an embryo slightly older than the above, such as that from which series B was taken, but still belonging to our first stage, a definite lumen appears in the anterior part of the Müllerian duct, which vanishes after a few sections. The duct terminates in a point which lies in a concavity of the wall of the Wolffian duct (Plate 27, Nos. 1 and 2, series G). The limits of the Wolffian wall and the pointed termination of the Müllerian duct are in many instances quite distinct; but the outline of the Wolffian duct appears to be carried round the Müllerian duct, and in some instances the terminal point of the Müllerian duct seems almost to form an integral part of the wall of the Wolffian duct.
The second of our stages corresponds with that in which the atrophy of the head-kidney is nearly complete (series D and H, Plate 28).
The Müllerian duct has by this stage made a very marked progress in its growth towards the cloaca, and, in contradistinction to the earlier stage, a lumen is now continued close up to the terminal point of the duct. In the two or three sections before it ends it appears as a distinct oval mass of cells (No. 10, series D, and No. 1, series H), without a lumen, lying between and touching the external wall of the Wolffian duct on the one hand, and the germinal epithelium on the other. It may either lie on the ventral side of the Wolffian duct (series D), or on the outer side (series H), but in either case is opposite the maximum thickening of that part of the germinal epithelium which always accompanies the Müllerian duct in its backward growth.
In the last section in which any trace of the Müllerian duct can be made out (series D, No. 11, and series H, No. 2), it has no longer an oval, well-defined contour, but appears to have completely fused with the wall of the Wolffian duct, which is accordingly very thick, and occupies the space which in the previous [Pg 630] section was filled by its own wall and the Müllerian duct. In the following section the thickening in the wall of the Wolffian duct has disappeared (Plate 28, series H, No. 3), and every trace of the Müllerian duct has vanished from view. The Wolffian duct is on one side in contact with the germinal epithelium.
The stage during which the condition above described lasts is not of long duration, but is soon succeeded by our third stage, in which a fresh mode of termination of the Müllerian duct is found. (Plate 28, series I.) This last stage remains up to about the close of the sixth day, beyond which our investigations do not extend.
A typical series of sections through the terminal part of the Müllerian duct at this stage presents the following features:
A few sections before its termination the Müllerian duct appears as a well-defined oval duct lying in contact with the wall of the Wolffian duct on the one hand and the germinal epithelium on the other (series I, No. 1). Gradually, however, as we pass backwards, the Müllerian duct dilates; the external wall of the Wolffian duct adjoining it becomes greatly thickened and pushed in in its middle part, so as almost to touch the opposite wall of the duct, and so form a bay in which the Müllerian duct lies (Plate 28, series I, Nos. 2 and 3). As soon as the Müllerian duct has come to lie in this bay its walls lose their previous distinctness of outline, and the cells composing them assume a curious vacuolated appearance. No well-defined line of separation can any longer be traced between the walls of the Wolffian duct and those of the Müllerian, but between the two is a narrow clear space traversed by an irregular network of fibres, in some of the meshes of which nuclei are present.
The Müllerian duct may be traced in this condition for a considerable number of sections, the peculiar features above described becoming more and more marked as its termination is approached. It continues to dilate and attains a maximum size in the section or so before it disappears. A lumen may be observed in it up to its very end, but is usually irregular in outline and frequently traversed by strands of protoplasm. The Müllerian duct finally terminates quite suddenly (Plate 28, series I, No. 4), and in the section immediately behind its termination the Wolffian duct assumes its normal appearance, and the part of [Pg 631] its outer wall on the level of the Müllerian duct comes into contact with the germinal epithelium (Plate 28, series I, No. 5).
We have traced the growing point of the Müllerian duct with the above features till not far from the cloaca, but we have not followed the last phases of its growth and its final opening into the cloaca.
In some of our embryos we have noticed certain rather peculiar structures, an example of which is represented at y in fig. K, taken from an embryo of 123 hours, in which all traces of the head-kidney had disappeared. It consists of a cord of cells, connecting the Wolffian duct and the hind end of the abdominal opening of the Müllerian duct. At the least one similar cord was met with in the same embryo, situated just behind the abdominal opening of the Müllerian duct. We have found similar structures in other embryos of about the same age, though never so well marked as in the embryo from which fig. K is taken. We have quite failed to make out the meaning, if any, of them.
Our interpretation of the appearances we have described in connection with the growth of the Müllerian duct can be stated in a very few words. Our second stage, where the solid point of the Müllerian duct terminates by fusing with the walls of the Wolffian duct, we interpret as meaning that the Müllerian is growing backwards as a solid rod of cells, split off from the outer wall of the Wolffian duct; in the same manner, in fact, as in Amphibia and Elasmobranchii. The condition of the terminal part of the Müllerian duct during our third stage cannot, we think, be interpreted in the same way, but the peculiarities of the cells of both Müllerian and Wolffian ducts, and the indistinctness of the outlines between them, appear to indicate that the Müllerian duct grows by cells passing from the Wolffian duct to it. In fact, although in a certain sense the growth of the two ducts is independent, yet the actual cells which assist in the growth of the Müllerian duct are, we believe, derived from the walls of the Wolffian duct.
General considerations.
The excretory system of a typical Vertebrate consists of the following parts:—
1. A head-kidney with the characters already described.
2. A duct for the head-kidney—the segmental duct.
3. A posterior kidney—(Wolffian body, permanent kidney, &c. The nature and relation of these parts we leave out of consideration, as they have no bearing upon our present investigations). The primitive duct for the Wolffian body is the segmental duct.
4. The segmental duct may become split into (a) a dorsal or inner duct, which serves as ureter (in the widest sense of the word); and (b) a ventral or outer duct, which has an opening into the body-cavity, and serves as the generative duct for the female, or for both sexes.
These parts exhibit considerable variations both in their structure and development, into some of which it is necessary for us to enter.
The head-kidney[438] attains to its highest development in the Marsipobranchii (Myxine, Bdellostoma). It consists of a longitudinal canal, from the ventral side of which numerous tubules pass. These tubules, after considerable subdivision, open by a large number of apertures into the pericardial cavity. From the longitudinal canal a few dorsal diverticula, provided with glomeruli, are given off. In the young the longitudinal canal is continued into the segmental duct; but this connection becomes [Pg 633] lost in the adult. The head-kidney remains, however, through life. In Teleostei and Ganoidei (?) the head-kidney is generally believed to remain through life, as the dilated cephalic portion of the kidneys when such is present. In Petromyzon and Amphibia the head-kidney atrophies. In Elasmobranchii the head-kidney, so far as is known, is absent.
The development of the segmental duct and head-kidney (when present) is still more important for our purpose than their adult structure.
In Myxine the development of these structures is not known. In Amphibia and Teleostei it takes place upon the same type, viz. by the conversion of a groove-like invagination of the peritoneal epithelium into a canal open in front. The head-kidney is developed from the anterior end of this canal, the opening of which remains in Teleostei single and closes early in embryonic life, but becomes in Amphibia divided into two, three, or four openings. In Elasmobranchii the development is very different.
The first trace of the urinary system makes its
appearance as a knob springing from the intermediate cell-mass opposite the
fifth protovertebra. This knob is the rudiment of the abdominal opening of
the segmental duct, and from it there grows backwards to the level of the
anus a solid column of cells, which constitutes the rudiment of the
segmental duct itself. The knob projects towards the epiblast, and the
column connected with it lies between the mesoblast and epiblast. The knob
and column do not long remain solid, but the former acquires an opening
into the body-cavity continuous with a lumen, which makes its appearance in
the latter.
The difference in the development of the segmental duct in the two types (Amphibia and Elasmobranchii) is very important. In the one case a continuous groove of the peritoneal epithelium becomes constricted into a canal, in the other a solid knob of cells is continued into a rod, at first solid, which grows backwards without any apparent relation to the peritoneal epithelium[439].
[Pg 634] The abdominal aperture of the segmental duct in Elasmobranchii, in that it becomes the permanent abdominal opening of the oviduct, corresponds physiologically rather with the abdominal opening of the Müllerian duct than with that of the segmental duct of Amphibia, which, after becoming divided up to form the pores of the head-kidney, undergoes atrophy. Morphologically, however, it appears to correspond with the opening of the segmental duct in Amphibia. We shall allude to this point more than once again, and give our grounds for the above view on p. 640.
The development of the segmental duct in Elasmobranchii as a solid rod is, we hope to shew, of special importance for the elucidation of the excretory system of Aves.
The development of these parts of Petromyzon is not fully known, but from W. Müller's account (Jenaische Zeitschrift, 1875) it would seem that an anterior invagination of the peritoneal epithelium is continued backwards as a duct (segmental duct), and that the anterior opening subsequently becomes divided up into the various apertures of the head-kidney. If this account is correct, Petromyzon presents a type intermediate between Amphibia and Elasmobranchii. In certain types, viz. Marsipobranchii and Teleostei, the segmental duct becomes the duct for the posterior kidney (segmental tubes), but otherwise undergoes no further differentiation. In the majority of types, [Pg 635] however, the case is different. In Amphibia[440], as has already been mentioned, a solid rod of cells is split off from its ventral wall, which afterwards becomes hollow, acquires an opening into the body-cavity, and forms the Müllerian duct.
In Elasmobranchii the segmental duct undergoes a more or less similar
division. It becomes longitudinally split into two complete ducts in the
female, and one complete duct and parts of a second in the male. The
resulting ducts are (1) the Wolffian duct dorsally, which remains
continuous with the excretory tubules of the kidney, and ventrally (2) the
oviduct or Müllerian duct in the female, and the rudiments of this duct in
the male. In the female the formation of these ducts takes place by a
nearly solid rod of cells, being gradually split off from the ventral side
of all but the foremost part of the original segmental duct, with the short
undivided anterior part of which duct it is continuous in front. Into it a
very small portion of the lumen of the original segmental duct is perhaps
continued. The remainder of the segmental duct (after the loss of its
anterior section and the part split off from its ventral side) forms the
Wolffian duct. The process of formation of the ducts in the male chiefly
differs from that in the female, in the fact of the anterior undivided part
of the segmental duct, which forms the front end of the Müllerian duct,
being shorter, and in the column of cells with which it is continuous being
from the first incomplete.
It will be seen from the above that the Müllerian duct consists of two distinct parts—an anterior part with the abdominal opening, and a posterior part split off from the segmental duct. This double constitution of the Müllerian duct is of great importance for a proper understanding of what takes place in the Bird.
The Müllerian duct appears therefore to develop in nearly the same manner in the Amphibian and Elasmobranch type, as a solid or nearly solid rod split off from the ventral wall of the segmental duct. But there is one important difference concerning the abdominal opening of the duct. In Amphibia this is a new formation, but in Elasmobranchii it is the original opening of the segmental duct. Although we admit that in a large [Pg 636] number of points, including the presence of a head-kidney, the urinogenital organs of Amphibia are formed on a lower type than those of the Elasmobranchii, yet it appears to us that this does not hold good for the development of the Müllerian duct.
The above description will, we trust, be sufficient to render clear our views upon the development of the excretory system in Aves.
In the bird the excretory system consists of the following parts (using the ordinary nomenclature) which are developed in the order below.
1. Wolffian duct. 2. Wolffian body. 3. Head-kidney. 4. Müllerian duct. 5. Permanent kidney and ureter.
About 2 and 5 we shall have nothing to say in the sequel.
We have already in the early part of the paper given an account of the head-kidney and Müllerian duct, but it will be necessary for us to say a few words about the development of the Wolffian duct (so called). Without entering into the somewhat extended literature on the subject, we may state that we consider that the recent paper of Dr Gasser[441] supplies us with the best extant account of the development of the Wolffian duct.
The first trace of it, which he finds, is visible in an embryo with eight protovertebræ as a slight projection from the intermediate cell mass towards the epiblast in the region of the three hindermost protovertebræ. In the next stage, with eleven protovertebræ, the solid rudiment of the duct extends from the fifth to the eleventh protovertebra, from the eighth to the eleventh protovertebra it lies between the epiblast and mesoblast, and is quite distinct from both, and Dr Gasser distinctly states that in its growth backwards from the eighth protovertebra the Wolffian duct never comes into continuity with the adjacent layers.
In the region of the fifth protovertebra, where the duct was originally continuous with the mesoblast, it has now become free, but is still attached in the region of the sixth and to the eighth protovertebra. In an embryo with fourteen protovertebræ the duct extends from the fourth to the fourteenth protovertebra, and is now free between epiblast and mesoblast for its whole extent. It is still for the most part solid though perhaps [Pg 637] a small lumen is present in its middle part. In the succeeding stages the lumen of the duct gradually extends backwards and forwards, the duct itself also passes inwards till it acquires its final position close to the peritoneal epithelium; at the same time its hind end elongates till it comes into connection with the cloacal section of the hind-gut. It should be noted that the duct in its backward growth does not appear to come into continuity with the subjacent mesoblast, but behaves in this respect exactly as does the segmental duct in Elasmobranchii (vide note on p. 634).
The question which we propose to ourselves is the following:—What are the homologies of the parts of the Avian urinogenital system above enumerated? The Wolffian duct appears to us morphologically to correspond in part to the segmental duct[442], or what Fürbringer would call the duct of the head-kidney. This may seem a paradox, since in birds it never comes into relation with the head-kidney. Nevertheless we consider that this homology is morphologically established, for the following reasons:—
(1) That the Wolffian duct gives rise (vide supra, p. 631) to the Müllerian duct as well as to the duct of the Wolffian body. In this respect it behaves precisely as does the segmental duct of Elasmobranchii and Amphibia. That it serves as the duct for the Wolffian body, before the Müllerian duct originates from it, is also in accordance with what takes place in other types.
(2) That it develops in a strikingly similar manner to the segmental duct of Elasmobranchii.
We stated expressly that the Wolffian duct corresponded only in part to the segmental duct. It does not, in fact, in our opinion, correspond to the whole segmental duct, but to the segmental duct minus the anterior abdominal opening in Elasmobranchii, which becomes the head-kidney in other types. In fact, we suppose that the segmental duct and head-kidney, which [Pg 638] in the Ichthyopsida develop as a single formation, develop in the Bird as two distinct structures—one of these known as the Wolffian duct, and the other the head-kidney. If our view about the head-kidney is accepted the above position will hardly require to be disputed, but we may point out that the only feature in which the Wolffian duct of the Bird differs in development from the segmental duct of Elasmobranchii is in the absence of the knob, which forms the commencement of the segmental duct, and in which the abdominal opening is formed; so that the comparison of the development of the duct in the two types confirms the view arrived at from other considerations.
The head-kidney and Müllerian duct in the Bird must be considered together. The parts which they eventually give rise to after the atrophy of the head-kidney have almost universally been regarded as equivalent to the Müllerian duct of the Ichthyopsida. By Braun[443], however, who from his researches on the Lizard satisfied himself of the entire independence of the Müllerian and Wolffian ducts in the Amniota, the Müllerian duct of these forms is regarded as a completely new structure with no genetic relations to the Müllerian duct of the Ichthyopsida. Semper[444], on the other hand, though he accepts the homology of the Müllerian duct in the Ichthyopsida and Amniota, is of opinion that the anterior part of the Müllerian duct in the Amniota is really derived from the Wolffian duct, though he apparently admits the independent growth of the posterior part of the Müllerian duct. We have been led by our observations, as well as by our theoretical deductions, to adopt a view exactly the reverse of that of Professor Semper. We believe that the anterior part of the Müllerian duct of Aves, which is at first the head-kidney, and subsequently becomes the abdominal opening of the duct, is developed from the peritoneal epithelium independently of all other parts of the excretory system; but that the posterior part of the duct is more or less completely derived from the walls of the Wolffian duct. This view is clearly in accordance with our account of the facts of development in Aves, and it fits in very well with the development of the Müllerian [Pg 639] duct in Elasmobranchii. We have already pointed out that in Elasmobranchii the Müllerian duct is formed of two factors—(1) of the whole anterior extremity of the segmental duct, including its abdominal opening; (2) of a rod split off from the ventral side of the segmental duct. In Birds the anterior part (corresponding to factor No. 1) of the Müllerian duct has a different origin from the remainder; so that if the development of the posterior part of the duct (factor No. 2) were to proceed in the same manner in Birds and Elasmobranchii, it ought to be formed at the expense of the Wolffian (i.e. segmental) duct, though in connection anteriorly with the head-kidney. And this is what actually appears to take place.
So far the homologies of the avian excretory system are fairly clear; but there are still some points which have to be dealt with in connection with the permanent opening of the Müllerian duct, and the relatively posterior position of the head-kidney. With reference to the first of these points the facts of the case are the following:—
In Amphibia the permanent opening of the Müllerian duct is formed as an independent opening after the atrophy of the head-kidney.
In Elasmobranchii the original opening of the segmental duct forms the permanent opening of the Müllerian duct and no head-kidney appears to be formed.
In Birds the anterior of the three openings of the head-kidney remains as the permanent opening of the Müllerian duct.
With reference to the difficulties involved in there being apparently three different modes in which the permanent opening of the Müllerian duct is formed, we would suggest the following considerations:
The history of the development of the excretory system teaches us that primitively the segmental duct must have served as efferent duct both for the generative products and kidney secretion (just as the Wolffian duct still does for the testicular products and secretion of the Wolffian body in Elasmobranchii and Amphibia); and further, that at first the generative products entered the segmental duct from the abdominal cavity by one or more of the abdominal openings of the kidney (almost certainly of the head-kidney). That the generative products did [Pg 640] not enter the segmental duct at first by an opening specially developed for them appears to us to follow from Dohrn's principle of the transmutation of function (Functionswechsel). As a consequence (by a process of natural selection) of the segmental duct having both a generative and a urinary function, a further differentiation took place, by which that duct became split into two—a ventral Müllerian duct and dorsal Wolffian duct.
The Müllerian duct without doubt was continuous with the head-kidney, and so with the abdominal opening or openings of the head-kidney which served as generative pores. At first the segmental duct was probably split longitudinally into two equal portions, but the generative function of the Müllerian duct gradually impressed itself more and more upon the embryonic development, so that, in the course of time, the Müllerian duct developed less and less at the expense of the Wolffian duct. This process appears partly to have taken place in Elasmobranchii, and still more in Amphibia; the Amphibia offering in this respect a less primitive condition than Elasmobranchii; while in Aves it has been carried even further. The abdominal opening no doubt also became specialised. At first it is quite possible that more than one abdominal pore may have served for the generative products; one of which, no doubt, eventually came to function alone. In Amphibia the specialisation of the opening appears to have gone so far that it no longer has any relation to the head-kidney, and even develops after the atrophy of the head-kidney. In Elasmobranchii, on the other hand, the functional opening appears at a period when we should expect the head-kidney to develop. This state is very possibly the result of a differentiation (along a different line to that in Amphibia) by which the head-kidney gradually ceased to become developed, but by which the primitive opening (which in the development of the head-kidney used to be divided into several pores leading into the body-cavity) remained undivided and served as the abdominal aperture of the Müllerian duct. Aves, finally, appear to have become differentiated along a third line; since in their ancestors the anterior pore of the head-kidney appears to have become specialised as the permanent opening of the Müllerian duct.
With reference to the posterior position of the head-kidney [Pg 641] in Aves we have only to remark, that a change in position of the head-kidney might easily take place after it acquired an independent development. The fact that it is slightly behind the glomerulus would seem to indicate, on the one hand, that it has already ceased to be of any functional importance; and, on the other, that the shifting has been due to its having a connection with the Müllerian duct.
We have made a few observations on the development of the Müllerian duct in Lacerta muralis, which have unfortunately led us to no decided conclusions. In a fairly young stage in the development of the Müllerian duct (the youngest we have met with), no trace of a head-kidney could be observed, but the character of the abdominal opening of the Müllerian duct was very similar to that figured by Braun[445]. As to the backward growth of the Müllerian duct, we can only state that the solid point of the duct in the young stages is in contact with the wall of the Wolffian duct, and the relation between the two is rather like that figured by Fürbringer (Pl. 1, figs. 14-15) in Amphibia.
DESCRIPTION OF PLATES 27 AND 28.
Complete List of Reference Letters.
ao. Aorta. cv. Cardinal vein. gl. Glomerulus. gr1. First groove of head-kidney. gr2. Second groove of head-kidney. gr3. Third groove of head-kidney. ge. Germinal epithelium. mrb. Malpighian body. me. Mesentery. md. Müllerian duct. r1. First ridge of head-kidney. r2. Second ridge of head-kidney. r3. Third ridge of head-kidney. Wd. Wolffian duct. x. Fold in germinal epithelium.
Plate 27.
Series A. Sections through the head-kidney at our second stage. Zeiss 2, ocul. 3 (reduced one-third). The second and third grooves are represented with the ridge connecting them, and the rod of cells running backwards for a short distance.
No. 1. Section through the second groove.
No. 2. Section through the ridge connecting the second and third grooves.
No. 3. Section passing through the same ridge at a point nearer the third groove.
Nos. 4, 5, 6. Sections through the third groove.
No. 7. Section through the point where the third groove passes into the solid rod of cells. [Pg 642]
No. 8. Section through the rod when quite separated from the germinal epithelium.
No. 9. Section very near the termination of the rod.
No. 10. Last section in which any trace of the rod is seen.
Series B. Sections passing through the head-kidney at our third stage. Zeiss C, ocul. 2. Our figures are representations of the following sections of the series, section 1 being the first which passes through the anterior groove of the head-kidney.
| No. | 1 | Section | 3. |
| " | 2 | " | 4. |
| " | 3 | " | 5. |
| " | 4 | " | 6. |
| " | 5 | " | 8. |
| " | 6 | " | 10. |
| " | 7 | " | 11. |
| " | 8 | " | 13. |
| " | 9 | " | 15. |
| " | 10 | " | 16. |
| " | 11 | " | 17. |
| " | 12 | " | 18. |
| " | 13 | " | 19. |
| " | 14 | " | 20. |
The Müllerian duct extends through eleven more sections.
The first groove (gr1.) extends to No. 3.
The second groove (gr2.) extends from No. 4 to No. 7.
The third groove (gr3.) extends from No. 11 to No. 13.
The first ridge (r1.) extends from No. 2 to No. 5.
The second ridge (r2.) extends from No. 8 to No. 11.
The third ridge (r3.) extends from No. 13 backwards through twelve sections, when it terminates by a pointed extremity.
Fig. C. Section through the ridge connecting the second and third grooves of the head-kidney of an embryo slightly younger than that from which Series B was taken. Zeiss C, ocul. 3 (reduced one-third).
The fold of the germinal epithelium, which gives rise to a deep groove (x.) external to the head-kidney is well marked.
Series G. Sections through the rod of cells constituting the termination of the Müllerian duct at a stage in which the head-kidney is still present. Zeiss C, ocul. 2.
Plate 28.
Series D. Sections chosen at intervals from a complete series traversing the peritoneal opening of the Müllerian duct, the remnant of the head-kidney, and the termination of the Müllerian duct. Zeiss C, ocul. 3 (reduced one-third).
Nos. 1 and 2. Sections through the persistent anterior opening of the head-kidney (abdominal opening of Müllerian duct). The approach of the Wolffian duct to the groove may be seen by a comparison of these two figures. In the sections in front of these (not figured) the two are much more widely separated than in No. 1.
No. 3. Section through the Müllerian duct, just posterior to the persistent opening.
Nos. 4 and 5. Remains of the ridges, which at an earlier stage connected the first and second grooves, are seen passing from the Müllerian duct to the peritoneal epithelium.
No. 6. Rudiment of the second groove (gr2.) of the head-kidney.
Between 6 and 7 is a considerable interval.
No. 7. All traces of this groove (gr2.) have vanished, and the Müllerian duct is quite disconnected from the epithelium.
[Pg 643] No. 8. Rudiment of the third groove (gr3.).
No. 9. Müllerian duct quite free in the space between the peritoneal epithelium and the Wolffian duct, in which condition it extends until near its termination. Between Nos. 9 and 10 is an interval of eight sections.
No. 10. The penultimate section, in which the Müllerian duct is seen. A lumen cannot be clearly made out.
No. 11. The last section in which any trace of the Müllerian duct is visible. No line of demarcation can be seen separating the solid end of the Müllerian duct from the ventral wall of the Wolffian duct.
Figs. E. and F. Sections through the glomerulus of the head-kidney from an embryo prior to the appearance of the head-kidney. Zeiss B, ocul. 2. A comparison of the two figures shows the variation in the thickness of the stalk of the glomerulus. E. Section anterior to the foremost Malpighian body. F. Section through both the glomerulus of the head-kidney and that of a Malpighian body. The two are seen to be connected.
Series H. Consecutive sections through the hind end of the Müllerian duct, from an embryo in which the head-kidney was only represented by a rudiment. (The embryo was, perhaps, very slightly older than that from which Series D was taken.) Zeiss C, ocul. 3 (reduced one-third).
No. 1. Müllerian duct is without a lumen, and quite distinct from the Wolffian wall.
No. 2. The solid end of the Müllerian duct is no longer distinct from the internal wall of the Wolffian duct.
No. 3. All trace of the Müllerian duct has vanished.
Series I. Sections through the hinder end of the Müllerian duct from an embryo of about the middle of the sixth day. Zeiss C, ocul. 2 (reduced one-third).
No. 1. The Müllerian duct is distinct and small.
No. 2. Is posterior by twelve sections to No. 1. The Müllerian duct is dilated, and its cells are vacuolated.
No. 3. Penultimate section, in which the Müllerian duct is visible; it is separated by three sections from No. 2.
No. 4. Last section in which any trace of the Müllerian duct is visible; the lumen, which was visible in the previous section, is now absent.
No. 5. No trace of Müllerian duct. Nos. 3, 4, and 5 are consecutive sections.
Fig. K. Section through the hind end of the abdominal opening of the Müllerian duct of a chick of 123 hours. Zeiss C, ocul. 2 (reduced one-third). It illustrates the peculiar cord connecting the Müllerian and Wolffian ducts.
[424] From the Quarterly Journal of Microscopical Science, Vol. XIX. 1879.
[425]
Das Urogenital-System der Plagiostomen,
Arbeiten a. d. zool.-zoot. Institut. Würzburg.
[426]
Zur vergl. Anat. u. Entwick. d. Excretionsorgane d.
Vertebraten,
Morphologisches Jahrbuch, Vol. IV.
[427]
On the Origin and History of the Urinogenital Organs of
Vertebrates,
Journal of Anat. and Phys., Vol. X. [This Edition
No. VII.]
[428] Arbeiten a. d. zool.-zoot. Institut. Würzburg, Vol. IV.
[429] Beitr. zur Anat. u. Entwick. d. Geschlechtsorgane, Inaug. Diss., Zürich, 1876.
[430] Proceedings of Royal Society, 1878.
[431] A deep focus of the rather thick section represented in No. 3 shewed the body much more nearly in the position it occupies in No. 4.
[432] Beiträge zur Entwicklungsgeschichte d. Allantois der Müller'schen Gange u. des Afters. Frankfurt, 1874.
[433] Loc. cit.
[434] Jenaische Zeitschrift, Vol. IX. 1875.
[435] Entwicklungsgeschichte d. Unke.
[436] Loc. cit.
[437]
Balfour, Origin and History of Urinogenital Organs of Vertebrates,
Journal of Anat. and
Phys. Vol. X., and Monograph on Elasmobranch
Fishes. [This edition Nos. VII. and X.]
[438]
I am inclined to give up the view I formerly expressed with reference to
the head-kidney and segmental duct, viz.
that they were to be regarded as the most anterior segmental tube, the
peritoneal opening of which had become divided, and which had become
prolonged backwards so as to serve as the duct for the posterior segmental
tubes,
and provisionally to accept the Gegenbaur-Fürbringer
view which has been fully worked out and ably argued for by Fürbringer (loc. cit. p. 96). According to this view the
head-kidney and its duct are to be looked on as the primitive and
unsegmented part of the excretory system, more or less similar to the
excretory system of many Trematodes and unsegmented Vermes. The segmental
tubes I regard as a truly segmental part of the excretory system acquired
subsequently.—F. M. B.
[439]
In a note on p. 50 of his memoir Fürbringer criticises my description
of the mode of growth of the segmental duct. The following is a free
translation of what he says: In Balfour's, as in other descriptions, an
account is given of a backward growth, which easily leads to the
supposition of a structure formed anteriorly forcing its way through the
tissues behind. This is, however, not the case, since, to my knowledge, no
author has ever detected a sharp boundary between the growing point of the
segmental duct (or Müllerian duct) and the surrounding tissues.
He
goes on to say that the growth in these cases really takes place by a
differentiation of tissue along a line in the region of the peritoneal
cavity.
Although I fully admit that it would be far easier to
homologise the development of the segmental duct in Amphibia and
Elasmobranchii according to this view, I must nevertheless vindicate the
accuracy of my original account. I have looked over my specimens again,
since the appearance of Dr Fürbringer's paper, and can find no evidence of
the end of the duct becoming continuous with the adjoining mesoblastic
tissues. In the section, before its disappearance, the segmental duct may,
so far as I can make out, be seen as a very small but distinct rod, which
is much more closely connected with the epiblast than with any other
layer. From Gasser's observations on the Wolffian duct in the bird, I am
led to conclude that it behaves in the same way as the segmental duct in
the Elasmobranchii. I will not deny that it is possible that the growth of
the duct takes place by wandering cells, but on this point I have no
evidence, and must therefore leave the question an open one.—F. M.
B.
[440] Fürbringer, loc. cit.
[441] Arch. für Mic. Anat. Vol. XIV.
[442]
The views here expressed about the Wolffian duct are nearly though not
exactly those which one of us previously put forward (Urinogenital
Organs of Vertebrates,
&c., pp. 45-46) [This edition, pp. 164, 165], and with which Fürbringer appears exactly to agree.
Possibly Dr Fürbringer would alter his view on this point were he to
accept the facts we believe ourselves to have discovered. Semper's view
also differs from ours, in that he believes the Wolffian duct to
correspond in its entirety with the segmental duct.
[443]
Urogenital-System d. Reptilien,
Arb. aus
d. zool.-zoot. Inst. Würzburg, Vol. IV.
[444] Loc. cit.
[445] Loc. cit.
(With Plate 29.)
Till quite recently no observations were recorded on the early developmental changes of the reptilian ovum. Not long ago Professors Kupffer and Benecke published a preliminary note on the early development of Lacerta agilis and Emys Europea[447]. I have myself also been able to make some observations on the embryo of Lacerta muralis. The number of my embryos has been somewhat limited, and most of those which I have had have been preserved in bichromate of potash, which has turned out a far from satisfactory hardening reagent. In spite of these difficulties I have been led on some points to very different results from those of the German investigators, and to results which are more in accordance with what we know of other Sauropsidan types. I commence with a short account of the results of Kupffer and Benecke.
Segmentation takes place exactly as in birds, and the resulting blastoderm, which is thickened at its edge, spreads rapidly over the yolk. Shortly before the yolk is half enclosed a small embryonic shield (area pellucida) makes its appearance in the centre of the blastoderm, which has, in the meantime, become divided into two layers. The upper of these is the epiblast, and the lower the hypoblast. The embryonic shield is mainly distinguished from the remainder of the blastoderm by the more columnar character of its constituent epiblast cells. It is somewhat pyriform in shape, the narrower end corresponding with [Pg 645] the future posterior end of the embryo. At the narrow end an invagination takes place, which gives rise to an open sac, the blind end of which is directed forwards. The opening of this sac is regarded by the authors as the blastopore. A linear thickening of epiblast arises in front of the blastopore, along the median line of which the medullary groove soon appears. In the caudal region the medullary folds spread out and enclose between them the blastopore, behind which they soon meet again. On the conversion of the medullary groove into a closed canal the blastopore becomes obliterated. The mesoblast grows out from the lip of the blastopore as four masses. Two of these are lateral: a third is anterior and median, and, although at first independent of the epiblast, soon attaches itself to it, and forms with it a kind of axis-cord. A fourth mass applied itself to the walls of the sac formed by invagination.
With reference to the very first developmental phenomena my observations are confined to two stages during the segmentation[448]. In the earliest of these the segmentation was about half completed, in the later one it was nearly over. My observations on these stages bear out generally the statements of Kupffer and Benecke. In the second of them the blastoderm was already imperfectly divided into two layers—a superficial epiblastic layer formed of a single row of cells, and a layer below this several rows deep. Below this layer fresh segments were obviously being added to the blastoderm from the subjacent yolk.
Between the second of these blastoderms and my next stage there is a considerable gap. The medullary plate is just established, and is marked by a shallow groove which becomes deeper in front. A section through the embryo is represented in Pl. 29, Series A, fig. 1. In this figure there may be seen the thickened medullary plate with a shallow medullary groove, below which are two independent plates of mesoblast (me.p.), one on each side of the middle line, very imperfectly divided into somatopleuric and splanchnopleuric layers. Below the mesoblast is a continuous layer of hypoblast (hy.), which develops a rod-like thickening along the axial line (ch.). This rod becomes in the next stage the notochord. Although this embryo is not well [Pg 646] preserved I feel very confident in asserting the continuity of the notochord with the hypoblast at this stage.
At the hind end of the embryo is placed a thickened ridge of tissue which continues the embryonic axis. In this ridge all the layers coalesce, and I therefore take it to be equivalent to the primitive streak of the avian blastoderm. It is somewhat triangular in shape, with the apex directed backward, the broad base placed in front.
At the junction between the primitive streak and the blastoderm is situated a passage, open at both extremities, leading from the upper surface of the blastoderm obliquely forwards to the lower.
The dorsal and anterior wall of this passage is formed of a distinct epithelial layer, continuous at its upper extremity with the epiblast, and at its lower with the notochordal plate, so that it forms a layer of cells connecting together the epiblast and hypoblast. The hinder and lower wall of the passage is formed by the cells of the primitive streak, which only assume a columnar form near the dorsal opening of the passage (vide fig. 4). This passage is clearly the blind sac of Kupffer and Benecke, who, if I am not mistaken, have overlooked its lower opening. As I hope to show in the sequel, it is also the equivalent of the neurenteric passage, which connects the neural and alimentary canals in the Ichthyopsida, and therefore represents the blastopore of Amphioxus, Amphibians, &c.
Series A, figs. 2, 3, 4, 5, illustrate the features of the passage and its relation to the embryo.
Fig. 2 passes through the ventral opening of the passage. The notochordal plate (ch´.) is vaulted over the opening, and on the left side is continuous with the mesoblast as well as the hypoblast. Figs. 3 and 4 are taken through the middle part of the passage (ne.), which is bounded above by a continuation of the notochordal plate, and below by the tissue of the primitive streak. The hypoblast (hy.), in the middle line, is imperfectly fused with the mesoblast of the primitive streak, which is now continuous across the middle line. The medullary groove has disappeared, but the medullary plate (mp.) is quite distinct.
In fig. 5 is seen the dorsal opening of the passage (ne.). If a section behind this had been figured, as is done for the next [Pg 647] series (B), it would have passed through the primitive streak, and, as in the chick, all the layers would have been fused together. The epiblast in the primitive streak completely coalesces with the mesoblast; but the hypoblast, though attached to the other layers in the middle line, can always be traced as a distinct stratum.
Fig. B is a surface view of my next oldest embryo. The medullary groove has become much deeper, especially in front. Behind it widens out to form a space equivalent to the sinus rhomboidalis of the embryo bird. The amnion forms a small fold covering over the cephalic extremity of the embryo, which is deeply embedded in the yolk. Some somites (protovertebræ) were probably present, but this could not be made out in the opaque embryo.
Fig. 1. Diagrammatic longitudinal section of an embryo of Lacerta. pp. body-cavity. am. Amnion. ne. Neurenteric canal. ch. Notochord. hy. Hypoblast. ep. Epiblast. pr. Primitive streak.
The woodcut (fig. 1) represents a diagrammatic longitudinal section through this embryo, and the sections belonging to Series B illustrate the features of the hind end of the embryo and of the primitive streak.
As is shown in fig. 1, the notochord (ch.) has now throughout the region of the embryo become separated from the subjacent hypoblast, and the lateral plates of mesoblast are distinctly divided into somatic and splanchnic layers. The medullary groove is continued as a deepish groove up to the opening of the neurenteric passage, which thus forms a perforation in the floor of the hinder end of the medullary groove (vide Series B, figs. 2, 3, and 4).
The passage itself is somewhat shorter than in the previous stage, and the whole of it is shown in a single section (fig. 4). This section must either have been taken somewhat obliquely, [Pg 648] or else the passage have been exceptionally short in this embryo, since in an older embryo it could not all be seen in one section.
The front wall of the passage is continuous with the notochord, which for two sections or so in front remains attached to the hypoblast (figs. 2 and 3). Behind the perforation in the floor of the medullary groove is placed the primitive streak (fig. 5), where all the layers become fused together, as in the earlier stage. Into this part a narrow diverticulum from the end of the medullary groove is continued for a very short distance (vide fig. 5, mc.).
The general features of the stage will best be understood by an examination of the diagrammatic longitudinal section, represented in woodcut, fig. 1. In front is shown the amnion (am.), growing over the head of the embryo. The notochord (ch.) is seen as an independent cord for the greater part of the length of the embryo, but falls into the hypoblast shortly in front of the neurenteric passage. The neurenteric passage is shown at ne., and behind it is shown the primitive streak.
In a still older stage, represented in surface view on Pl. 29, fig. C, the medullary folds have nearly met above, but have not yet united. The features of the passage from the neural groove to the hypoblast are precisely the same in the embryo just described, although the lumen of the passage has become somewhat narrower. There is still a short primitive streak behind the embryo.
The neurenteric passage persists but a very short time after the complete closure of the medullary canal. It is in no way connected with the allantois, as conjectured by Kupffer and Benecke, but the allantois is formed, as I have satisfied myself by longitudinal sections of a later stage, in the manner already described by Dobrynin, Gasser, and Kölliker for the bird and mammal.
The general results of Kupffer's and Benecke's observations, with the modifications introduced by my own observations, are as follows:—After the segmentation and the formation of the embryonic shield (area pellucida) the blastoderm becomes distinctly divided into epiblast and hypoblast[449]. At the hind end of the shield a somewhat triangular primitive streak is formed by [Pg 649] the fusion of the epiblast and hypoblast with a number of cells between them, which are probably derived from the lower rows of the segmentation cells. At the front end of the streak a passage arises, open at both extremities, leading obliquely forwards through the epiblast to the space below the hypoblast. The walls of the passage are formed of a layer of columnar cells continuous both with epiblast and hypoblast. In front of the primitive streak the body of the embryo becomes first differentiated by the formation of a medullary plate, and at the same time there grows out from the primitive streak a layer of mesoblast, which spreads out in all directions between the epiblast and hypoblast. In the axis of the embryo the mesoblast plate is stated by Kupffer and Benecke to be continuous across the middle line, but this appears very improbable. In a slightly later stage the medullary plate becomes marked by a shallow groove, and the mesoblast of the embryo is then undoubtedly constituted of two lateral plates, one on each side of the median line. In the median line the notochord arises as a ridge-like thickening of the hypoblast, which becomes very soon quite separated from the hypoblast, except at the hind end, where it is continued into the front wall of the neurenteric passage. It is interesting to notice the remarkable relation of the notochord to the walls of the neurenteric passage. More or less similar relations are also well marked in the case of the goose and the fowl (Gasser)[450], and support the conclusion deducible from the lower forms of vertebrata, that the notochord is essentially hypoblastic.
The passage at the front end of the primitive streak forms the posterior boundary of the medullary plate, though the medullary groove is not at first continued back to it. The anterior wall of this passage connects together the medullary plate and the notochordal ridge of the hypoblast. In the succeeding stages the medullary groove becomes continued back to the opening of the passage, which then becomes enclosed in the medullary folds, and forms a true neurenteric passage. It becomes narrowed as the medullary folds finally unite to form the medullary canal, and eventually disappears.
[Pg 650] I conclude this paper with a concise statement of what appears to me the probable nature of the much-disputed organ, the primitive streak, and of the arguments in support of my view.
In a paper on the primitive streak in the Quart. Journ. of Mic. Sci.,
in 1873 (p. 280) [This edition, p. 45], I
made the following statement with reference to this subject:—It is
clear, therefore, that the primitive groove must be the rudiment of some
ancestral feature.... It is just possible that it is the last trace of that
involution of the epiblast by which the hypoblast is formed in most of the
lower animals.
At a later period, in July, 1876, after studying the development of Elasmobranch fishes, I enlarged the hypothesis in a review of the first part of Prof. Kölliker's Entwicklungsgeschichte. The following is the passage in which I speak of it[451]:
"In treating of the exact relation of the primitive groove to the formation of the embryo, Professor Kölliker gives it as his view that though the head of the embryo is formed independently of the primitive groove, and only secondarily unites with this, yet that the remainder of the body is without doubt derived from the primitive groove. With this conclusion we cannot agree, and the very descriptions of Professor Kölliker appear to us to demonstrate the untenable nature of his results. We believe that the front end of the primitive groove at first occupies the position eventually filled by about the third pair of protovertebræ, but that as the protovertebræ are successively formed, and the body of the embryo grows in length, the primitive groove is carried further and further back, so as always to be situated immediately behind the embryo. As Professor Kölliker himself has shewn it may still be seen in this position even later than the fortieth hour of incubation.
"Throughout the whole period of its existence it retains a character which at once distinguishes it in sections from the medullary groove.
"Beneath it the epiblast and mesoblast are always fused, though they are always separate elsewhere; this fact, which was [Pg 651] originally shewn by ourselves, has been very clearly brought out by Professor Kölliker's observations.
"The features of the primitive groove which throw special light on its meaning are the following:—
"(1) It does not enter directly into the formation of the embryo.
"(2) The epiblast and mesoblast always become fused beneath it.
"(3) It is situated immediately behind the embryo.
"Professor Kölliker does not enter into any speculations as to the meaning of the primitive groove, but the above-mentioned facts appear to us clearly to prove that the primitive groove is a rudimentary structure, the origin of which can only be completely elucidated by a knowledge of the development of the Avian ancestors.
"In comparing the blastoderm of a bird with that of any anamniotic vertebrate, we are met at the threshold of our investigations by a remarkable difference between the two. Whereas in all the lower vertebrates the embryo is situated at the edge of the blastoderm, it is in birds and mammals situated in the centre. This difference of position at once suggests the view that the primitive groove may be in some way connected with the change of position in the blastoderm which the ancestors of birds must have undergone. If we carry our investigations amongst the lower vertebrates a little further, we find that the Elasmobranch embryo occupies at first the normal position at the edge of the blastoderm, but that in the course of development the blastoderm grows round the yolk far more slowly in the region of the embryo than elsewhere. Owing to this, the embryo becomes left in a bay, the two sides of which eventually meet and coalesce in a linear fashion immediately behind the embryo, thus removing the embryo from the edge of the blastoderm and forming behind it a linear streak not unlike the primitive streak. We would suggest the hypothesis that the primitive groove is a rudiment which gives the last indication of a change made by the Avian ancestors in their position in the blastoderm, like that made by Elasmobranch embryos when removed from the edge of the blastoderm and placed in a central situation similar to that of the embryo bird. On this hypothesis the [Pg 652] situation of the primitive groove immediately behind the embryo, as well as the fact of its not becoming converted into any embryonic organ would be explained. The central groove might probably also be viewed as the groove naturally left between the coalescing edges of the blastoderm.
Would the fusion of epiblast and mesoblast also
receive its explanation on this hypothesis? We are of opinion that it
would. At the edge of the blastoderm which represents the blastopore mouth
of Amphioxus all the layers become fused together in the anamniotic
vertebrates. So that if the primitive groove is in reality a rudiment of
the coalesced edges of the blastoderm, we might naturally expect the layers
to be fused there, and the difficulty presented by the present condition of
the primitive groove would rather be that the hypoblast is not fused with
the other layers than that the mesoblast is indissolubly united with the
epiblast. The fact that the hypoblast is not fused with the other layers
does not appear to us to be fatal to our hypothesis, and in Mammalia, where
the primitive and medullary grooves present precisely the same relations as
in birds, all three layers are, according to Hensen's account, fused
together. This, however, is denied by Kölliker, who states that in Mammals,
as in Birds, only the epiblast and mesoblast fuse together. Our hypothesis
as to the origin of the primitive groove appears to explain in a fairly
satisfactory manner all the peculiarities of this very enigmatical organ;
it also relieves us from the necessity of accepting Professor Kölliker's
explanation of the development of the mesoblast, though it does not, of
course, render that explanation in any way untenable.
At a somewhat later period Rauber arrived at a more or less similar conclusion, which, however, he mixes up with a number of opinions from which I am compelled altogether to dissent[452].
The general correctness of my view, as explained in my second quotation, appears to me completely established by Gasser's beautiful researches on the early development of the chick and goose[453], and by my own observations just recorded on the lizard. While at the same time the parallel between the blastopore of Elasmobranchii and of the Sauropsida, is rendered [Pg 653] more complete by the discovery of the neurenteric passage in the latter group, which was first of all made by Gasser.
The following paragraphs contain a detailed attempt to establish the above view by a careful comparison of the primitive streak and its adjuncts in the amniotic vertebrates with the blastopore in Elasmobranchii.
In Elasmobranchii the blastopore consists of the following parts:—(1), a section at the end of the medullary plate, which becomes converted into the neurenteric canal[454]; (2), a section forming what may be called the yolk blastopore, which eventually constitutes a linear streak connecting the embryo with the edge of the blastoderm (vide monograph on Elasmobranch fishes, pp. 281 and 296). In order to establish my hypothesis on the nature of the primitive streak, it is necessary to find the representatives of both these parts in the primitive streak of the amniotic vertebrates. The first section ought to appear as a passage from the neural to the enteric side of the blastoderm at the posterior end of the medullary plate. At its front edge the epiblast and hypoblast should be continuous, as they are at the hind end of the embryo in Elasmobranchii, and, finally, the passage should, on the closure of the medullary groove, become converted into the neurenteric canal. All these conditions are exactly fulfilled by the opening at the front end of the primitive streak of the lizard (vide woodcut, fig. 1, p. 647). In the chick there is at first no such opening, but, as I hope to shew in a future paper, it is replaced by the epiblast and hypoblast falling into one another at the front end of the primitive streak. At a later period, as has been shewn by Gasser[455], there is a distinct rudiment of the neurenteric canal in the chick, and a complete canal in the goose. Finally, in mammals, as has been shewn by Schäffer[456] for the guinea-pig, there is at the front end of the primitive streak a complete continuity between epiblast and hypoblast. The continuity of the epiblast and hypoblast at the hind end of the embryo in the bird and the mammal is a [Pg 654] rudiment of the continuity of these layers at the dorsal lip of the blastopore in Elasmobranchii, Amphibia, &c. The second section of the blastopore in Elasmobranchii or yolk blastopore is, I believe, partly represented by the primitive streak. The yolk blastopore in Elasmobranchii is the part of the blastopore belonging to the yolk sac as opposed to that belonging to the embryo, and it is clear that the primitive streak cannot correspond to the whole of this, since the primitive streak is far removed from the edge of the blastoderm long before the yolk is completely enclosed. Leaving this out of consideration the primitive streak, in order that the above comparison may hold good, should satisfy the following conditions:
1. It should connect the embryo with the edge of the blastoderm.
2. It should be constituted as if formed of the fused edges of the blastoderm.
3. The epiblast of it should eventually not form part of the medullary plate of the embryo, but be folded over on to the ventral side.
The first of these conditions is only partially fulfilled, but, considering the rudimentary condition of the whole structure, no great stress can, it seems to me, be laid on this fact.
The second condition seems to me very completely satisfied. Where the two edges of the blastoderm become united we should expect to find a complete fusion of the layers such as takes place in the primitive streak; and the fact that in the primitive streak the hypoblast does not so distinctly coalesce with the mesoblast as the mesoblast with the epiblast cannot be urged as a serious argument against me.
The growth outwards of the mesoblast from the axis of the primitive streak is probably a remnant of the invagination of the hypoblast and mesoblast from the lip of the blastopore in Amphibia, &c.
The groove in the primitive streak may with great plausibility be regarded as the indication of a depression which would naturally be found along the line where the thickened edges of the blastoderm became united.
With reference to the third condition, I will make the following observations. The neurenteric canal, as it is placed at the [Pg 655] extreme end of the embryo, must necessarily, with reference to the embryo, be the hindermost section of the blastopore, and therefore the part of the blastopore apparently behind this can only be so owing to the embryo not being folded off from the yolk sac; and as the yolk sac is in reality a specialised part of the ventral wall of the body, the yolk blastopore must also be situated on the ventral side of the embryo.
Kölliker and other distinguished embryologists have believed that the epiblast of the whole of the primitive streak became part of the neural plate. If this view were correct, which is accepted even by Rauber, the hypothesis I am attempting to establish would fall to the ground. I have, however, no doubt that these embryologists are mistaken. The very careful observations of Gasser shew that the part of the primitive streak adjoining the embryo becomes converted into the tail-swelling, and that the posterior part is folded in on the ventral side of the embryo, and, losing its characteristic structure, forms part of the ventral wall of the body. On this point my own observations confirm those of Gasser. In the lizard the early appearance of the neurenteric canal at the front end of the primitive streak clearly shews that here also the primitive streak can take no share in forming the neural plate.
The above considerations appear to me sufficient to establish my hypothesis with reference to the nature of the primitive streak, which has the merit of explaining, not only the structural peculiarities of the primitive streak, but also the otherwise inexplicable position of the embryo of the amniotic vertebrates in the centre of the blastoderm.
[Pg 656] DESCRIPTION OF PLATE 29.
Complete List of Reference Letters.
am. Amnion. ch. Notochord. ch´. Notochordal thickening of hypoblast. ep. Epiblast. hy. Hypoblast. m.g. Medullary groove. me.p. Mesoblastic plate. ne. Neurenteric canal (blastopore). pr. Primitive streak.
Series A. Sections through an embryo shortly after the formation of the medullary groove. x 120[457].
Fig. 1. Section through the trunk of the embryo.
Figs. 2-5. Sections through the neurenteric canal.
Fig. B. Surface view of a somewhat older embryo than that from which Series A is taken. x 30.
Series B. Sections through the embryo represented in Fig. B. x 120.
Fig. 1. Section through the trunk of the embryo.
Figs. 2, 3. Sections through the hind end of the medullary groove.
Fig. 4. Section through the neurenteric canal.
Fig. 5. Section through the primitive streak.
Fig. C. Surface view of a somewhat older embryo than that represented in Fig. B. x 30.
[446] From the Quarterly Journal of Microscopical Science, Vol. XIX. 1879.
[447] Die Erste Entwicklungsvorgänge am Ei der Reptilien, Königsberg, 1878.
[448] For these two specimens, which were hardened in picric acid, I am indebted to Dr Kleinenberg.
[449] This appears to me to take place before the formation of the embryonic shield.
[450] Gasser, Der Primitivstreifen bei Vogelembryonen, Marburg, 1878.
[451] Journal of Anat. and Phys., Vol. X. pp. 790 and 791. Compare also my Monograph on Elasmobranch Fishes, note on p. 68 [This edition, p. 281].
[452]
Primitivrinne u. Urmund,
Morphologisches
Jahrbuch, Band II. p. 551.
[453] Gasser, Der Primitivstreifen bei Vogelembryonen, Marburg, 1878.
[454] I use this term for the canal connecting the neural and alimentary tract, which was first discovered by Kowalevsky.
[455] Loc. cit.
[456]
A contribution to the history of the development in the Guinea-pig,
Journal of Anat. and
Phys. Vol. XI. pp. 332-336.
[457] The spaces between the layers in these sections are due to the action of the hardening reagent.
The discovery by Mr Moseley[459] of
a tracheal system in Peripatus must be reckoned as one of the most
interesting results obtained by the naturalists of the Challenger.
The discovery clearly proves that the genus Peripatus, which is widely
distributed over the globe, is the persisting remnant of what was probably
a large group of forms, from which the present tracheate Arthropoda are
descended.
The affinities of Peripatus render any further light on its anatomy a matter of some interest; and through the kindness of Mr Moseley I have had an opportunity of making investigations on some well preserved examples of Peripatus capensis, a few of the results of which I propose to lay before the Society.
I shall confine my observations to three organs. (1) The segmental organs, (2) the nervous system, (3) the so-called fat bodies of Mr Moseley.
In all the segments of the body, with the exception of the first two or three postoral ones, there are present glandular bodies, apparently equivalent to the segmental organs of Annelids.
These organs have not completely escaped the attention of previous observers. The anterior of them were noticed by Grube[460], but their relations were not made out. By Saenger[461], as I gather from Leuckart's Bericht for the years 1868-9, these structures were also noticed, and they were interpreted as segmental [Pg 658] organs. Their external openings were correctly identified. They are not mentioned by Moseley, and no notice of them is to be found in the text-books. The observations of Grube and Saenger seem, in fact, to have been completely forgotten.
The organs are placed at the bases of the feet in two lateral divisions of the body-cavity shut off from the main central median division of the body-cavity by longitudinal septa of transverse muscles.
Each fully developed organ consists of three parts:
(1) A dilated vesicle opening externally at the base of a foot.
(2) A coiled glandular tube connected with this and subdivided again into several minor divisions.
(3) A short terminal portion opening at one extremity into the coiled tube (2) and at the other, as I believe, into the body-cavity. This section becomes very conspicuous in stained preparations by the intensity with which the nuclei of its walls absorb the colouring matter.
The segmental organs of Peripatus, though formed on a type of their own, more nearly resemble those of the Leech than of any other form with which I am acquainted. The annelidan affinities shewn by their presence are of some interest. Around the segmental organs in the feet are peculiar cells richly supplied with tracheæ, which appear to me to be similar to the fat bodies in insects. There are two glandular bodies in the feet in addition to the segmental organs.
The more obvious features of the nervous system have been fully made out by previous observers, who have shewn that it consists of large paired supra-œsophageal ganglia connected with two widely separated ventral cords—stated by them not to be ganglionated. Grube describes the two cords as falling into one another behind the anus—a feature the presence of which is erroneously denied by Saenger. The lateral cords are united by numerous (5 or 6 for each segment) transverse cords.
The nervous system would appear at first sight to be very lowly organised, but the new points I believe myself to have made out, as well as certain previously known features in it appear to me to shew that this is not the case.
[Pg 659] The following is a summary of the fresh points I have observed in the nervous system:
(1) Immediately underneath the œsophagus the œsophageal commissures dilate and form a pair of ganglia equivalent to the annelidan and arthropodan sub-œsophageal ganglia. These ganglia are closely approximated and united by 5 or 6 commissures. They give off large nerves to the oral papillæ.
(2) The ventral nerve cords are covered on their ventral side by a thick ganglionic layer[462], and at each pair of feet they dilate into a small but distinct ganglionic swelling. From each ganglionic swelling are given off a pair of large nerves[463] to the feet; and the ganglionic swellings of the two cords are connected together by a pair of commissures containing ganglion cells[464]. The other commissures connecting the two cords together do not contain ganglion cells.
The chief feature in which Peripatus was supposed to differ from normal Arthropoda and Annelida, viz. the absence of ganglia on the ventral cords, does not really exist. In other particulars, as in the amount of nerve cells in the ventral cords and the completeness of the commissural connections between the two cords, &c., the organisation of the nervous system of Peripatus ranks distinctly high. The nervous system lies within the circular and longitudinal muscles, and is thus not in proximity with the skin. In this respect also Peripatus shews no signs of a primitive condition of the nervous system.
A median nerve is given off from the posterior border of the supra-œsophageal ganglion to the œsophagus, which probably forms a rudimentary sympathetic system. I believe also that I have found traces of a paired sympathetic system.
The organ doubtfully spoken of by Mr Moseley as a fat body, and by Grube as a lateral canal, is in reality a glandular tube, lined by beautiful columnar cells containing secretion globules, which opens by means of a non-glandular duct into the mouth. It lies close above the ventral nerve cords in a lateral compartment [Pg 660] of the body-cavity, and extends backwards for a varying distance.
This organ may perhaps be best compared with the simple salivary gland of Julus. It is not to be confused with the slime glands of Mr Moseley, which have their opening in the oral papillæ. If I am correct in regarding it as homologous with the salivary glands so widely distributed amongst the Tracheata, its presence indicates a hitherto unnoticed arthropodan affinity in Peripatus.
[458] From the Proceedings of the Cambridge Philosophical Society, Vol. III. 1879.
[459]
On the Structure and Development of Peripatus Capensis,
Phil. Trans.,
Vol. CLXIV.
1874.
[460]
Bau von Perip. Edwardsii,
Archiv f. Anat. u. Phys. 1853.
[461] Moskauer Naturforscher Sammlung, Abth. Zool. 1869.
[462] This was known to Grube, loc. cit.
[463] These nerves were noticed by Milne-Edwards, but Grube failed to observe that they were much larger than the nerves given off between the feet.
[464] These commissures were perhaps observed by Saenger, loc. cit.
Professor Schulze's[466] last memoir on the development of Calcareous Sponges, confirms and enlarges Metschnikoff's[467] earlier observations, and gives us at last a fairly complete history of the development of one form of Calcareous Sponge. The facts which have been thus established have suggested to me a view of the morphology and systematic position of the Spongida, somewhat different to that now usually entertained. In bringing forward this view, I would have it understood that it does not claim to be more than a mere suggestion, which if it serves no other function may, perhaps, be of use in stimulating research.
To render clear what I have to say, I commence with a very brief statement of the facts which may be considered as established with reference to the development of Sycandra raphanus, the form which was studied by both Metschnikoff and Schulze. The segmentation of the ovum, though in many ways remarkable, is of no importance for my present purpose, and I take up the development at the close of the segmentation, while the embryo is still encapsuled in the parental tissues. It is at this stage lens-shaped, with a central segmentation cavity. An equatorial plane divides it into two parts, which have equal shares in bounding the segmentation cavity. One of these halves is formed of about thirty-two large, round, granular cells, the other of a larger number of ciliated clear columnar cells. While the embryo is still encapsuled a partial invagination of the [Pg 662] granular cells takes place, reducing the segmentation cavity to a mere slit; this invagination is, however, quite temporary and unimportant, and on the embryo becoming free, which shortly takes place, no trace of it is visible; but, on the contrary, the segmentation cavity becomes larger, and the granular cells project very much more prominently than in the encapsuled state.
Fig. 1.
Two free stages in the development of Sycandra raphanus (copied from Schulze).
A. Amphiblastula stage; B. a later stage after the ciliated cells have commenced to become invaginated; cs. segmentation cavity; ec. granular cells which will form the ectoderm; en. ciliated cells which become invaginated to form the entoderm
The larva, after it has left the parental tissues, has an oval form and is transversely divided into two areas (fig. 1, A). One of these areas is formed of the elongated, clear, ciliated cells, with a small amount of pigment near the inner ends (en), and the other and larger area of the thirty-two granular cells already mentioned (ec). Fifteen or sixteen of these are arranged as a special ring on the border of the clear cells. In the centre of the embryo is a segmentation cavity (cs) which lies between the granular and the clear cells, but is mainly bounded by the vaulted inner surface of the latter. This stage is known as the amphiblastula stage. After the larva has for some time enjoyed a free existence, a remarkable series of changes takes place, which result in the invagination of the half of it formed of the clear [Pg 663] cells, and form a prelude to the permanent attachment of the larva. The entire process of invagination is completed in about half an hour. The whole embryo first becomes flattened, but especially the ciliated half which gradually becomes less prominent (fig. 1, B), and still later the cells composing it undergo a true process of invagination. As a result of this invagination the segmentation cavity is obliterated and the larva assumes a compressed plano-convex form with a central gastrula cavity, and a blastopore in the middle of the flattened surface. The two layers of the gastrula may now be spoken of as ectoderm and entoderm. The blastopore becomes gradually narrowed by the growth over it of the outer row of granular cells. When it has become very small the attachment of the larva takes place by the flat surface where the blastopore is situated. It is effected by protoplasmic processes of the outer ring of ectoderm cells, which, together with the other ectoderm cells, now become amœboid. At the same time they become clearer and permit a view of the interior of the gastrula. Between the ectoderm cells and the entoderm cells which line the gastrula cavity there arises a hyaline structureless layer, which is more closely attached to the ectoderm than to the entoderm, and is probably derived from the former. A view of the gastrula stage after the larva has become fixed is given in fig. 2.
Fig. 2.
Fixed Gastrula stage of Sycandra raphanus (copied from Schulze).
The figure shews the amœboid ectoderm cells (ec) derived from the granular cells of the earlier stage, and the columnar entoderm cells, lining the gastrula cavity, derived from the ciliated cells of the earlier stage. The larva is fixed by the amœboid cells on the side on which the blastopore is situated.
The young of Sycandra raphanus shortly after the
development of the spicula
(copied from Schulze).
A. View from the side; B. view from the free extremity; os. osculum; ec. ectoderm; en. entoderm composed of collared ciliated cells. The terminal osculum and lateral pores are represented as oval white spaces.
After invagination the cilia of the entoderm cells can no longer be seen, and are probably absorbed, and their disappearance is nearly coincident with the complete obliteration of the blastopore, an event which takes place shortly after the attachment of the larva. After the formation of the structureless layer between the ectoderm and entoderm, calcareous spicules make their appearance in it as delicate unbranched rods pointed at both extremities. The larva when once fixed rapidly grows in length and assumes a cylindrical form (fig. 3, A). The sides of the cylinder are beset with calcareous spicules which project beyond the surface, and in addition to the unbranched forms, spicules are developed with three and four rays as well as some with a blunt extremity and serrated edge. The extremity of the cylinder opposite the attached surface is flattened, and [Pg 665] though surrounded by a ring of four-rayed spicules is itself free from them. At this extremity a small perforation is formed leading into the gastric cavity which rapidly increases in size and forms an exhalent osculum (os). A series of inhalent apertures are also formed at the sides of the cylinder. The relative times of appearance of the single osculum and smaller apertures is not constant for the different larvæ. On the central gastrula cavity of the sponge becoming placed in communication with the external water, the entoderm cells lining it become ciliated afresh (fig. 3, B, en) and develop the peculiar collar characteristic of the entoderm cells of the Spongida. When this stage of development is reached we have a fully developed sponge of the type made known by Haeckel as Olynthus.
Till the complete development of other forms of Spongida has been worked out it is not possible to feel sure how far the phenomena observable in Sycandra hold good in all cases. Quite recently the Russian embryologist, M. Ganin[468], has given an account, without illustrations, of the development of Spongilla fluviatilis, which does not appear reconcileable with that of Sycandra. Considering the difficulties of observation it appears better to assume for this and some other descriptions that the observations are in error rather than that there is a fundamental want of uniformity in development amongst the Spongida.
The first point in the development of Sycandra which deserves notice is the character of the free swimming larva. The peculiar larval form, with one half of the body composed of amœboid granular cells and the other of clear ciliated cells is nearly constant amongst the Calcispongiæ, and widely distributed in a somewhat modified condition amongst the Fibrospongiæ and Myxospongiæ. Does this larva retain the characters of an ancestral type of the Spongida, and if so what does its form mean? It is, of course, possible that it has no ancestral meaning but has been secondarily acquired; I prefer myself to think that this is not the case, more especially as it appears to me that the characters of the larva may be plausibly explained by regarding it as a transitional form between the Protozoa and Metazoa. According to this view the larva is to be considered [Pg 666] as a colony of Protozoa, one half of the individuals of which have become differentiated into nutritive forms, and the other half into locomotor and respiratory forms. The granular amœboid cells represent the nutritive forms, and the ciliated cells represent the locomotor and respiratory forms. That the passage from the Protozoa to the Metazoa may have been effected by such a differentiation is not improbable on à priori grounds, and fits in very well with the condition of the free swimming larva of Spongida, though another and perhaps equally plausible suggestion as to this passage has been put forward by my friend Professor Lankester[469].
While the above view seems fairly satisfactory for the free swimming stage of the larval Sponge there arises in the subsequent development a difficulty which appears at first sight fatal to it. This difficulty is the invagination of the ciliated cells instead of the granular ones. If the granular cells represent the nutritive individuals of the colony, they and not the ciliated cells ought most certainly to give rise to the lining of the gastrula cavity, according to the generally accepted views of the morphology of the Spongida. The suggestion which I would venture to put forward in explanation of this paradox involves a completely new view of the nature and functions of the germinal layers of adult Sponges.
It is as follows:—When the free swimming ancestor of the Spongida became fixed, the ciliated cells by which its movements used to be effected must have to a great extent become functionless. At the same time the amœboid nutritive cells would need to expose as large a surface as possible. In these two considerations there may, perhaps, be found a sufficient explanation of the invagination of the ciliated cells, and the growth of the amœboid cells over them. Though respiration was, no doubt, mainly effected by the ciliated cells, it is improbable that it was completely localised in them, but the continuation of their function was provided for by the formation [Pg 667] of an osculum and pores. The ciliated collared cells which line the ciliated chambers, or in some cases the radial tubes, are undoubtedly derived from the invaginated cells, and if there is any truth in the above suggestion, the collared cells in the adult Sponge must be mainly respiratory and not digestive in function, while the normal epithelial cells which cover the surface of the sponge, and in most cases line the greater part of the passages through its substance, must carry on the digestion[470]. If the reverse is the case the whole theory falls to the ground. It has not, so far as I know, been definitely made out where the digestion is carried on. Lieberkühn would appear to hold the view that the amœboid lining cells of the passages are mainly concerned with digestion, while Carter holds that digestion is carried on by the collared cells of the ciliated chambers.
If it is eventually proved by actual experiments on the nutrition of Sponges, that digestion is carried on by the general cells lining the passages, and not by the ciliated cells, it is clear that neither the ectoderm nor entoderm of Sponges will correspond with the similarly named layers in the Cœlenterata and the Metazoa[TN13]. The invaginated entoderm will be the respiratory layer and the ectoderm the digestive and sensory layer; the sensory function being probably mainly localised in the epithelium on the surface, and the digestive one in the epithelium lining the passages. Such a fundamental difference in the germinal layers between the Spongida and the other Metazoa, would necessarily involve the creation of a special division of the Metazoa for the reception of the former group.
[465] From the Quarterly Journ. of Microscopical Science, Vol. XIX. 1879.
[466]
Untersuchungen über d. Bau u. d. Entwicklung der
Spongien,
Zeit. f. wiss. Zool. Bd. XXXI. 1878.
[467]
Zur Entwicklungsgeschichte der Kalkschwämme,
Zeit. f. wiss. Zool. Bd. XXIV. 1874.
[468]
Zur Entwicklung d. Spongilla fluviatilis,
Zoologischer Anzeiger, Vol.
I. No. 9,
1878.
[469]
Notes on Embryology and Classification.
Quarterly Journal of
Microscopical Science, Vol. XVII. 1877. It
seems not impossible, if the speculations in this paper have any
foundation that while the views here put forward as to the passage from
the Protozoon to the Metazoon condition may hold true for the Spongida,
some other mode of passage may have taken place in the case of the other
Metazoa.
[470] That the flat cells which line the greater part of the passages of most Sponges are really derived from ectodermic invaginations appears to me clearly proved by Schulze's and Barrois' observations on the young fixed stages of Halisarea. Ganin appears, however, to maintain a contrary view for Spongilla.
(With Plates 30, 31, 32.)
The following observations do not profess to contain a complete history of the development even of a single species of spider. They are the result of investigations carried on at intervals during rather more than two years, on the ova of Agelena labyrinthica; and I should not have published them now, if I had any hope of being able to complete them before the appearance of the work I am in the course of publishing on Comparative Embryology. It appeared to me, however, desirable to publish in full such parts of my observations as are completed before the appearance of my treatise, since the account of the development of the Araneina is mainly founded upon them.
My investigations on the germinal layers and organs have been chiefly conducted by means of sections. To prepare the embryos for sections, I employed the valuable method first made known by Bobretzky. I hardened the embryos in bichromate of potash, after placing them for a short time in nearly boiling water. They were stained as a whole with hæmatoxylin after the removal of the membranes, and embedded for cutting in coagulated albumen.
The number of investigators who have studied the development of spiders is inconsiderable. A list of them is given at the end of the paper.
The earliest writer on the subject is Herold (No. 4); he was followed after a very considerable interval of time by Claparède [Pg 669] (No. 3), whose memoir is illustrated by a series of beautiful plates, and contains a very satisfactory account of the external features of development.
Balbiani (No. 1) has gone with some detail into the history of the early stages; and Ludwig (No. 5) has published some very important observations on the development of the blastoderm. Finally, Barrois (No. 2) has quite recently taken up the study of the group, and has added some valuable observations on the development of the germinal layers.
In addition to these papers on the true spiders, important investigations have been published by Metschnikoff on other groups of the Arachnida, notably the scorpion. Metschnikoff's observations on the formation of the germinal layers and organs accord in most points with my own.
The development of the Araneina may be divided into four periods: (1) the segmentation; (2) the period from the close of the segmentation up to the period when the segments commence to be formed; (3) the period from the commencing formation of the segments to the development of the full number of limbs; (4) the subsequent stages up to the attainment of the adult form.
In my earliest stage the segmentation was already completed, and the embryo was formed of a single layer of large flattened cells enveloping a central mass of polygonal yolk-segments.
Each yolk-segment is formed of a number of large clear somewhat oval yolk-spherules. In hardened specimens the yolk-spherules become polygonal, and in ova treated with hot water prior to preservation are not unfrequently broken up. Amongst the yolk-segments are placed a fair number of nucleated bodies of a very characteristic appearance. Each of them is formed of (1) a large, often angular, nucleus, filled with deeply staining bodies (nucleoli?); (2) [TN14] a layer of protoplasm surrounding the nucleus, prolonged into a protoplasmic reticulum. The exact relation of these nucleated bodies to the yolk-segments is not very easy to make out, but the general tendency of my observations is to shew (1) that each nucleated body belongs to a yolk-sphere, and (2) that it is generally placed not at the centre, but to one side of a yolk-sphere. If the above conclusions are correct each complete yolk-segment is a cell, and each such [Pg 670] cell consists of a normal nucleus, protoplasm, and yolk-spherules. There is a special layer of protoplasm surrounding the nucleus, while the remainder of the protoplasm consists of a reticulum holding together the yolk-spherules. Yolk-cells of this character are seen in Pls. 31 and 32, figs. 10-21.
The nuclei of the yolk-cells are probably derived by division from the nuclei of the segmentation rosettes (vide Ludwig, No. 5), and it is probable that they take their origin at the time when the superficial layer of protoplasm separates from the yolk-columns below to form the blastoderm.
The protoplasm of the yolk-cells undergoes rapid division, as is shewn by the fact that there are often two nucleated bodies close together, and sometimes two nuclei in a single mass of protoplasm (fig. 10). It is probable that in some cases the yolk-spheres divide at the same time as the protoplasm belonging to them; the division of the nucleated bodies is, however, in the main destined to give rise to fresh cells which enter the blastoderm.
I have not elucidated to my complete satisfaction the next stage or two in the development of the embryo; and have not succeeded in completely reconciling the results of my own observations with those of Claparède and Balbiani. In order to shew exactly where my difficulties lie it is necessary briefly to state the results arrived at by the above authors.
According to Claparède the first differentiation in Pholcus consists in the accumulation of the cells over a small area to form a protuberance, which he calls the primitive cumulus. Owing to its smaller specific gravity the part of the ovum with the cumulus always turns upwards, like the blastodermic pole of a fowl's egg.
After a short time the cumulus elongates itself on one side, and becomes connected by a streak with a white patch, which appears on the surface of the egg, about 90° from the cumulus. This patch gradually enlarges, and soon covers the whole surface of the ovum except the region where the cumulus is placed. It becomes the ventral plate or germinal streak of the embryo, its extremity adjoining the cumulus is the anal extremity, and its opposite extremity the cephalic one. The cumulus itself is placed in a depression on the dorsal surface of the ovum. [Pg 671] Claparède compares the cumulus to the dorsal organ of many Crustacea.
Balbiani (No. 1) describes the primitive cumulus in Tegenaria domestica, Epeira diadema, and Agelena labyrinthica, as originating as a protuberance at the centre of the ventral surface, surrounded by a specialised portion of the blastoderm (p. 57), which I will call the ventral plate. In Tegenaria domestica he finds that it encloses the so-called yolk-nucleus, p. 62. By an unequal growth of the ventral plate the primitive cumulus comes to be placed at the cephalic pole of the ventral plate. The cumulus now becomes less prominent, and in a few cases disappears. In the next stage the central part of the ventral plate becomes very prominent and forms the procephalic lobe, close to the anterior border of which is usually placed the primitive cumulus (p. 67). The space between the cumulus and the procephalic lobe grows larger, so that the latter gradually travels towards the dorsal surface and finally vanishes. Behind the procephalic lobe the first traces of the segments make their appearance, as three transverse bands, before a distinct anal lobe becomes apparent.
The points which require to be cleared up are, (1) what is the nature of the primitive cumulus? (2) where is it situated in relation to the embryo? Before attempting to answer these questions I will shortly describe the development, so far as I have made it out, for the stages during which the cumulus is visible.
The first change that I find in the embryo (when examined after it has been hardened)[472] is the appearance of a small, whitish spot, which is at first very indistinct. A section through such an ovum (Pl. 31, fig. 10) shews that the cells of about one half of the ovum have become more columnar than those of the other half, and that there is a point (pr.c.) near one end of the thickened half where the cells are more columnar, and about two layers or so deep. It appears to me probable that this point is the whitish spot visible in the hardened ovum. In a somewhat later stage (Pl. 30, fig. 1) the whitish spot becomes more conspicuous [Pg 672] (pc.), and appears as a distinct prominence, which is, without doubt, the primitive cumulus, and from it there proceeds on one side a whitish streak. The prominence, as noticed by Claparède and Balbiani, is situated on the flatter side of the ovum. Sections at this stage shew the same features as the previous stage, except that (1) the cells throughout are smaller, (2) those of the thickened hemisphere of the ovum more columnar, and (3) the cumulus is formed of several rows of cells, though not divided into distinct layers. In the next stage the appearances from the surface are rather more obscure, and in some of my best specimens a coagulum, derived from the fluid surrounding the ovum, covers the most important part of the blastoderm. In Pl. 30, fig. 2, I have attempted to represent, as truly as I could, the appearances presented by the ovum. There is a well-marked whitish side of the ovum, near one end of which is a prominence (pc.), which must, no doubt, be identified with the cumulus of the earlier stages. Towards the opposite end, or perhaps rather nearer the centre of the white side of the ovum, is an imperfectly marked triangular white area. There can be no doubt that the line connecting the cumulus with the triangular area is the future long axis of the embryo, and the white area is, without doubt, the procephalic lobe of Balbiani.
A section of the ovum at this stage is represented in Pl. 31, fig. 11. It is not quite certain in what direction the section is taken, but I think it probable it is somewhat oblique to the long axis. However this may be, the section shews that the whitish hemisphere of the blastoderm is formed of columnar cells, for the most part two or so layers deep, but that there is, not very far from the middle line, a wedge-shaped internal thickening of the blastoderm where the cells are several rows deep. With what part visible in surface view this thickened portion corresponds is not clear. To my mind it most probably corresponds to the larger white patch, in which case I have not got a section through the terminal prominence. In the other sections of the same embryo the wedge-shaped thickening was not so marked, but it, nevertheless, extended through all the sections. It appears to me probable that it constitutes a longitudinal thickened ridge of the blastoderm. In any case, it is clear that the white hemisphere of the blastoderm is a thickened portion of the [Pg 673] blastoderm, and that the thickening is in part due to the cells being more columnar, and, in part, to their being more than one row deep, though they have not become divided into two distinct germinal layers. It is further clear that the increase in the number of cells in the thickened part of the blastoderm is, in the main, a result of the multiplication of the original single row of cells, while a careful examination of my sections proves that it is also partly due to cells, derived from the yolk, having been added to the blastoderm.
In the following stage which I have obtained (which cannot be very much older than the previous stage, because my specimens of it come from the same batch of eggs), a distinct and fairly circumscribed thickening forming the ventral surface of the embryo has become established. Though its component parts are somewhat indistinct, it appears to consist of a procephalic lobe, a less prominent caudal lobe, and an intermediate portion divided into about three segments; but its constituents cannot be clearly identified with the structures visible in the previous stage. I am inclined, however, to identify the anterior thickened area of the previous stage with the procephalic lobe, and a slight protuberance of the caudal portion (visible from the surface) with the primitive cumulus. I have, however, failed to meet with any trace of the cumulus in my sections.
To this stage, which forms the first of the second period of the larval history, I shall return, but it is necessary now to go back to the observations of Claparède and Balbiani.
There can, in the first place, be but little doubt that what I have called the primitive cumulus in my description is the structure so named by Claparède and Balbiani.
It is clear that Balbiani and Claparède have both failed to appreciate the importance of the organ, which my observations shew to be the part of the ventral thickening of the blastoderm where two rows of cells are first established, and therefore the point where the first traces of the future mesoblast becomes visible.
Though Claparède and Balbiani differ somewhat as to the position of the organ, they both make it last longer than I do: I feel certainly inclined to doubt whether Claparède is right in considering a body he figures after six segments are present, to [Pg 674] be the same as the dorsal organ of the embryo before the formation of any segments, especially as all the stages between the two appear to have escaped him. In Agelena there is undoubtedly no organ in the position he gives when six segments are found.
Balbiani's observations accord fairly with my own up to the stage represented in fig. 2. Beyond this stage my own observations are not satisfactory, but I must state that I feel doubtful whether Balbiani is correct in his description of the gradual separation of the procephalic lobe and the cumulus, and the passage of the latter to the dorsal surface, and think it possible that he may have made a mistake as to which side of the procephalic lobe, in relation to the parts of the embryo, the cumulus is placed.
Although there appear to be grounds for doubting whether either Balbiani and Claparède are correct in the position they assign to the cumulus, my observations scarcely warrant me in being very definite in my statements on this head, but, as already mentioned, I am inclined to place the organ near the posterior end (and therefore, as will be afterwards shewn, in a somewhat dorsal situation) of the ventral embryonic thickening.
In my earliest stage of the third period there is present, as has already been stated, a procephalic lobe, and an indistinct and not very prominent caudal portion, and about three segments between the two. The definition of the parts of the blastoderm at this stage is still very imperfect, but from subsequent stages it appears to me probable that the first of the three segments is that of the first pair of ambulatory limbs, and that the segments of the cheliceræ and pedipalpi are formed later than those of the first three ambulatory appendages.
Balbiani believes that the segment of the cheliceræ is formed later than that of the six succeeding segments. He further concludes, from the fact that this segment is cut off from the procephalic portion in front, that it is really part of the procephalic lobe. I cannot accept the validity of this argument; though I am glad to find myself in, at any rate, partial harmony with the distinguished French embryologist as to the facts. Balbiani denies for this stage the existence of a caudal lobe. There is certainly, as is very well shewn in my longitudinal [Pg 675] sections, a thickening of the blastoderm in the caudal region, though it is not so prominent in surface views as the procephalic lobe.
A transverse section through an embryo at this stage (Pl. 31, fig. 12) shews that there is a ventral plate of somewhat columnar cells more than one row deep, and a dorsal portion of the blastoderm formed of a single row of flattened cells. Every section at this stage shews that the inner layer of cells of the ventral plate is receiving accessions of cells from the yolk, which has not to any appreciable extent altered its constitution. A large cell, passing from the yolk to the blastoderm, is shewn in fig. 12 at y.c.
The cells of the ventral plate are now divided into two distinct layers. The outer of these is the epiblast, the inner the mesoblast. The cells of both layers are quite continuous across the median line, and exhibit no trace of a bilateral arrangement.
This stage is an interesting one on account of the striking similarity which (apart from the amnion) exists between a section through the blastoderm of a spider and that of an insect immediately after the formation of the mesoblast. The reader should compare Kowalevsky's (Mém. Acad. Pétersbourg, Vol. XVI. 1871) fig. 26, Pl. IX. with my fig. 12. The existence of a continuous ventral plate of mesoblast has been noticed by Barrois (p. 532), who states that the two mesoblastic bands originate from the longitudinal division of a primitive single band.
In a slightly later stage (Pl. 30, fig. 3a and 3b) six distinct segments are interpolated between the procephalic and the caudal lobes. The two foremost, ch and pd (especially the first), of these are far less distinct than the remainder, and the first segment is very indistinctly separated from the procephalic lobe. From the indistinctness of the first two somites, I conclude that they are later formations than the four succeeding ones. The caudal and procephalic lobes are very similar in appearance, but the procephalic lobe is slightly the wider of the two. There is a slight protuberance on the caudal lobe, which is possibly the remnant of the cumulus. The superficial appearance of segmentation is produced by a series of transverse valleys, separating raised intermediate portions which form the segments. [Pg 676] The ventral thickening of the embryo now occupies rather more than half the circumference of the ovum.
Transverse sections shew that considerable changes have been effected in the constitution of the blastoderm. In the previous stage, the ventral plate was formed of an uniform external layer of epiblast, and a continuous internal layer of mesoblast. The mesoblast has now become divided along the whole length of the embryo, except, perhaps, the procephalic lobes, into two lateral bands which are not continuous across the middle line (Pl. 31, fig. 13, me). It has, moreover, become a much more definite layer, closely attached to the epiblast. Between each mesoblastic band and the adjoining yolk there are placed a few scattered cells, which in a somewhat later stage become the splanchnic mesoblast. These cells are derived from the yolk-cells; and almost every section contains examples of such cells in the act of joining the mesoblast.
The epiblast of the ventral plate has not, to any great extent, altered in constitution. It is, perhaps, a shade thinner in the median line than it is laterally. The division of the mesoblast plate into two bands, together, perhaps, with the slight reduction of the epiblast in the median ventral line, gives rise at this stage to an imperfectly marked median groove.
The dorsal epiblast is still formed of a single layer of flat cells. In the neighbourhood of this layer the yolk nuclei are especially concentrated. The yolk itself remains as before.
The segments continue to increase regularly, each fresh segment being added in the usual way between the last formed segment and the unsegmented caudal lobe. At the stage when about nine or ten segments have become established, the first rudiments of appendages become visible. At this period (Pl. 30, fig. 4) there is a distinct median ventral groove, extending through the whole length of the embryo, which becomes, however, considerably shallower behind. The procephalic region is distinctly bilobed. The first segment (that of the cheliceræ) is better marked off from it than in the previous stage, but is without a trace of an appendage, and exhibits therefore, in respect to the development of its appendages, the same retardation that characterised its first appearance. The next five segments, viz. those of the pedipalpi and four ambulatory appendages, present [Pg 677] a very well-marked swelling at each extremity. These swellings are the earliest traces of the appendages. Of the three succeeding segments, only the first is well differentiated. The caudal lobe, though less broad than the procephalic lobe, is still a widish structure. The most important internal changes concern the mesoblast, which is now imperfectly though distinctly divided into somites, corresponding with segments visible externally. Each mesoblastic somite is formed of a distinct somatic layer closely attached to the epiblast, and a thinner and less well-marked splanchnic layer. In the appendage-bearing segments the somatic layer is continued up into the appendages.
The epiblast is distinctly thinner in the median line than at the two sides.
The next stage figured (Pl. 30, figs. 5 and 6) is an important one, as it is characterized by the establishment of the full number of appendages. The whole length of the ventral plate has greatly increased, so that it embraces nearly the circumference of the ovum, and there is left uncovered but a very small arc between the two extremities of the plate (Pl. 30, fig. 6; Pl. 31, fig. 15). This arc is the future dorsal portion of the embryo, which lags in its development immensely behind the ventral portion.
There is a very distinctly bilobed procephalic region (pr.l) well separated from the segment with the cheliceræ (ch). It is marked by a shallow groove opening behind into a circular depression (st.)—the earliest rudiment of the stomodæum. The six segments behind the procephalic lobes are the six largest, and each of them bears two prominent appendages. They constitute the six appendage-bearing segments of the adult. The four future ambulatory appendages are equal in size: they are slightly larger than the pedipalpi, and these again than the cheliceræ. Behind the six somites with prominent appendages there are four well-marked somites, each with a small protuberance. These four protuberances are provisional appendages. They have been found in many other genera of Araneina (Claparède, Barrois). The segments behind these are rudimentary and difficult to count, but there are, at any rate, five, and at a slightly later stage probably six, including the anal lobe. These fresh segments have been formed by the continued segmentation of [Pg 678] the anal lobe, which has greatly altered its shape in the process. The ventral groove of the earlier stage is still continued along the whole length of the ventral plate.
By the close of this stage the full number of post-cephalic segments has become established. They are best seen in the longitudinal section (Pl. 31, fig. 15). There are six anterior appendage-bearing segments, followed by four with rudimentary appendages (not seen in this figure), and six without appendages behind. There are, therefore, sixteen in all. This number accords with the result arrived at by Barrois, but is higher by two than that given by Claparède.
The germinal layers (vide Pl. 31, fig. 14) have by this stage undergone a further development. The mesoblastic somites are more fully developed. The general relations of these somites is shewn in longitudinal section in Pl. 31, fig. 15, and in transverse section in Pl. 31, fig. 14. In the tail, where they are simplest (shewn on the upper side in fig. 14), each mesoblastic somite is formed of a somatic layer of more or less cubical cells attached to the epiblast, and a splanchnic layer of flattened cells. Between the two is placed a completely circumscribed cavity, which constitutes part of the embryonic body-cavity. Between the yolk and the splanchnic layer are placed a few scattered cells, which form the latest derivatives of the yolk-cells, and are to be reckoned as part of the splanchnic mesoblast. The mesoblastic somites do not extend outwards beyond the edge of the ventral plate, and the corresponding mesoblastic somites of the two sides do not nearly meet in the middle line. In the limb-bearing somites the mesoblast has the same general characters as in the posterior somites, but the somatic layer is prolonged as a hollow papilliform process into the limb, so that each limb has an axial cavity continuous with the section of the body-cavity of its somite. The description given by Metschnikoff of the formation of the mesoblastic somites in the scorpion, and their continuation into the limbs, closely corresponds with the history of these parts in spiders. In the region of each procephalic lobe the mesoblast is present as a continuous layer underneath the epiblast, but in the earlier part of the stage, at any rate, is not formed of two distinct layers with a cavity between them.
[Pg 679] The epiblast at this stage has also undergone important changes. Along the median ventral groove it has become very thin. On each side of this groove it exhibits in each appendage-bearing somite a well-marked thickening, which gives in surface views the appearance of a slightly raised area (Pl. 30, fig. 5), between each appendage and the median line. These thickenings are the first rudiments of the ventral nerve ganglia. The ventral nerve cord at this stage is formed of two ridge-like thickenings of the epiblast, widely separated in the median line, each of which is constituted of a series of raised divisions—the ganglia—united by shorter, less prominent divisions (fig. 14, vg). The nerve cords are formed from before backwards, and are not at this stage found in the hinder segments. There is a distinct ganglionic thickening for the cheliceræ quite independent of the procephalic lobes.
In the procephalic lobes the epiblast is much thickened, and is formed of several rows of cells. The greater part of it is destined to give rise to the supra-œsophageal ganglia.
During the various changes which have been described the blastoderm cells have been continually dividing, and, together with their nuclei, have become considerably smaller than at first. The yolk cells have in the meantime remained much as before, and are, therefore, considerably larger than the nuclei of the blastoderm cells. They are more numerous than in the earlier stages, but are still surrounded by a protoplasmic body, which is continued into a protoplasmic reticulum. The yolk is still divided up into polygonal segments, but from sections it would appear that the nuclei are more numerous than the segments, though I have failed to arrive at quite definite conclusions on this point.
As development proceeds the appendages grow longer, and gradually bend inwards. They become very soon divided by a series of ring-like constrictions which constitute the first indications of the future joints (Pl. 30, fig. 6). The full number of joints are not at once reached, but in the ambulatory appendages five only appear at first to be formed. There are four joints in the pedipalpi, while the cheliceræ do not exhibit any signs of becoming jointed till somewhat later. The primitive presence of only five joints in the ambulatory appendages [Pg 680] is interesting, as this number is permanent in Insects and in Peripatus.
The next stage figured forms the last of the third period (Pl. 30, figs. 7 and 7a). The ventral plate is still rolled round the egg (fig. 7), and the end of the tail and the procephalic lobes nearly meet dorsally, so that there is but a very slight development of the dorsal region. There are the same number of segments as before, and the chief differences in appearance between the present and the previous stage depend upon the fact (1) that the median ventral integument between the nerve ganglia has become wider, and at the same time thinner; (2) that the limbs have become much more developed; (3) that the stomodæum is definitely established; (4) that the procephalic lobes have undergone considerable development.
Of these features, the three last require a fuller description. The limbs of the two sides are directed towards each other, and nearly meet in the ventral line. The cheliceræ are two-jointed, and terminate in what appear like rudimentary chelæ, a fact which perhaps indicates that the spiders are descended from ancestors with chelate cheliceræ. The four embryonic post-ambulatory appendages are now at the height of their development.
The stomodæum (Pl. 30, fig. 7, and Pl. 31, fig. 17, st) is a deepish pit between the two procephalic lobes, and distinctly in front of the segment of the cheliceræ. It is bordered in front by a large, well-marked, bilobed upper lip, and behind by a smaller lower lip. The large upper lip is a temporary structure, to be compared, perhaps, with the gigantic upper lip of the embryo of Chelifer (cf. Metschnikoff). On each side of and behind the mouth two whitish masses are visible, which are the epiblastic thickenings which constitute the ganglia of the cheliceræ (Pl. 30, fig. 7, ch.g).
The procephalic lobes (pr.l) now form two distinct masses, and each of them is marked by a semicircular groove, dividing them into a narrower anterior and a broader posterior division.
In the region of the trunk the general arrangement of the germinal layers has not altered to any great extent. The ventral ganglionic thickenings are now developed in all the segments in the abdominal as well as in the thoracic region. The individual [Pg 681] thickenings themselves, though much more conspicuous than in the previous stage (Pl. 31, fig. 16, v.c), are still integral parts of the epiblast. They are more widely separated than before in the middle line. The mesoblastic somites retain their earlier constitution (Pl. 31, fig. 16). Beneath the procephalic lobes the mesoblast has, in most respects, a constitution similar to that of a mesoblastic somite in the trunk. It is formed of two bodies, one on each side, each composed of a splanchnic and somatic layer (Pl. 31, fig. 17, sp. and so), enclosing between them a section of the body-cavity. But the cephalic somites, unlike those of the trunk, are united by a median bridge of mesoblast, in which no division into two layers can be detected. This bridge assists in forming a thick investment of mesoblast round the stomodæum (st).
The existence of a section of the body-cavity in the præoral region is a fact of some interest, especially when taken in connection with the discovery, by Kleinenberg, of a similar structure in the head of Lumbricus. The procephalic lobe represents the præoral lobe of Chætopod larvæ, but the prolongation of the body-cavity into it does not, in my opinion, necessarily imply that it is equivalent to a post-oral segment.
The epiblast of the procephalic lobes is a thick layer several cells deep, but without any trace of a separation of the ganglionic portion from the epidermis.
The nuclei of the yolk have increased in number, but the yolk, in other respects, retains its earlier characters.
The next period in the development is that in which the body of the embryo gradually acquires the adult form. The most important event which takes place during this period is the development of the dorsal region of the embryo, which, up to its commencement, is practically non-existent. As a consequence of the development of the dorsal region, the embryo, which has hitherto had what may be called a dorsal flexure, gradually unrolls itself, and acquires a ventral flexure. This change in the flexure of the embryo is in appearance a rather complicated phenomenon, and has been somewhat differently described by the two naturalists who have studied it in recent times.
For Claparède the prime cause of the change of flexure is [Pg 682] the translation dorsalwards of the limbs. He compares the dorsal region of the embryo to the arc of a circle, the two ends of which are united by a cord formed by the line of insertion of the limbs. He points out that if you bring the middle of the cord, so stretched between the two ends of the arc, nearer to the summit of the arc, you necessarily cause the two ends of the arc to approach each other, or, in other words, if the insertion of the limbs is drawn up dorsally, the head and tail must approach each other ventrally.
Barrois takes quite a different view to that of Claparède, which will
perhaps be best understood if I quote a translation of his own words. He
says: At the period of the last stage of the embryonic band (the stage
represented in Pl. 31, fig. 7, in the present
paper) this latter completely encircles the egg, and its posterior
extremity nearly approaches the cephalic region. Finally, the germinal
bands, where they unite at the anal lobe (placed above on the dorsal
surface), form between them a very acute angle. During the following stages
one observes the anal segment separate further and further from the
cephalic region, and approach nearer and nearer to the ventral region. This
displacement of the anal segment determines, in its turn, a modification in
the divergence of the anal bands; the angle which they form at their
junction tends to become more obtuse. The same processes continue regularly
till the anal segment comes to occupy the opposite extremity to the
cephalic region, a period at which the two germinal bands are placed in the
same plane and the two sides of the obtuse angle end by meeting in a
straight line. If we suppose a continuation of the same phenomenon it is
clear that the anal segment will come to occupy a position on the ventral
surface, and the germinal bands to approach, but in the inverse way, so as
to form an angle opposite to that which they formed at first. This
condition ends the process by which the posterior extremity of the
embryonic band, at first directed towards the dorsal side, comes to bend in
towards the ventral region.
Neither of the above explanations is to my mind perfectly satisfactory. The whole phenomenon appears to me to be very simple, and to be caused by the elongation of the dorsal region, i.e. the region on the dorsal surface between the anal and procephalic [Pg 683] lobes. Such an elongation necessarily separates the anal and procephalic lobes; but, since the ventral plate does not become shortened in the process, and the embryo cannot straighten itself on account of the egg-shell, it necessarily becomes flexed, and such flexure can only be what I have already called a ventral flexure. If there were but little food yolk this flexure would cause the whole embryo to be bent in, so as to have the ventral surface concave, but instead of this the flexure is confined at first to the two bands which form the ventral plate. These bands are bent in the natural way (Pl. 30, fig. 8b), but the yolk forms a projection, a kind of yolk-sack as Barrois calls it, distending the thin integument between the two ventral bands. This yolk-sack is shewn in surface view in Pl. 30, fig. 8, and in section in Pl. 32, fig. 18. At a later period, when the yolk has become largely absorbed in the formation of various organs, the true nature of the ventral flexure becomes apparent, and the abdomen of the young Spider is found to be bent over so as to press against the ventral surface of the thorax (Pl. 30, fig. 9). This flexure is shewn in section in Pl. 32, fig. 21.
At the earliest stage of this period of which I have examples, the dorsal region has somewhat increased, though not very much. The limbs have grown very considerably and now cross in the middle line.
The ventral ganglia, though not the supra-œsophageal, have become separated from the epiblast.
The yolk nuclei, each surrounded by protoplasm as before, are much more numerous.
In other respects there are no great changes in the internal features.
In my next stage, represented in Pl. 30, figs. 8a, and 8b, a very considerable advance has become effected. In the first place the dorsal surface has increased in length to rather more than one half the circumference of the ovum. The dorsal region has, however, not only increased in length, but also in definiteness, and a series of transverse markings (figs. 8a and b), which are very conspicuous in the case of the four anterior abdominal segments (the segments with rudimentary appendages), have appeared, indicating the limits of segments dorsally. The terga of the somites may, in fact, be said to have become formed. [Pg 684] The posterior terga (fig. 8a) are very narrow compared to the anterior.
The caudal protuberance is more prominent than it was, and somewhat bilobed; it is continued on each side into one of the bands, into which the ventral plate is divided. These bands, as is best seen in side view (fig. 8b), have a ventral curvature, or, perhaps more correctly, are formed of two parts, which meet at a large angle open towards the ventral surface. The posterior of these parts bears the four still very conspicuous provisional appendages, and the anterior the six pairs of thoracic appendages. The four ambulatory appendages are now seven-jointed, as in the adult, but though longer than in the previous stage they do not any longer cross or even meet in the middle line, but are, on the contrary, separated by a very considerable interval. This is due to the great distension by the yolk of the ventral part of the body, in the interval between the two parts of the original ventral plate. The amount of this yolk may be gathered from the section (Pl. 32, fig. 18). The pedipalpi carry a blade on their basal joint. The cheliceræ no longer appear to spring from an independent postoral segment.
There is a conspicuous lower lip, but the upper is less prominent than before. Sections at this stage shew that the internal changes have been nearly as considerable as the external.
The dorsal region is now formed of a (1) flattened layer of epiblast cells, and a (2) fairly thick layer of large and rather characteristic cells which any one who has studied sections of spider's embryos will recognize as derivatives of the yolk. These cells are not, therefore, derived from prolongations of the somatic and splanchnic layers of the already formed somites, but are new formations derived from the yolk. They commenced to be formed at a much earlier period, and some of them are shewn in the longitudinal section (Pl. 31, fig. 15). In the next stage these cells become differentiated into the somatic and splanchnic mesoblast layers of the dorsal region of the embryo.
In the dorsal region of the abdomen the heart has already become established. So far as I have been able to make out it is formed from a solid cord of the cells of the dorsal region. [Pg 685] The peripheral layer of this cord gives rise to the walls of the heart, while the central cells become converted into the corpuscles of the blood.
The rudiment of the heart is in contact with the epiblast above, and there is no greater evidence of its being derived from the splanchnic than from the somatic mesoblast; it is, in fact, formed before the dorsal mesoblast has become differentiated into two layers.
In the abdomen three or four transverse septa, derived from the splanchnic mesoblast, grow a short way into the yolk. They become more conspicuous during the succeeding stage, and are spoken of in detail in the description of that stage. In the anterior part of the thorax a longitudinal and vertical septum is formed, which grows downwards from the median dorsal line, and divides the yolk in this region into two parts. In this septum there is formed at a later stage a vertical muscle attached to the suctorial part of the stomodæum.
The mesoblastic somites of the earlier stage are but little modified; and there are still prolongations of the body-cavity into the limbs (Pl. 32, fig. 18).
The lateral parts of the ventral nerve cords are now at their maximum of separation (Pl. 32, fig. 18, v.g.). Considerable differentiation has already set in in the constitution of the ganglia themselves, which are composed of an outer mass of ganglion cells enclosing a kernel of nerve fibres, which lie on the inner side and connect the successive ganglia. There are still distinct thoracic and abdominal ganglia for each segment, and there is also a pair of separate ganglion for the cheliceræ, which assists, however, in forming the œsophageal commissures.
The thickenings of the præoral lobe which form the supra-œsophageal ganglia are nearly though not quite separated from the epiblast. The semicircular grooves of the earlier stages are now deeper than before, and are well shewn in sections nearly parallel to the outer anterior surface of the ganglion (Pl. 32, fig. 19). The supra-œsophageal ganglia are still entirely formed of undifferentiated cells, and are without commissural tissue like that present in the ventral ganglia.
The stomodæum has considerably increased in length, and the proctodæum has become formed as a short, posteriorly [Pg 686] directed involution of the epiblast. I have seen traces of what I believe to be two outgrowths from it, which form the Malpighian bodies.
The next stage constitutes (Pl. 30, fig. 9) the last which requires to be dealt with so far as the external features are concerned. The yolk has now mainly passed into the abdomen, and the constriction separating the thorax and abdomen has begun to appear. The yolk-sack has become absorbed, so that the two halves of the ventral plate in the thorax are no longer widely divaricated. The limbs have to a large extent acquired their permanent structure, and the rings of which they are formed in the earlier stages are now replaced by definite joints. A delicate cuticle has become formed, which is not figured in my sections. The four rudimentary appendages have disappeared, unless, which seems to me in the highest degree improbable, they remain as the spinning mammillæ, two pairs of which are now present. Behind is the anal lobe, which is much smaller and less conspicuous than in the previous stage. The spinnerets and anal lobe are shewn as five papillæ in Pl. 30, fig. 9. Dorsally the heart is now very conspicuous, and in front of the cheliceræ may be seen the supra-œsophageal ganglia.
The indifferent mesoblast has now to a great extent become converted into the permanent tissues. On the dorsal surface there was present in the last stage a great mass of unformed mesoblast cells. This mass of cells has now become divided into a somatic and splanchnic layer (Pl. 32, fig. 22). It has, moreover, in the abdominal region at any rate, become divided up into somites. At the junction between the successive somites the splanchnic mesoblast on each side of the abdomen dips down into the yolk and forms a septum (Pl. 32, fig. 22, s). The septa so formed, which were first described by Barrois, are not complete. The septa of the two sides do not, in the first place, quite meet along the median dorsal or ventral lines, and in the second place they only penetrate the yolk for a certain distance. Internally they usually end in a thickened border.
Along the line of insertion of each of these septa there is developed a considerable space between the somatic and splanchnic layers of mesoblast. The parts of the body-cavity so established [Pg 687] are transversely directed channels passing from the heart outwards. They probably constitute the venous spaces, and perhaps also contain the transverse aortic branches.
In the intervals between these venous spaces the somatic and splanchnic layers of mesoblast are in contact with each other.
I have not been able to work out satisfactorily the later stages of development of the septa, but I have found that they play an important part in the subsequent development of the abdomen. In the first place they send off lateral offshoots, which unite the various septa together, and divide up the cavity of the abdomen into a number of partially separated compartments. There appears, however, to be left a free axial space for the alimentary tract, the mesoblastic walls of which are, I believe, formed from the septa.
At the present stage the splanchnic mesoblast, apart from the septa, is a delicate membrane of flattened cells (fig. 22, sp). The somatic mesoblast is thicker, and is formed of scattered cells (so).
The somatic layer is in part converted, in the posterior region of the abdomen, into a delicate layer of longitudinal muscles, the fibres of which are not continuous for the whole length of the body, but are interrupted at the lines of junction of the successive segments. They are not present in the anterior part of the abdomen. The longitudinal direction of these fibres, and their division with myotomes, is interesting, since both these characters, which are preserved in Scorpions, are lost in the abdomen of the adult Spider.
The original mesoblastic somites have undergone quite as important changes as the dorsal mesoblast. In the abdominal region the somatic layer constitutes two powerful bands of longitudinal muscles, inserted anteriorly at the root of the fourth ambulatory appendage, and posteriorly at the spinning mammillæ. Between these two bands are placed the nervous bands. The relation of these parts are shewn in the section in Pl. 32, fig. 20d, which cuts the abdomen horizontally and longitudinally. The mesoblastic bands are seen at m., and the nervous bands within them at ab.g. In the thoracic region the part of the somatic layer in each limb is converted into muscles, which are continued into dorsal and ventral muscles [Pg 688] in the thorax (vide fig. 20c). There are, in addition to these, intrinsic transverse fibres on the ventral side of the thorax. Besides these muscles there are in the thorax, attached to the suctorial extremity of the stomodæum, three powerful muscles, which I believe to be derived from the somatic mesoblast. One of these passes vertically down from the dorsal surface, in the septum the commencement of which was described in the last stage. The two other muscles are lateral, one on each side (Pl. 31, fig. 20c.).
The heart has now, in most respects, reached its full development. It is formed of an outer muscular layer, within which is a doubly-contoured lining, containing nuclei at intervals, which is probably of the nature of an epithelioid lining (Pl. 32, fig. 22, ht). In its lumen are numerous blood-corpuscles (not represented in my figure). The heart lies in a space bound below by the splanchnic mesoblast, and to the sides by the somatic mesoblast. This space forms a kind of pericardium (fig. 22, pc), but dorsally the heart is in contact with the epiblast. The arterial trunks connected with it are fully established.
The nervous system has undergone very important changes.
In the abdominal region the ganglia of each side have fused together into a continuous cord (fig. 21, ab.g). In fig. 20, in which the abdomen is cut horizontally and longitudinally, there are seen the two abdominal cords (ab.g) united by two transverse commissures; and I believe that there are at this stage three or four transverse commissures at any rate, which remain as indications of the separate ganglia, from the coalescence of which the abdominal cords are formed. The two abdominal cords are parallel and in close contact.
In the thoracic region changes of not less importance have taken place. The ganglia are still distinct. The two cords formed of these ganglia are no longer widely separated in median line, but meet, in the usual way, in the ventral line. Transverse commissures have become established (fig. 20c) between the ganglia of the two sides. There is as little trace at this, as at the previous stages, of an ingrowth of epiblast, to form a median portion of the central nervous system. Such a median structure has been described by Hatschek for Lepidoptera, and he states that it gives rise to the transverse commissures [Pg 689] between the ganglia. My observations shew that for the spider, at any rate, nothing of the kind is present.
As shewn in the longitudinal section (Pl. 32, fig. 21), the ganglion of the cheliceræ has now united with the supra-œsophageal ganglion. It forms, as is shewn in fig. 20b (ch.g.), a part of the œsophageal commissure, and there is no sub-œsophageal commissure uniting the ganglia of the cheliceræ, but the œsophageal ring is completed below by the ganglia of the pedipalpi (fig. 20c, pd.g.).
The supra-œsophageal ganglia have become completely separated from the epiblast.
I have unfortunately not studied their constitution in the adult, so that I cannot satisfactorily identify the parts which can be made out at this stage.
I distinguish, however, the following regions:
(1) A central region containing the commissural part, and continuous below with the ganglia of the cheliceræ.
(2) A dorsal region formed of two hemispherical lobes.
(3) A ventral anterior region.
The central region contains in its interior the commissural portion, forming a punctiform, rounded mass in each ganglion. A transverse commissure connects the two (vide fig. 20b).
The dorsal hemispherical lobes are derived from the part which, at the earlier stage, contained the semicircular grooves. When the supra-œsophageal ganglia become separated from the epidermis the cells lining these grooves become constricted off with them, and form part of these ganglia. Two cavities are thus formed in this part of the supra-œsophageal ganglia. These cavities become, for the most part, obliterated, but persist at the outer side of the hemispherical lobes (figs. 20a and 21).
The ventral lobe of the brain is a large mass shewn in longitudinal section in fig. 21. It lies immediately in front of and almost in contact with the ganglia of the cheliceræ.
The two hemispherical lobes agree in position with the fungiform body (pilzhutförmige Körpern), which has attracted so much the attention of anatomists, in the supra-œsophageal ganglia of Insects and Crustacea; but till the adult brain of Spiders has been more fully studied it is not possible to state whether the hemispherical lobes become fungiform bodies.
[Pg 690] Hatschek[473] has described a special epiblastic invagination in the supra-œsophageal ganglion of Bombyx, which is probably identical with the semicircular groove of Spiders and Scorpions, but in the figure he gives the groove does not resemble that in the Arachnida. A similar groove is found in Peripatus, and there forms, as I have found, a large part of the supra-œsophageal ganglia. It is figured by Moseley, Phil. Trans., Vol. CLXIV. pl. lxxv, fig. 9.
The stomodæum is considerably larger than in the last stage, and is lined by a cuticle; it is a blind tube, the blind end of which is the suctorial pouch of the adult. To this pouch are attached the vertical dorsal, and two lateral muscles spoken of above.
The proctodæum[TN15] (pr.) has also grown in length, and the two Malpighian vessels which grow out from its blind extremity (fig. 20e, mp.g.) have become quite distinct. The part now formed is the rectum of the adult. The proctodæum is surrounded by a great mass of splanchnic mesoblast. The mesenteron has as yet hardly commenced to be developed. There is, however, a short tube close to the proctodæum (fig. 20e, mes), which would seem to be the commencement of it. It ends blindly on the side adjoining the rectum, but is open anteriorly towards the yolk, and there can be very little doubt that it owes its origin to cells derived from the yolk. On its outer surface is a layer of mesoblast.
From the condition of the mesenteron at this stage there can be but little doubt that it will be formed, not on the surface, but in the interior of the yolk. I failed to find any trace of an anterior part of the mesenteron adjoining the stomodæum. In the posterior part of the thorax (vide fig. 20d), there is undoubtedly no trace of the alimentary tract.
The presence of this rudiment shews that Barrois is mistaken in supposing that the alimentary canal is formed entirely from the stomodæum and proctodæum, which are stated by him to grow towards each other, and to meet at the junction of the thorax and abdomen. My own impression is that the stomodæum and proctodæum have reached their full extension at the [Pg 691] present stage, and that both the stomach in the thorax and the intestine in the abdomen are products of the mesenteron.
The yolk retains its earlier constitution, being divided into polygonal segments, formed of large yolk vesicles. The nuclei are more numerous than before. In the thorax the yolk is anteriorly divided into two lobes by the vertical septum, which contains the vertical muscle of the suctorial pouch. In the posterior part of the thorax it is undivided.
I have not yet been able clearly to make out the eventual fate of the yolk. At a subsequent stage, when the cavity of the abdomen is cut up into a series of compartments by the growth of the septa, described above, the yolk fills these compartments, and there is undoubtedly a proliferation of yolk cells round the walls of these compartments. It would not be unreasonable to conclude from this that the compartments were destined to form the hepatic cæca, each cæcum being enclosed in a layer of splanchnic mesoblast, and its hypoblastic wall being derived from the yolk cells. I think that this hypothesis is probably correct, but I have met with some facts which made me think it possible that the thickenings at the ends of the septa, visible in Pl. 32, fig. 22, were the commencing hepatic cæca.
I must, in fact, admit that I have hitherto failed to work out satisfactorily the history of the mesenteron and its appendages. The firm cuticle of young spiders is an obstacle both in the way of making sections and of staining, which I have not yet overcome.
General Conclusions.
Without attempting to compare at length the development of the spiders with that of other Arthropoda, I propose to point out a few features in the development of spiders, which appear to shew that the Arachnida are undoubtedly more closely related to the other Tracheata than to the Crustacea.
The whole history of the formation of the mesoblast is very similar to that in insects. The mesoblast in both groups is formed by a thickening of the median line of the ventral plate (germinal streak).
[Pg 692] In insects there is usually formed a median groove, the walls of which become converted into a plate of mesoblast. In spiders there is no such groove, but a median keel-like thickening of the ventral plate (Pl. 31, fig. 11), is very probably an homologous structure. The unpaired plate of mesoblast formed in both insects and Arachnida is exactly similar, and becomes divided, in both groups, into two bands, one on each side of the middle line. Such differences as there are between Insects and Arachnida sink into insignificance compared with the immense differences in the origin of the mesoblast between either group, and that in the Isopoda, or, still more, the Malacostraca and most Crustacea. In most Crustacea we find that the mesoblast is budded off from the walls of an invagination, which gives rise to the mesenteron.
In both spiders and Myriopoda, and probably insects, the mesoblast is subsequently divided into somites, the lumen of which is continued into the limbs. In Crustacea mesoblastic somites have not usually been found, though they appear occasionally to occur, e.g. Mysis, but they are in no case similar to those in the Tracheata.
In the formation of the alimentary tract, again, the differences between the Crustacea and Tracheata are equally marked, and the Arachnida agree with the Tracheata. There is generally in Crustacea an invagination, which gives rise to the mesenteron. In Tracheata this never occurs. The proctodæum is usually formed in Crustacea before or, at any rate, not later than the stomodæum[474]. The reverse is true for the Tracheata. In Crustacea the proctodæum and stomodæum, especially the former, are very long, and usually give rise to the greater part of the alimentary tract, while the mesenteron is usually short.
In the Tracheata the mesenteron is always considerable, and the proctodæum is always short. The derivation of the Malpighian bodies from the proctodæum is common to most Tracheata. Such organs are not found in the Crustacea.
With reference to other points in my investigations, the evidence which I have got that the cheliceræ are true postoral appendages supplied in the embryo from a distinct postoral [Pg 693] ganglion, confirms the conclusions of most previous investigators, and shews that these appendages are equivalent to the mandibles, or possibly the first pair of maxillæ of other Tracheata. The invagination, which I have found, of part of a groove of epiblast in the formation of the supra-œsophageal ganglia is of interest, owing to the wide extension of a similar occurrence amongst the Tracheata.
The wide divarication of the ventral nerve cords in the embryo renders it easy to prove that there is no median invagination of epiblast between them, and supports Kleinenberg's observations on Lumbricus as to the absence of this invagination. I have further satisfied myself as to the absence of such an invagination in Peripatus. It is probable that Hatschek and other observers who have followed him are mistaken in affirming the existence of such an invagination in either the Chætopoda or the Arthropoda.
The observations recorded in this paper on the yolk cells and their derivations are, on the whole, in close harmony with the observations of Dohrn, Bobretzky, and Graber, on Insects. They shew, however, that the first formed mesoblastic plate does not give rise to the whole of the mesoblast, but that during the whole of embryonic life the mesoblast continues to receive accessions of cells derived from the cells of the yolk.
Araneina.
1. Balbiani, Mémoire sur le Développement des Araneides,
Ann. Sci. Nat., series v, Vol. XVII. 1873.
2. J. Barrois, Recherches s. l. Développement des Araignées,
Journal de l'Anat. et de la Physiol., 1878.
3. E. Claparède, Recherches s. l'Evolution des Araignées, Utrecht, 1860.
4. Herold, De Generatione Araniorum in Ovo, Marburg, 1824.
5. H. Ludwig, Ueb.
d. Bildung des Blastoderm bei d. Spinnen,
Zeit. f.
wiss. Zool., Vol. XXVI. 1876.
[Pg 694] EXPLANATION OF PLATES 30, 31, AND 32.
Plate 30.
Complete List of Reference Letters.
ch. Cheliceræ. ch.g. Ganglion of cheliceræ. c.l. Caudal lobe. p.c. Primitive cumulus. pd. Pedipalpi. pr.l. Præoral lobe. pp1. pp2. etc. Provisional appendages. sp. Spinnerets. st. Stomodæum.
I-IV. Ambulatory appendages. 1-16. Postoral segments.
Fig. 1. Ovum, with primitive cumulus and streak proceeding from it.
Fig. 2. Somewhat later stage, in which the primitive cumulus is still visible. Near the opposite end of the blastoderm is a white area, which is probably the rudiment of the procephalic lobe.
Fig. 3a and 3b. View of an embryo from the ventral surface and from the side when six segments have become established.
Fig. 4. View of an embryo, ideally unrolled, when the first rudiments of the appendages become visible.
Fig. 5. Embryo ideally unrolled at the stage when all the appendages have become established.
Fig. 6. Somewhat older stage, when the limbs begin to be jointed. Viewed from the side.
Fig. 7. Later stage, viewed from the side.
Fig. 7a. Same embryo as fig. 7, ideally unrolled.
Figs. 8a and 8b. View from the ventral surface and from the side of an embryo, after the ventral flexure has considerably advanced.
Fig. 9. Somewhat older embryo, viewed from the ventral surface.
PLATES 31 AND 32.
Complete List of Reference Letters.
ao. Aorta. ab.g. Abdominal nerve cord. ch. Cheliceræ. ch.g. Ganglion of cheliceræ. ep. Epiblast. hs. Hemispherical lobe of supra-œsophageal ganglion. ht. Heart. l.l. Lower lip. m. Muscles. me. Mesoblast. mes. Mesenteron. mp.g. Malpighian tube. ms. Mesoblastic somite. œ. Œsophagus. p.c. Pericardium. pd. Pedipalpi. pd.g. Ganglion of pedipalpi. pr. Proctodæum (rectum). pr.c. Primitive cumulus. s. Septum in abdomen. so. Somatopleure. sp. Splanchnopleure. [Pg 695] st. Stomodæum. su. Suctorial apparatus. su.g. Supra-œsophageal ganglion. th.g. Thoracic ganglion. v.g. Ventral nerve cord. y.c. Cells derived from yolk. yk. Yolk. y.n. Nuclei of yolk cells.
Ig-IVg. Ganglia of ambulatory limbs. 1-16. Postoral segments.
Fig. 10. Section through an ovum, slightly younger than fig. 1. Shewing the primitive cumulus and the columnar character of the cells of one half of the blastoderm.
Fig. 11. Section through an embryo of the same age as fig. 2. Shewing the median thickening of the blastoderm.
Fig. 12. Transverse section through the ventral plate of a somewhat older embryo. Shewing the division of the ventral plate into epiblast and mesoblast.
Fig. 13. Section through the ventral plate of an embryo of the same age as fig. 3, shewing the division of the mesoblast of the ventral plate into two mesoblastic bands.
Fig. 14. Transverse section through an embryo of the same age as fig. 5, passing through an abdominal segment above and a thoracic segment below.
Fig. 15. Longitudinal section slightly to one side of the middle line through an embryo of the same age.
Fig. 16. Transverse section through the ventral plate in the thoracic region of an embryo of the same age as fig. 7.
Fig. 17. Transverse section through the procephalic lobes of an embryo of the same age. gr. Section of hemicircular groove in procephalic lobe.
Fig. 18. Transverse section through the thoracic region of an embryo of the same age as fig. 8.
Fig. 19. Section through the procephalic lobes of an embryo of the same age.
Fig. 20a, b, c, d, e. Five sections through an embryo of the same age as fig. 9. a and b are sections through the procephalic lobes, c through the front part of the thorax. d cuts transversely the posterior parts of the thorax, and longitudinally and horizontally the ventral surface of the abdomen. e cuts the posterior part of the abdomen longitudinally and horizontally, and shews the commencement of the mesenteron.
Fig. 21. Longitudinal and vertical section of an embryo of the same age. The section passes somewhat to one side of the middle line, and shews the structure of the nervous system.
Fig. 22. Transverse section through the dorsal part of the abdomen of an embryo of the same stage as fig. 9.
[471] From the Quarterly Journ. of Microscopical Science, Vol. XX. 1880.
[472] I was unfortunately too much engaged, at the time when the eggs were collected, to study them in the fresh condition; a fact which has added not a little to my difficulties in elucidating the obscure points in the early stages.
[473]
Beiträge z. Entwick. d. Lepidopteren,
Jenaische Zeit., Vol. XI.
p. 124.
[474] If Grobben's account of the development of Moina is correct this statement must be considered not to be universally true.
In an interesting memoir devoted to the elucidation of a series of points in the anatomy and development of the Vertebrata, Schneider[476] has described what he believes to be motor nerves in Amphioxus, which spring from the anterior side of the spinal cord. According to Schneider these nerves have been overlooked by all previous observers except Stieda.
I[477] myself attempted to shew some time ago that anterior roots were absent in Amphioxus; and in some speculations on the cranial nerves, I employed this peculiarity of the nervous system of Amphioxus to support a view that Vertebrata were primitively provided only with nerves of mixed function springing from the posterior side of the spinal cord. Under these circumstances, Schneider's statement naturally attracted my attention, and I have made some efforts to satisfy myself as to its accuracy. The nerves, as he describes them, are very peculiar. They arise from a number of distinct roots in the hinder third of each segment. They form a flat bundle, of which part passes upwards and part downwards. When they meet the muscles they bend backwards, and fuse with the free borders of the muscle-plates. The fibres, which at first sight appear to form the nerve, are, however, transversely striated, and are regarded by Schneider as muscles; and he holds that each muscle-plate sends a process to the edge of the spinal cord, which there receives its innervation. A considerable body of evidence is requisite to justify a belief in the existence of such very extraordinary and unparalleled motor nerves; and for my part I cannot say that Schneider's observations are convincing to me. I have attempted to repeat his observations, employing the methods he describes.
[Pg 697] In the first place, he states that by isolating the spinal cord by boiling in acetic acid, the anterior roots may be brought into view as numerous conical processes of the spinal cord in each segment. I find by treating the spinal cord in this way, that processes more or less similar, but more irregular than those which he figures, are occasionally present; but I cannot persuade myself that they are anything but parts of the sheath of the spinal cord which is not completely dissolved by treatment with acetic acid. By treatment with nitric acid no such processes are to be seen, though the whole length and very finest branches of the posterior nerves are preserved.
By treating with nitric acid and clarifying by oil of cloves, and subsequently removing one half of the body so as to expose the spinal cord in sitû, the origin and distribution of the posterior nerves is very clearly exhibited. But I have failed to detect any trace of the anterior nerve-roots. Horizontal section, which ought also to bring them clearly into view, failed to shew me anything which I could interpret as such. I agree with Schneider that a process of each muscle-plate is prolonged up to the anterior border of the spinal cord, but I can find no trace of a connection between it and the cord.
Schneider has represented a transverse section in which the anterior nerves are figured. I am very familiar with an appearance in section such as that represented in his figure, but I satisfied myself when I previously studied the nerves in Amphioxus, that the body supposed to be a nerve by Schneider was nothing else than part of the intermuscular septum, and after re-examining my sections I see no reason to alter my view.
A very satisfactory proof that the ventral nerves do not exist would be found, if it could be established that the dorsal nerves contained both motor and sensory fibres. So far I have not succeeded in proving this; I have not, however, had fresh specimens to assist me in the investigation. Langerhans[478], whose careful observations appear to me to have been undervalued by Schneider, figures a branch distributed to the muscles, which passes off from the dorsal roots. Till the inaccuracy of this observation is demonstrated, the balance of evidence appears to me to be opposed to Schneider's view.
[475] From the Quarterly Journal of Microscopical Science, Vol. XX. 1880.
[476] Beiträge z. Anat. u. Entwick. d. Wirbelthiere, Berlin, 1879.
[477]
On the Spinal Nerves of Amphioxus,
Journ. of Anat. and Phys. Vol. X. 1876. [This
edition, No. IX. p. 197.]
[478] Archiv f. Mikros. Anatomie, Vol. XII.
In the spring of the present year, Professor Huxley delivered an address at the Royal Institution, to which he gave the felicitous title of 'The coming of age of the origin of species.' It is, as he pointed out, twenty-one years since Mr Darwin's great work was published, and the present occasion is an appropriate one to review the effect which it has had on the progress of biological knowledge.
There is, I may venture to say, no department of biology the growth of which has not been profoundly influenced by the Darwinian theory. When Messrs Darwin and Wallace first enunciated their views to the scientific world, the facts they brought forward seemed to many naturalists insufficient to substantiate their far-reaching conclusions. Since that time an overwhelming mass of evidence has, however, been rapidly accumulating in their favour. Facts which at first appeared to be opposed to their theories have one by one been shewn to afford striking proofs of their truth. There are at the present time but few naturalists who do not accept in the main the Darwinian theory, and even some of those who reject many of Darwin's explanations still accept the fundamental position that all animals are descended from a common stock.
To attempt in the brief time which I have at my disposal to trace the influence of the Darwinian theory on all the branches of anatomy and physiology would be wholly impossible, and I shall confine myself to an attempt to do so for a small section only. There is perhaps no department of Biology which has been so revolutionised, if I may use the term, by the theory of animal evolution, as that of Development or Embryology. The reason of this is not far to seek. According to the Darwinian [Pg 699] theory, the present order of the organic world has been caused by the action of two laws, known as the laws of heredity and of variation. The law of heredity is familiarly exemplified by the well-known fact that offspring resemble their parents. Not only, however, do the offspring belong to the same species as their parents, but they inherit the individual peculiarities of their parents. It is on this that the breeders of cattle depend, and it is a fact of every-day experience amongst ourselves. A further point with reference to heredity to which I must call your attention is the fact that the characters, which display themselves at some special period in the life of the parent, are acquired by the offspring at a corresponding period. Thus, in many birds the males have a special plumage in the adult state. The male offspring is not, however, born with the adult plumage, but only acquires it when it becomes adult.
The law of variation is in a certain sense opposed to the law of heredity. It asserts that the resemblance which offspring bear to their parents is never exact. The contradiction between the two laws is only apparent. All variations and modifications in an organism are directly or indirectly due to its environments; that is to say, they are either produced by some direct influence acting upon the organism itself, or by some more subtle and mysterious action on its parents; and the law of heredity really asserts that the offspring and parent would resemble each other if their environments were the same. Since, however, this is never the case, the offspring always differ to some extent from the parents. Now, according to the law of heredity, every acquired variation tends to be inherited, so that, by a summation of small changes, the animals may come to differ from their parent stock to an indefinite extent.
We are now in a position to follow out the consequences of these two laws in their bearing on development. Their application will best be made apparent by taking a concrete example. Let us suppose a spot on the surface of some very simple organism to become, at a certain period of life, pigmented, and therefore to be especially sensitive to light. In the offspring of this form, the pigment-spot will reappear at a corresponding period; and there will therefore be a period in the life of the offspring during which there is no pigment-spot, and a second period in [Pg 700] which there is one. If a naturalist were to study the life-history, or, in other words, the embryology of this form, this fact about the pigment-spot would come to his notice, and he would be justified, from the laws of heredity, in concluding that the species was descended from an ancestor without a pigment-spot, because a pigment-spot was absent in the young. Now, we may suppose the transparent layer of skin above the pigment-spot to become thickened, so as gradually to form a kind of lens, which would throw an image of external objects on the pigment-spot. In this way a rudimentary eye might be evolved out of the pigment-spot. A naturalist studying the embryology of the form with this eye would find that the pigment-spot was formed before the lens, and he would be justified in concluding, by the same process of reasoning as before, that the ancestors of the form he was studying first acquired a pigment-spot and then a lens. We may picture to ourselves a series of steps by which the simple eye, the origin of which I have traced, might become more complicated; and it is easy to see how an embryologist studying the actual development of this complicated eye would be able to unravel the process of its evolution.
The general nature of the methods of reasoning employed by embryologists, who accept the Darwinian theory, is exemplified by the instance just given. If this method is a legitimate one, and there is no reason to doubt it, we ought to find that animals, in the course of their development, pass through a series of stages, in each of which they resemble one of their remote ancestors; but it is to be remembered that, in accordance with the law of variation, there is a continual tendency to change, and that the longer this tendency acts the greater will be the total effect. Owing to this tendency, we should not expect to find a perfect resemblance between an animal, at different stages of its growth, and its ancestors; and the remoter the ancestors, the less close ought the resemblance to be. In spite, however, of this limitation, it may be laid down as one of the consequences of the law of inheritance that every animal ought, in the course of its individual development, to repeat with more or less fidelity the history of its ancestral evolution.
A direct verification of this proposition is scarcely possible. There is ample ground for concluding that the forms from which [Pg 701] existing animals are descended have in most instances perished; and although there is no reason why they should not have been preserved in a fossil state, yet, owing to the imperfection of the geological record, palæontology is not so often of service as might have been hoped.
While, for the reasons just stated, it is not generally possible to prove by direct observation that existing forms in their embryonic state repeat the characters of their ancestors, there is another method by which the truth of this proposition can be approximately verified.
A comparison of recent and fossil forms shews that there are actually living at the present day representatives of a considerable proportion of the groups which have in previous times existed on the globe, and there are therefore forms allied to the ancestors of those living at the present day, though not actually the same species. If therefore it can be shewn that the embryos of existing forms pass through stages in which they have the characters of more primitive groups, a sufficient proof of our proposition will have been given.
That such is often the case is a well-known fact, and was even known before the publication of Darwin's works. Von Baer, the greatest embryologist of the century, who died at an advanced age but a few years ago, discussed the proposition at considerable length in a work published between the years 1830 and 1840. He came to the conclusion that the embryos of higher forms never actually resemble lower forms, but only the embryos of lower forms; and he further maintained that such resemblances did not hold at all, or only to a very small extent, beyond the limits of the larger groups. Thus he believed that, though the embryos of Vertebrates might agree amongst themselves, there was no resemblance between them and the embryos of any invertebrate group. We now know that these limitations of Von Baer do not hold good, but it is to be remembered that the meaning now attached by embryologists to such resemblances was quite unknown to him.
These preliminary remarks will, I trust, be sufficient to demonstrate how completely modern embryological reasoning is dependent on the two laws of inheritance and variation, which constitute the keystones of the Darwinian theory.
[Pg 702] Before the appearance of the Origin of Species many very valuable embryological investigations were made, but the facts discovered were to their authors merely so many ultimate facts, which admitted of being classified, but could not be explained. No explanation could be offered of why it is that animals, instead of developing in a simple and straightforward way, undergo in the course of their growth a series of complicated changes, during which they often acquire organs which have no function, and which, after remaining visible for a short time, disappear without leaving a trace.
No explanation, for instance, could be offered of why it is that a frog in the course of its growth has a stage in which it breathes like a fish, and then why it is like a newt with a long tail, which gradually becomes absorbed, and finally disappears. To the Darwinian the explanation of such facts is obvious. The stage when the tadpole breathes by gills is a repetition of the stage when the ancestors of the frog had not advanced in the scale of development beyond a fish, while the newt-like stage implies that the ancestors of the frog were at one time organized very much like the newts of to-day. The explanation of such facts has opened out to the embryologist quite a new series of problems. These problems may be divided into two main groups, technically known as those of phylogeny and those of organogeny. The problems of phylogeny deal with the genealogy of the animal kingdom. A complete genealogy would form what is known as a natural classification. To attempt to form such a classification has long been the aim of a large number of naturalists, and it has frequently been attempted without the aid of embryology. The statements made in the earlier part of my address clearly shew how great an assistance embryology is capable of giving in phylogeny; and as a matter of fact embryology has been during the last few years very widely employed in all phylogenetic questions, and the results which have been arrived at have in many cases been very striking. To deal with these results in detail would lead me into too technical a department of my subject; but I may point out that amongst the more striking of the results obtained entirely by embryological methods is the demonstration that the Vertebrata are not, as was nearly universally believed by older [Pg 703] naturalists, separated by a wide gulf from the Invertebrata, but that there is a group of animals, known as the Ascidians, formerly united with the Invertebrata, which are now universally placed with the Vertebrata.
The discoveries recently made in organogeny, or the genesis of organs, have been quite as striking, and in many respects even more interesting, than those in phylogeny, and I propose devoting the remainder of my address to a history of results which have been arrived at with reference to the origin of the nervous system.
To render clear the nature of these results I must say a few words as to the structure of the animal body. The body is always built of certain pieces of protoplasm, which are technically known to biologists as cells. The simplest organisms are composed either of a single piece of this kind, or of several similar pieces loosely aggregated together. Each of these pieces or cells is capable of digesting and assimilating food, and of respiring; it can execute movements, and is sensitive to external stimuli, and can reproduce itself. All the functions of higher animals can, in fact, be carried on in this single cell. Such lowly organized forms are known to naturalists as the Protozoa. All other animals are also composed of cells, but these cells are no longer complete organisms in themselves. They exhibit a division of labour: some carrying on the work of digestion; some, which we call nerve-cells, receiving and conducting stimuli; some, which we call muscle-cells, altering their form—in fact, contracting in one direction—under the action of the stimuli brought to them by the nerve-cells. In most cases a number of cells with the same function are united together, and thus constitute a tissue. Thus the cells which carry on the work of digestion form a lining membrane to a tube or sack, and constitute a tissue known as a secretory epithelium. The whole of the animals with bodies composed of definite tissues of this kind are known as the Metazoa.
A considerable number of early developmental processes are common to the whole of the Metazoa.
In the first place every Metazoon commences its existence as a simple cell, in the sense above defined; this cell is known as the ovum. The first developmental process which takes [Pg 704] place consists in the division or segmentation of the single cell into a number of smaller cells. The cells then arrange themselves into two groups or layers known to embryologists as the primary germinal layers. These two layers are usually placed one within the other round a central cavity. The inner of the two is called the hypoblast, the outer the epiblast. The existence of these two layers in the embryos of vertebrated animals was made out early in the present century by Pander, and his observations were greatly extended by Von Baer and Remak. But it was supposed that these layers were confined to vertebrated animals. In the year 1849, and at greater length in 1859, Huxley demonstrated that the bodies of all the polype tribe or Cœlenterata—that is to say of the group to which the common polype, jelly-fish and the sea-anemone belong—were composed of two layers of cells, and stated that in his opinion these two layers were homologous with the epiblast and hypoblast of vertebrate embryos. This very brilliant discovery came before its time. It fell upon barren ground, and for a long time bore no fruit. In the year 1866 a young Russian naturalist named Kowalevsky began to study by special histological methods the development of a number of invertebrated forms of animals, and discovered that at an early stage of development the bodies of all these animals were divided into germinal layers like those in vertebrates. Biologists were not long in recognizing the importance of these discoveries, and they formed the basis of two remarkable essays, one by our own countryman, Professor Lankester, and the other by a distinguished German naturalist, Professor Haeckel, of Jena.
In these essays the attempt was made to shew that the stage in development already spoken of, in which the cells are arranged in the form of two layers enclosing a central cavity has an ancestral meaning, and that it is to be interpreted to signify that all the Metazoa are descended from an ancestor which had a more or less oval form, with a central digestive cavity provided with a single opening, serving both for the introduction of food and for the ejection of indigestible substances. The body of this ancestor was supposed to have been a double-walled sack formed of an inner layer, the hypoblast, lining the digestive [Pg 705] cavity, and an outer layer, the epiblast. To this form Haeckel gave the name of gastræa or gastrula.
There is every reason to think that Lankester and Haeckel were quite justified in concluding that a form more or less like that just described was the ancestor of the Metazoa; but the further speculations contained in their essays as to the origin of this form from the Protozoa can only be regarded as suggestive feelers, which, however, have been of great importance in stimulating and directing embryological research. It is, moreover, very doubtful whether there are to be found in the developmental histories of most animals any traces of this gastræa ancestor, other than the fact of their passing through a stage in which the cells are divided into two germinal layers.
The key to the nature of the two germinal layers is to be found in Huxley's comparison between them, and the two layers in the fresh-water polype and the sea-anemone. The epiblast is the primitive skin, and the hypoblast is the primitive epithelial wall of the alimentary tract.
In the whole of the polype group, or Cœlenterata, the body remains through life composed of the two layers, which Huxley recognized as homologous with the epiblast and hypoblast of the Vertebrata; but in all the higher Metazoa a third germinal layer, known as the mesoblast, early makes its appearance between the two primary layers. The mesoblast originates as a differentiation of one or of both the primary germinal layers; but although the different views which have been held as to its mode of origin form an important section of the history of recent embryological investigations, I must for the moment confine myself to saying that from this layer there take their origin—the whole of the muscular system, of the vascular system, and of that connective-tissue system which forms the internal skeleton, tendons, and other parts.
We have seen that the epiblast represents the skin or epidermis of the simple sack-like ancestor common to all the Metazoa. In all the higher Metazoa it gives rise, as might be expected, to the epidermis, but it gives rise at the same time to a number of other organs; and, in accordance with the principles laid down in the earlier part of my address, it is to be concluded that the organs so derived have been formed as differentiations of [Pg 706] the primitive epidermis. One of the most interesting of recent embryological discoveries is the fact that the nervous system is, in all but a very few doubtful cases, derived from the epiblast. This fact was made out for vertebrate animals by the great embryologist Von Baer; and the Russian naturalist Kowalevsky, to whose researches I have already alluded, shewed that this was true for a large number of invertebrate animals. The derivation of the nervous system from the epiblast has since been made out for a sufficient number of forms satisfactorily to establish the generalization that it is all but universally derived from the epiblast.
In any animal in which there is no distinct nervous system, it is obvious that the general surface of the body must be sensitive to the action of its surroundings, or to what are technically called stimuli. We know experimentally that this is so in the case of the Protozoa, and of some very simple Metazoa, such as the freshwater Polype or Hydra, where there is no distinct nervous system. The skin or epidermis of the ancestor of the Metazoa was no doubt similarly sensitive; and the fact of the nervous system being derived from the epiblast implies that the functions of the central nervous system, which were originally taken by the whole skin, became gradually concentrated in a special part of the skin which was step by step removed from the surface, and finally became a well-defined organ in the interior of the body.
What were the steps by which this remarkable process took place? How has it come about that there are nerves passing from the central nervous system to all parts of the skin, and also to the muscles? How have the arrangements for reflex actions arisen by which stimuli received on the surface of the body are carried to the central part of the nervous system, and are thence transmitted to the appropriate muscles, and cause them to contract? All these questions require to be answered before we can be said to possess a satisfactory knowledge of the origin of the nervous system. As yet, however, the knowledge of these points derived from embryology is imperfect, although there is every hope that further investigation will render it less so. Fortunately, however, a study of comparative anatomy, especially that of the Cœlenterata, fills up some of the gaps left from our study of embryology.
[Pg 707] From embryology we learn that the ganglion-cells of the central part of the nervous system are originally derived from the simple undifferentiated epithelial cells of the surface of the body. We further learn that the nerves are out-growths of the central nervous system. It was supposed till quite recently that the nerves in Vertebrates were derived from parts of the middle germinal layer or mesoblast, and that they only became secondarily connected with the central nervous system. This is now known not to be the case, but the nerves are formed as processes growing out from the central part of the nervous system.
Another important fact shewn by embryology is that the central nervous system, and percipient portion of the organs of special sense, are often formed from the same part of the primitive epidermis. Thus, in ourselves and in other vertebrate animals the sensitive part of the eye, known as the retina, is formed from two lateral lobes of the front part of the primitive brain. The crystalline lens and cornea of the eye are, however, subsequently formed from the skin.
The same is true for the peculiar compound eyes of crabs or Crustacea. The most important part of the central nervous system of these animals is the supra-œsophageal ganglia, often known as the brain, and these are formed in the embryo from two thickened patches of the skin at the front end of the body. These thickened patches become gradually detached from the surface, remaining covered over by a layer of skin. They then constitute the supra-œsophageal ganglia; but they form not only the ganglia, but also the rhabdons or retinal elements of the eye—the parts in fact which correspond to the rods and cones in our own retina. The layer of epidermis or skin which lies immediately above the supra-œsophageal ganglia becomes gradually converted into the refractive media of the crustacean eye. A cuticle which lies on its surface forms the peculiar facets on the surface of the eye, which are known as the corneal lenses, while the cells of the epidermis give rise to lens-like bodies known as the crystalline cones.
It would be easy to quote further instances of the same kind, but I trust that the two which I have given will be sufficient to shew the kind of relation which often exists between the organs [Pg 708] of special sense, especially those of vision, and the central nervous system. It might have been anticipated à priori that organs of special sense would only appear in animals provided with a well-developed central nervous system. This, however, is not the case. Special cells, with long delicate hairs, which are undoubtedly highly sensitive structures, are present in animals in which as yet nothing has been found which could be called a central nervous system; and there is every reason to think that the organs of special sense originated pari passu with the central nervous system. It is probable that in the simplest organisms the whole body is sensitive to light, but that with the appearance of pigment-cells in certain parts of the body, the sensitiveness to light became localised to the areas where the pigment-cells were present. Since, however, it was necessary that stimuli received by such organs should be communicated to other parts of the body, some of the epidermic cells in the neighbourhood of the pigment-spots, which were at first only sensitive, in the same manner as other cells of the epidermis, became gradually differentiated into special nerve-cells. As to the details of this differentiation, embryology does not as yet throw any great light; but from the study of comparative anatomy there are grounds for thinking that it was somewhat as follows:—Cells placed on the surface sent protoplasmic processes of a nervous nature inwards, which came into connection with nervous processes from similar cells placed in other parts of the body. The cells with such processes then became removed from the surface, forming a deeper layer of the epidermis below the sensitive cells of the organ of vision. With these cells they remained connected by protoplasmic filaments, and thus they came to form a thickening of the epidermis underneath the organ of vision, the cells of which received their stimuli from those of the organ of vision, and transmitted the stimuli so received to other parts of the body. Such a thickening would obviously be the rudiment of a central nervous system, and it is easy to see by what steps it might become gradually larger and more important, and might gradually travel inwards, remaining connected with the sense organ at the surface by protoplasmic filaments, which would then constitute nerves. The rudimentary eye would at first merely consist partly of cells sensitive to light, and partly of optical structures [Pg 709] constituting the lens, which would throw an image of external objects upon it, and so convert the whole structure into a true organ of vision. It has thus come about that, in the development of the individual, the retina or sensitive part of the eye is first formed in connection with the central nervous system, while the lenses of the eye are independently evolved from the epidermis at a later period.
The general features of the origin of the nervous system which have so far been made out by means of the study of embryology are the following:—
(1) That the nervous system of the higher Metazoa has been developed in the course of a long series of generations by a gradual process of differentiation of parts of the epidermis.
(2) That part of the central nervous system of many forms arose as a local collection of nerve-cells in the epidermis, in the neighbourhood of rudimentary organs of vision.
(3) That ganglion cells have been evolved from simple epithelial cells of the epidermis.
(4) That the primitive nerves were outgrowths of the original ganglion cells; and that the nerves of the higher forms are formed as outgrowths of the central nervous system.
The points on which embryology has not yet thrown a satisfactory light are:—
(1) The steps by which the protoplasmic processes, from the primitive epidermic cells, became united together so as to form a network of nerve-fibres, placing the various parts of the body in nervous communication.
(2) The process by which nerves became connected with muscles, so that a stimulus received by a nerve-cell could be communicated to and cause a contraction in a muscle.
Recent investigations on the anatomy of the Cœlenterata, especially of jelly-fish and sea-anemones, have thrown some light on these points, although there is left much that is still obscure.
In our own country Mr Romaines has conducted some interesting physiological experiments on these forms; and Professor Schäfer has made some important histological investigations upon them. In Germany a series of interesting researches have also been made on them by Professors Kleinenberg, Claus and [Pg 710] Eimer, and more especially by the brothers Hertwig, of Jena. Careful histological investigations, especially those of the last-named authors, have made us acquainted with the forms of some very primitive types of nervous system. In the common sea-anemones there are, for instance, no organs of special sense, and no definite central nervous system. There are, however, scattered throughout the skin, and also throughout the lining of the digestive tract, a number of specially modified epithelial cells, which are no doubt delicate organs of sense. They are provided at their free extremity with a long hair, and are prolonged on their inner side into a fine process which penetrates the deeper part of the epithelial layer of the skin or digestive wall. They eventually join a fine network of protoplasmic fibres which forms a special layer immediately within the epithelium. The fibres of this network are no doubt essentially nervous. In addition to fibres there are, moreover, present in the network cells of the same character as the multipolar ganglion-cells in the nervous system of Vertebrates, and some of these cells are characterized by sending a process into the superjacent epithelium. Such cells are obviously epithelial cells in the act of becoming nerve-cells; and it is probable that the nerve-cells are, in fact, sense-cells which have travelled inwards and lost their epithelial character.
There is every reason to think that the network just described is not only continuous with the sense-cells in the epithelium, but that it is also continuous with epithelial cells which are provided with muscular prolongations. The nervous system thus consists of a network of protoplasmic fibres, continuous on the one hand with sense-cells in the epithelium, and on the other with muscular cells. The nervous network is generally distributed both beneath the epithelium of the skin and that of the digestive tract, but is especially concentrated in the disc-like region between the mouth and tentacles. The above observations have thrown a very clear light on the characters of the nervous system at an early stage of its evolution, but they leave unanswered the questions (1) how the nervous network first arose, and (2) how its fibres became continuous with muscles. It is probable that the nervous network took its origin from processes of the sense-cells. The processes of the different cells probably first met and then fused [Pg 711] together, and, becoming more arborescent, finally gave rise to a complicated network.
The connection between this network and the muscular cells also probably took place by a process of contact and fusion.
Epithelial cells with muscular processes were discovered by Kleinenberg before epithelial cells with nervous processes were known, and he suggested that the epithelial part of such cells was a sense-organ, and that the connecting part between this and the contractile processes was a rudimentary nerve. This ingenious theory explained completely the fact of nerves being continuous with muscles; but on the further discoveries being made which I have just described, it became obvious that this theory would have to be abandoned, and that some other explanation would have to be given of the continuity between nerves and muscles. The hypothetical explanation just offered is that of fusion.
It seems very probable that many of the epithelial cells were originally provided with processes the protoplasm of which, like that of the Protozoa, carried on the functions of nerves and muscles at the same time, and that these processes united amongst themselves into a network. By a process of differentiation parts of this network may have become specially contractile, and other parts may have lost their contractility and become solely nervous. In this way the connection between nerves and muscles might be explained, and this hypothesis fits in very well with the condition of the neuro-muscular system as we find it in the Cœlenterata.
The nervous system of the higher Metazoa appears then to have originated from a differentiation of some of the superficial epithelial cells of the body, though it is possible that some parts of the system may have been formed by a differentiation of the alimentary epithelium. The cells of the epithelium were most likely at the same time contractile and sensory, and the differentiation of the nervous system may very probably have commenced, in the first instance, from a specialization in the function of part of a network formed of neuro-muscular prolongations of epithelial cells. A simultaneous differentiation of other parts of the network into muscular fibres may have led to the continuity at present obtaining between nerves and muscles.
[Pg 712] Local differentiations of the nervous network, which was no doubt distributed over the whole body, took place on the formation of organs of special sense, and such differentiations gave rise to the formation of a central nervous system. The central nervous system was at first continuous with the epidermis, but became separated from it and travelled inwards. Ganglion-cells took their origin from sensory epithelial cells, provided with prolongations, continuous with the nervous network. Such epithelial cells gradually lost their epithelial character, and finally became completely detached from the epidermis.
Nerves, such as we find them in the higher types, originated from special differentiations of the nervous network, radiating from the parts of the central nervous system.
Such, briefly, is the present state of our knowledge as to the genesis of the nervous system. I ought not, however, to leave this subject without saying a few words as to the hypothetical views which the distinguished evolutionist Mr Herbert Spencer has put forward on this subject in his work on Psychology.
For Herbert Spencer nerves have originated, not as processes of
epithelial cells, but from the passage of motion along the lines of least
resistance. The nerves would seem, according to this view, to have been
formed in any tissue from the continuous passage of nervous impulses
through it. A wave of molecular disturbance,
he says, passing
along a tract of mingled colloids closely allied in composition, and
isomerically transforming the molecules of one of them, will be apt at the
same time to form some new molecules of the same type,
and thus a nerve
becomes established.
A nervous centre is formed, according to Herbert Spencer, at the point
in the colloid in which nerves are generated, where a single nervous wave
breaks up, and its parts diverge along various lines of least resistance.
At such points some of the nerve-colloid will remain in an amorphous state,
and as the wave of molecular motion will there be checked, it will tend to
cause decompositions amongst the unarranged molecules. The decompositions
must, he says, cause additional molecular motion to be disengaged; so
that along the outgoing lines there will be discharged an augmented wave.
Thus there will arise at this point something having the character of a
ganglion corpuscle.
[Pg 713]These hypotheses of Herbert Spencer, which have been widely adopted in this country, are, it appears to me, not borne out by the discoveries to which I have called your attention to-day. The discovery that nerves have been developed from processes of epithelial cells, gives a very different conception of their genesis to that of Herbert Spencer, which makes them originate from the passage of nervous impulses through a tract of mingled colloids; while the demonstration that ganglion-cells arose as epithelial cells of special sense, which have travelled inwards from the surface, admits still less of a reconciliation with Herbert Spencer's view on the same subject.
Although the present state of our knowledge on the genesis of the nervous system is a great advance on that of a few years ago, there is still much remaining to be done to make it complete.
The subject is well worth the attention of the morphologist, the physiologist, or even of the psychologist, and we must not remain satisfied by filling up the gaps in our knowledge by such hypotheses as I have been compelled to frame. New methods of research will probably be required to grapple with the problems that are still unsolved; but when we look back and survey what has been done in the past, there can be no reason for mistrusting our advance in the future.
(With Plate 33.)
Some years ago the study of the development of the soft parts of the fins in several Elasmobranch types, more especially in Torpedo, led me to the conclusion that the vertebrate limbs were remnants of two continuous lateral fins[480]. More or less similar views (which I was not at that time acquainted with) had been previously held by Maclise, Humphrey, and other anatomists; these views had not, however, met with much acceptance, and diverge in very important points from those put forward by me. Shortly after the appearance of my paper, J. Thacker published two interesting memoirs comparing the skeletal parts of the paired and unpaired fins[481].
In these memoirs Thacker arrives at conclusions as to the nature of the fins in the main similar to mine, but on entirely independent grounds. He attempts to shew that the structure of the skeleton of the paired fins is essentially the same as that of the unpaired fins, and in this comparison lays special stress on the very simple skeleton of the pelvic fin in the cartilaginous Ganoids, more especially in Acipenser and Polyodon. He points out that the skeleton of the pelvic fin of Polyodon consists essentially of a series of nearly isolated rays, which have a strikingly similar arrangement to that of the rays of the skeleton in [Pg 715] many unpaired fins. He sums up his views in the following way[482]:—
"As the dorsal and anal fins were specializations of the median folds of Amphioxus, so the paired fins were specializations of the two lateral folds which are supplementary to the median in completing the circuit of the body. These lateral folds, then, are the homologues of Wolffian ridges, in embryos of higher forms. Here, as in the median fins, there were formed chondroid and finally cartilaginous rods. These became at least twice segmented. The orad ones, with more or less concrescence proximally, were prolonged inwards. The cartilages spreading met in the middle line; and a later extension of the cartilages dorsad completed the limb-girdle.
The limbs of the Protognathostomi consisted of a
series of parallel articulated cartilaginous rays. They may have coalesced
somewhat proximally and orad. In the ventral pair they had extended
themselves mesiad until they had nearly or quite met and formed the
hip-girdle; they had not here extended themselves dorsad. In the pectoral
limb the same state of things prevailed, but was carried a step further,
namely, by the dorsal extension of the cartilage constituting the scapular
portion, thus more nearly forming a ring or girdle.
The most important point in Thacker's theories which I cannot accept is the derivation of the folds, of which the paired fins of the Vertebrata are supposed to be specializations, from the lateral folds of Amphioxus; and Thacker himself recognizes that this part of his theory stands on quite a different footing to the remainder.
Not long after the publication of Thacker's paper, an important memoir was published by Mivart in the Transactions of this Society[483]. The object of the researches recorded in this paper was, as Mivart explains, to test how far the hard parts of the limbs and of the azygos fins may have arisen through centripetal chondrifications or calcifications, and so be genetically exoskeletal[484].
[Pg 716] Mivart's investigations and the majority of his views were independent of Thacker's memoir; but he acknowledges that he has derived from Thacker the view that pelvic and pectoral girdles, as well as the skeleton of the limbs, may have arisen independently of the axial skeleton.
The descriptive part of Mivart's paper contains an account of the structure of a great variety of interesting and undescribed types of paired and unpaired fins, mainly of Elasmobranchii. The following is the summary given by Mivart of the conclusions at which he has arrived[485]:—
"1. Two continuous lateral longitudinal folds were developed, similar to dorsal and ventral median longitudinal folds.
"2. Separate narrow solid supports (radials), in longitudinal series, and with their long axes directed more or less outwards at right angles with the long axis of the body, were developed in varying extents in all these four longitudinal folds.
"3. The longitudinal folds became interrupted variously, but so as to form two prominences on each side, i.e. the primitive paired limbs.
"4. Each anterior paired limb increased in size more rapidly than the posterior limb.
"5. The bases of the cartilaginous supports coalesced as was needed, according to the respective practical needs of the different separate portions of the longitudinal folds, i.e. the respective needs of the several fins.
"6. Occasionally the dorsal radials coalesced (as in Notidanus, &c.) and sought centripetally (Pristis, &c.) adherence to the skeletal axis.
"7. The radials of the hinder paired limb did so more constantly, and ultimately prolonged themselves inwards by mesiad growth from their coalesced base, till the piscine pelvic structure arose, as, e.g., in Squatina.
"8. The pectoral radials with increasing development also coalesced proximally, and thence prolonging themselves inwards to seek a point d'appui, shot dorsad and ventrad to obtain a firm support, and at the same time to avoid the visceral cavity. [Pg 717] Thus they came to abut dorsally against the axial skeleton, and to meet ventrally together in the middle line below.
"9. The lateral fins, as they were applied to support the body on the ground, became elongated, segmented, and narrowed, so that probably the line of the propterygium, or possibly that of the mesopterygium, became the cheiropterygial axis.
"10. The distal end of the incipient cheiropterygium either preserved and enlarged preexisting cartilages or developed fresh ones to serve fresh needs, and so grew into the developed cheiropterygium; but there is not yet enough evidence to determine what was the precise course of this transformation.
"11. The pelvic limb acquired a solid connection with the axial skeleton (a pelvic girdle) through its need of a point d'appui as a locomotive organ on land.
12. The pelvic limb became also elongated; and
when its function was quite similar to that of the pectoral limb, its
structure became also quite similar (e.g. Ichthyosaurus,
Plesiosaurus, Chelydra, &c.); but for the ordinary quadrupedal mode of
progression it became segmented and inflected in a way generally parallel
with, but (from its mode of use) in part inversely to, the inflections of
the pectoral limb.
Günther[486] has propounded a theory on the primitive character of the fins, which, on the whole, fits in with the view that the paired fins are structures of the same nature as the unpaired fins. The interest of Günther's views on the nature of the skeleton of the fins more especially depends upon the fact that he attempts to evolve the fin of Ceratodus from the typical Selachian type of pectoral fin. His own statement on this subject is as follows[487]:—
"On further inquiry into the more distant relations of the Ceratodus-limb, we may perhaps be justified in recognizing in it a modification of the typical form of the Selachian pectoral fin. Leaving aside the usual treble division of the carpal cartilage (which, indeed, is sometimes simple), we find that this shovel-like carpal forms the base for a great number of phalanges, which are arranged in more or less regular transverse rows (zones) and in longitudinal rows (series). The number of phalanges of [Pg 718] the zones and series varies according to the species and the form of the fin; in Cestracion philippi the greater number of phalanges is found in the proximal zones and middle series, all the phalanges decreasing in size from the base of the fin towards the margins. In a Selachian with a long, pointed, scythe-shaped pectoral fin, like that of Ceratodus, we may, from analogy, presume that the arrangement of the cartilages might be somewhat like that shewn in the accompanying diagram, which I have divided into nine zones and fifteen series.
When we now detach the outermost phalanx from each
side of the first horizontal zone, and with it the other phalanges of the
same series, when we allow the remaining phalanges of this zone to coalesce
into one piece (as, in nature, we find coalesced the carpals of
Ceratodus and many phalanges in Selachian fins), and when we repeat
this same process with the following zones and outer series, we arrive at
an arrangement identical with what we actually find in
Ceratodus.
While the researches of Thacker and Mivart are strongly confirmatory of the view at which I had arrived with reference to the nature of the paired fins, other hypotheses as to the nature of the skeleton of the fins have been enunciated, both before and after the publication of my memoir, which are either directly or indirectly opposed to my view.
Huxley in his memoir on Ceratodus, which throws light on so many important morphological problems, has dealt with the nature of paired fins[488].
He holds, in accordance with a view previously adopted by Gegenbaur,
that the limb of Ceratodus presents us with the nearest known
approximation to the fundamental form of vertebrate limb or
archipterygium,
and is of opinion that in a still more archaic fish than
Ceratodus the skeleton of the fin would be made up of homologous
segments, which might be termed pteromeres, each of which would consist of
a mesomere with a preaxial and a postaxial paramere.
He considers that
the pectoral fins of Elasmobranchii, more especially the fin of
Notidanus, which he holds to be the most primitive form of
Elasmobranch fin, results in the simplest possible manner from the [Pg 719]
shortening of the axis of such a fin-skeleton as that of Ceratodus,
and the coalescence of some of its elements.
Huxley does not enter into
the question of the origin of the skeleton of the pelvic fin of
Elasmobranchii.
It will be seen that Huxley's idea of the primitive structure of the archipterygium is not easily reconcilable with the view that the paired fins are parts of a once continuous lateral fin, in that the skeleton of such a lateral fin, if it has existed, must necessarily have consisted of a series of parallel rays.
Gegenbaur[489] has done more than any other living anatomist to elucidate the nature of the fins; and his views on this subject have undergone considerable changes in the course of his investigations. After Günther had worked out the structure of the fin of Ceratodus, Gegenbaur suggested that it constituted the most primitive persisting type of fin, and has moreover formed a theory as to the origin of the fins founded on this view, to the effect that the fins, together with their respective girdles, are to be derived from visceral arches with their rays.
His views on this subject are clearly explained in the subjoined passages quoted from the English translation of his Elements of Comparative Anatomy, pp. 473 and 477.
"The skeleton of the free appendage is attached to the extremity of the girdle. When simplest, this is made up of cartilaginous rods (rays), which differ in their size, segmentation, and relation to one another. One of these rays is larger than the rest, and has a number of other rays attached to its sides. I have given the name of archipterygium to the ground-form of the skeleton which extends from the limb-bearing girdle into the free appendage. The primary ray is the stem of this archipterygium, the characters of which enable us to follow out the lines of development of the skeleton of the appendage. Cartilaginous arches beset with the rays form the branchial skeleton. The form of skeleton of the appendages may be compared with [Pg 720] them; and we are led to the conclusion that it is possible that they may have been derived from such forms. In the branchial skeleton of the Selachii the cartilaginous bars are beset with simple rays. In many a median one is developed to a greater size. As the surrounding rays become smaller, and approach the larger one, we get an intermediate step towards that arrangement in which the larger median ray carries a few smaller ones. This differentiation of one ray, which is thereby raised to a higher grade, may be connected with the primitive form of the appendicular skeleton; and as we compare the girdle with a branchial arch, so we may compare the median ray and its secondary investment of rays with the skeleton of the free appendage.
"All the varied forms which the skeleton of the free appendages exhibits may be derived from a ground-form which persists in a few cases only, and which represents the first, and consequently the lowest, stage of the skeleton in the fin—the archipterygium. This is made up of a stem which consists of jointed pieces of cartilage, which is articulated to the shoulder-girdle and is beset on either side with rays which are likewise jointed. In addition to the rays of the stem there are others which are directly attached to the limb-girdle.
"Ceratodus has a fin-skeleton of this form; in it there is a stem beset with two rows of rays. But there are no rays in the shoulder-girdle. This biserial investment of rays on the stem of the fin may also undergo various kinds of modifications. Among the Dipnoi, Protopterus retains the medial row of rays only, which have the form of fine rods of cartilage; in the Selachii, on the other hand, the lateral rays are considerably developed. The remains of the medial row are ordinarily quite small, but they are always sufficiently distinct to justify us in supposing that in higher forms the two sets of rays might be better developed. Rays are still attached to the stem and are connected with the shoulder-girdle by means of larger plates. The joints of the rays are sometimes broken up into polygonal plates which may further fuse with one another; concrescence of this kind may also affect the pieces which form the base of the fin. By regarding the free rays, which are attached to these basal pieces, as belonging to these basal portions, we are able to [Pg 721] divide the entire skeleton of the fin into three segments—pro-, meso-, and metapterygium.
The metapterygium represents the stem of the
archipterygium and the rays on it. The propterygium and the mesopterygium
are evidently derived from the rays which still remain attached to the
shoulder-girdle.
Since the publication of the memoirs of Thacker, Mivart, and myself, a pupil of Gegenbaur's, M. v. Davidoff[490], has made a series of very valuable observations, in part directed towards demonstrating the incorrectness of our theoretical views, more especially Thacker's and Mivart's view of the genesis of the skeleton of the limbs. Gegenbaur[491] has also written a short paper in connection with Davidoff's memoir, in support of his own as against our views.
It would not be possible here to give an adequate account of Davidoff's observations on the skeleton, muscular system, and nerves of the pelvic fins. His main argument against the view that the paired fins are the remains of a continuous lateral fin is based on the fact that a variable but often considerable number of the spinal nerves in front of the pelvic fin are united by a longitudinal commissure with the true plexus of the nerves supplying the fin. From this he concludes that the pelvic fin has shifted its position, and that it may once therefore have been situated close behind the visceral arches. Granting, however, that Davidoff's deduction from the character of the pelvic plexus is correct, there is, so far as I see, no reason in the nature of the lateral-fin theory why the pelvic fins should not have shifted; and, on the other hand, the longitudinal cord connecting some of the ventral roots in front of the pelvic fin may have another explanation. It may, for instance, be a remnant of the time when the pelvic fin had a more elongated form than at present, and accordingly extended further forwards.
In any case our knowledge of the nature and origin of nervous plexuses is far too imperfect to found upon their characters such conclusions as those of Davidoff.
[Pg 722] Gegenbaur, in his paper above quoted, further urges against Thacker and Mivart's views the fact that there is no proof that the fin of Polyodon is a primitive type; and also suggests that the epithelial line which I have found connecting the embryonic pelvic and pectoral fins in Torpedo may be a rudiment indicating a migration backwards of the pelvic fin.
With reference to the development of the pectoral fin in the Teleostei there are some observations of 'Swirski[492], which unfortunately do not throw very much light upon the nature of the limb.
'Swirski finds that in the Pike the skeleton of the limb is formed of a plate of cartilage continuous with the pectoral girdle, which soon becomes divided into a proximal and a distal portion. The former is subsequently segmented into five basal rays, and the latter into twelve parts, the number of which subsequently becomes reduced.
* * * * *
The observations which I have to lay before the Society were made with the object of determining how far the development of the skeleton of the limbs throws light on the points on which the anatomists whose opinions have just been quoted are at variance.
They were made, in the first instance, to complete a chapter in my work on comparative embryology; and, partly owing to the press of other engagements, but still more to the difficulty of procuring material, my observations are confined to the two British species of the genus Scyllium, viz. Sc. stellare and Sc. canicula; yet I venture to believe that the results at which I have arrived are not wholly without interest.
Before dealing with the development of the skeleton of the fin, it will be convenient to describe with great brevity the structure of the pectoral and pelvic fins of the adult. The pectoral fins consist of broad plates inserted horizontally on the sides of the body; so that in each there may be distinguished a dorsal and a ventral surface, and an anterior and a posterior border. Their shape may best be gathered from the woodcut (fig. 1); and it is to be especially noted that the narrowest part [Pg 723] of the fin is the base, where it is[TN16] attached to the side of the body. The cartilaginous skeleton only occupies a small zone at the base of the fin, the remainder being formed of a fringe supported by radiately arranged horny fibres[493].
Fig. 1.
Pectoral fins and girdle of an adult of Scyllium canicula (natural size, seen from behind and above).
co. Coracoid. sc. scapula. pp. propterygium. mep. mesopterygium. mp. metapterygium. fn. part of fin supported by horny fibre.
Fig. 2.
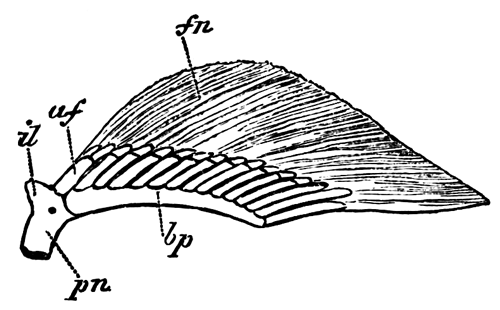
Right pelvic fin and part of pelvic girdle of an adult female of Scyllium canicula (natural size).
il. iliac process. pn. pubic process, cut across below. bp. basipterygium. af. anterior cartilaginous fin-ray articulated to pelvic girdle. fn. part of fin supported by horny fibres.
[Pg 724] The true skeleton consists of three basal pieces articulating with the pectoral girdle; on the outer side of which there is a series of more or less segmented cartilaginous fin-rays. Of the basal cartilages one (pp) is anterior, a second (mep) is placed in the middle, and a third is posterior (mp). They have been named by Gegenbaur the propterygium, the mesopterygium, and the metapterygium; and these names are now generally adopted.
The metapterygium is by far the most important of the three, and in Scyllium canicula supports 12 or 13 rays[494]. It forms a large part of the posterior boundary of the fin, and bears rays only on its anterior border.
The mesopterygium supports 2 or 3 rays, in the basal parts of which the segmentation into distinct rays is imperfect; and the propterygium supports only a single ray.
The pelvic fins are horizontally placed, like the pectoral fins, but differ from the latter in nearly meeting each other along the median ventral line of the body. They also differ from the pectoral fins in having a relatively much broader base of attachment to the sides of the body. Their cartilaginous skeleton (woodcut, fig. 2) consists of a basal bar, placed parallel to the base of the fin, and articulated in front with the pelvic girdle.
On its outer border it articulates with a series of cartilaginous fin-rays. I shall call the basal bar the basipterygium. The rays which it bears are most of them less segmented than those of the pectoral fin, being only divided into two; and the posterior ray, which is placed in the free posterior border of the fin, continues the axis of the basipterygium. In the male it is modified in connection with the so-called clasper.
The anterior fin-ray of the pelvic fin, which is broader than the other rays, articulates directly with the pelvic girdle, instead of with the basipterygium. This ray, in the female of Scyllium canicula and in the male of Scyllium catulus (Gegenbaur), is peculiar in the fact that its distal segment is longitudinally divided into two or more pieces, instead of being single as is the case with the remaining rays. It is probably equivalent to two of the posterior rays.
[Pg 725] Development of the paired Fins.—The first rudiments of the limbs appear in Scyllium, as in other fishes, as slight longitudinal ridge-like thickenings of the epiblast, which closely resemble the first rudiments of the unpaired fins.
These ridges are two in number on each side—an anterior immediately behind the last visceral fold, and a posterior on the level of the cloaca. In most Fishes they are in no way connected; but in some Elasmobranch embryos, more especially in that of Torpedo, they are connected together at their first development by a line of columnar epiblast cells. This connecting line of columnar epiblast, however, is a very transitory structure. The rudimentary fins soon become more prominent, consisting of a projecting ridge both of epiblast and mesoblast, at the outer edge of which is a fold of epiblast only, which soon reaches considerable dimensions. At a later stage the mesoblast penetrates into this fold, and the fin becomes a simple ridge of mesoblast covered by epiblast. The pectoral fins are at first considerably ahead of the pelvic fins in development.
The direction of the original epithelial line which connected the two fins of each side is nearly, though not quite, longitudinal, sloping somewhat obliquely ventralwards. It thus comes about that the attachment of each pair of limbs is somewhat on a slant, and that the pelvic pair nearly meet each other in the median ventral line shortly behind the anus.
The embryonic muscle-plates, as I have elsewhere shewn, grow into the bases of the fins; and the cells derived from these ingrowths, which are placed on the dorsal and ventral surfaces in immediate contact with the epiblast, probably give rise to the dorsal and ventral muscular layers of the limb, which are shewn in section in Plate 33, fig. 1, m, and in Plate 33, fig. 7, m.
The cartilaginous skeleton of the limbs is developed in the indifferent mesoblast cells between the two layers of muscles. Its early development in both the pectoral and the pelvic fins is very similar. When first visible it differs histologically from the adjacent mesoblast simply in the fact of its cells being more concentrated; while its boundary is not sharply marked.
At this stage it can only be studied by means of sections. It arises simultaneously and continuously with the pectoral and pelvic girdles, and consists, in both fins, of a bar springing at [Pg 726] right angles from the posterior side of the pectoral or pelvic girdle, and running parallel to the long axis of the body along the base of the fin. The outer side of this bar is continued into a thin plate, which extends into the fin.
The structure of the skeleton of the fin slightly after its first differentiation will be best understood from Plate 33, fig. 1, and Plate 33, fig. 7. These figures represent transverse sections through the pelvic and pectoral fins of the same embryo on the same scale. The basal bar is seen at bp, and the plate at this stage (which is considerably later than the first differentiation) already partially segmented into rays at br. Outside the region of the cartilaginous plate is seen the fringe with the horny fibres (h.f.); and dorsally and ventrally to the cartilaginous skeleton are seen the already well-differentiated muscles (m).
The pectoral fin is shewn in horizontal section in Plate 33, fig. 6, at a somewhat earlier stage than that to which the transverse sections belong. The pectoral girdle (p.g.) is cut transversely, and is seen to be perfectly continuous with the basal bar (vp) of the fin. A similar continuity between the basal bar of the pelvic fin and the pelvic girdle is shewn in Plate 33, fig. 2, at a somewhat later stage. The plate continuous with the basal bar of the fin is at first, to a considerable extent in the pectoral, and to some extent in the pelvic fin, a continuous lamina, which subsequently segments into rays. In the parts of the plate which eventually form distinct rays, however, almost from the first the cells are more concentrated than in those parts which will form the tissue between the rays; and I am not inclined to lay any stress whatever upon the fact of the cartilaginous fin-rays being primitively part of a continuous lamina, but regard it as a secondary phenomenon, dependent on the mode of conversion of embryonic mesoblast cells into cartilage. In all cases the separation into distinct rays is to a large extent completed before the tissue of which the plates are formed is sufficiently differentiated to be called cartilage by an histologist.
The general position of the fins in relation to the body, and their relative sizes, may be gathered from Plate 33, figs. 4 and 5, which represent transverse sections of the same embryo as that from which the transverse sections shewing the fin on a larger scale were taken.
[Pg 727] During the first stage of its development the skeleton of both fins may thus be described as consisting of a longitudinal bar running along the base of the fin, and giving off at right angles series of rays which pass into the fin. The longitudinal bar may be called the basipterygium; and it is continuous in front with the pectoral or pelvic girdle, as the case may be.
The further development of the primitive skeleton is different in the case of the two fins.
The Pelvic Fin.—The changes in the pelvic fin are comparatively slight. Plate 33, fig. 2, is a representation of the fin and its skeleton in a female of Scyllium stellare shortly after the primitive tissue is converted into cartilage, but while it is still so soft as to require the very greatest care in dissection. The fin itself forms a simple projection of the side of the body. The skeleton consists of a basipterygium (bp), continuous in front with the pelvic girdle. To the outer side of the basipterygium a series of cartilaginous fin-rays are attached—the posterior ray forming a direct prolongation of the basipterygium, while the anterior ray is united rather with the pelvic girdle than with the basipterygium. All the cartilaginous fin-rays except the first are completely continuous with the basipterygium, their structure in section being hardly different from that shewn in Plate 33, fig. 1.
The external form of the fin does not change very greatly in the course of the further development; but the hinder part of the attached border is, to some extent, separated off from the wall of the body, and becomes the posterior border of the adult fin. With the exception of a certain amount of segmentation in the rays, the character of the skeleton remains almost as in the embryo. The changes which take place are illustrated by Plate 33, fig. 3, shewing the fin of a young male of Scyllium stellare. The basipterygium has become somewhat thicker, but is still continuous in front with the pelvic girdle, and otherwise retains its earlier characters. The cartilaginous fin-rays have now become segmented off from it and from the pelvic girdle, the posterior end of the basipterygial bar being segmented off as the terminal ray.
The anterior ray is directly articulated with the pelvic girdle, and the remaining rays continue articulated with the basipterygium. Some of the latter are partially segmented.
[Pg 728] As may be gathered by comparing the figure of the fin at the stage just described with that of the adult fin (woodcut, fig. 2), the remaining changes are very slight. The most important is the segmentation of the basipterygial bar from the pelvic girdle.
The pelvic fin thus retains in all essential points its primitive structure.
The Pectoral Fin.—The earliest stage of the pectoral fin differs, as I have shewn, from that of the pelvic fin only in minor points (Pl. 33, fig. 6). There is the same longitudinal or basipterygial bar (bp), to which the fin-rays are attached, which is continuous in front with the pectoral girdle (pg). The changes which take place in the course of the further development, however, are very much more considerable in the case of the pectoral than in that of the pelvic fin.
The most important change in the external form of the fin is caused by a reduction in the length of its attachment to the body. At first (Pl. 33, fig. 6), the base of the fin is as long as the greatest breadth of the fin; but it gradually becomes shortened by being constricted off from the body at its hinder end. In connection with this process the posterior end of the basipterygial bar is gradually rotated outwards, its anterior end remaining attached to the pectoral girdle. In this way this bar comes to form the posterior border of the skeleton of the fin (Pl. 33, figs. 8 and 9), constituting the metapterygium (mp). It becomes eventually segmented off from the pectoral girdle, simply articulating with its hinder edge.
The plate of cartilage, which is continued outwards from the basipterygium, or, as we may now call it, the metapterygium, into the fin, is not nearly so completely divided up into fin-rays as the homologous part of the pelvic fin; and this is especially the case with the basal part of the plate. This basal part becomes, in fact, at first only divided into two parts (Pl. 33, fig. 8)—a small anterior part at the front end (me.p), and a larger posterior along the base of the metapterygium (mp); and these two parts are not completely segmented from each other. The anterior part directly joins the pectoral girdle at its base, resembling in this respect the anterior fin-ray of the pelvic girdle. It constitutes the (at this stage undivided) rudiment of the mesopterygium [Pg 729] and propterygium of Gegenbaur. It bears in my specimen of this age four fin-rays at its extremity, the anterior not being well marked. The remaining fin-rays are prolongations outwards of the edge of the plate continuous with the metapterygium. These rays are at the stage figured more or less transversely segmented; but at their outer edge they are united together by a nearly continuous rim of cartilage. The spaces between the fin-rays are relatively considerably larger than in the adult.
The further changes in the cartilages of the pectoral limb are, morphologically speaking, not important, and are easily understood by reference to Pl. 33, fig. 9 (representing the skeleton of the limb of a nearly ripe embryo). The front end of the anterior basal cartilage becomes segmented off as a propterygium (pp), bearing a single fin-ray, leaving the remainder of the cartilage as a mesopterygium (mes). The remainder of the now considerably segmented fin-rays are borne by the metapterygium.
* * * * *
General Conclusions.—From the above observations, conclusions of a positive kind may be drawn as to the primitive structure of the skeleton; and the observations have also, it appears to me, important bearings on the theories of my predecessors in this line of investigation.
The most obvious of the positive conclusions is to the effect that the embryonic skeleton of the paired fins consists of a series of parallel rays similar to those of the unpaired fins. These rays support the soft parts of the fins, which have the form of a longitudinal ridge; and they are continuous at their base with a longitudinal bar. This bar, from its position at the base of the fin, can clearly never have been a median axis with the rays on both sides. It becomes the basipterygium in the pelvic fin, which retains its embryonic structure much more completely than the pectoral fin; and the metapterygium in the pectoral fin. The metapterygium of the pectoral fin is thus clearly homologous with the basipterygium of the pelvic fin, as originally supposed by Gegenbaur, and as has since been maintained by Mivart. The propterygium and mesopterygium are obviously relatively unimportant parts of the skeleton as compared with the metapterygium.
[Pg 730] My observations on the development of the skeleton of the fins certainly do not of themselves demonstrate that the paired fins are remnants of a once continuous lateral fin; but they support this view in that they shew the primitive skeleton of the fins to have exactly the character which might have been anticipated if the paired fins had originated from a continuous lateral fin. The longitudinal bar of the paired fins is believed by both Thacker and Mivart to be due to the coalescence of the bases of the primitively independent rays of which they believe the fin to have been originally composed. This view is probable enough in itself, and is rendered more so by the fact, pointed out by Mivart, that a longitudinal bar supporting the cartilaginous rays of unpaired fins is occasionally formed; but there is no trace in the embryo Scylliums of the bar in question being formed by the coalescence of rays, though the fact of its being perfectly continuous with the bases of the fin-rays is somewhat in favour of such coalescence.
Thacker and Mivart both hold that the pectoral and pelvic girdles are developed by ventral and dorsal growths of the anterior end of the longitudinal bar supporting the fin-rays.
There is, so far as I see, no theoretical objection to be taken to this view; and the fact of the pectoral and pelvic girdles originating continuously and long remaining united with the longitudinal bars of their respective fins is in favour of it rather than the reverse. The same may be said of the fact that the first part of each girdle to be formed is that in the neighbourhood of the longitudinal bar (basipterygium) of the fin, the dorsal and ventral prolongations being subsequent growths.
On the whole my observations do not throw much light on the theories of Thacker and Mivart as to the genesis of the skeleton of the paired fin; but, so far as they bear on the subject, they are distinctly favourable to those theories.
The main results of my observations appear to me to be decidedly adverse to the views recently put forward on the structure of the fin by Gegenbaur and Huxley, both of whom, as stated above, consider the primitive type of fin to be most nearly retained in Ceratodus, and to consist of a central multisegmented axis with numerous lateral rays.
[Pg 731] Gegenbaur derives the Elasmobranch pectoral fin from a form which he calls the archipterygium, nearly like that of Ceratodus, with a median axis and two rows of rays—but holds that in addition to the rays attached to the median axis, which are alone found in Ceratodus, there were other rays directly articulated to the shoulder-girdle. He considers that in the Elasmobranch fin the majority of the lateral rays on the posterior (or median according to his view of the position of the limb) side have become aborted, and that the central axis is represented by the metapterygium; while the pro- and mesopterygium and their rays are, he believes, derived from those rays of the archipterygium which originally articulated directly with the shoulder-girdle.
This view appears to me to be absolutely negatived by the facts of development of the pectoral fin in Scyllium—not so much because the pectoral fin in this form is necessarily to be regarded as primitive, but because what Gegenbaur holds to be the primitive axis of the biserial fin is demonstrated to be really the base, and it is only in the adult that it is conceivable that a second set of lateral rays could have existed on the posterior side of the metapterygium. If Gegenbaur's view were correct, we should expect to find in the embryo, if anywhere, traces of the second set of lateral rays; but the fact is that, as may easily be seen by an inspection of figs. 6 and 7, such a second set of lateral rays could not possibly have existed in a type of fin like that found in the embryo. With this view of Gegenbaur's it appears to me that the theory held by this anatomist to the effect that the limbs are modified gill-arches also falls, in that his method of deriving the limbs from gill-arches ceases to be admissible, while it is not easy to see how a limb, formed on the type of the embryonic limb of Elasmobranchii, could be derived from a gill-arch with its branchial rays.
Gegenbaur's older view, that the Elasmobranch fin retains a primitive uniserial type, appears to me to be nearer the truth than his more recent view on this subject; though I hold the fundamental point established by the development of these parts in Scyllium to be that the posterior border of the adult Elasmobranch pectoral fin is the primitive base-line,i.e.line of attachment of the fin to the side of the body.
[Pg 732] Huxley holds that the mesopterygium is the proximal piece of the axial skeleton of the limb of Ceratodus, and derives the Elasmobranch fin from that of Ceratodus by the shortening of its axis and the coalescence of some of its elements. The entirely secondary character of the mesopterygium, and its total absence in the young embryo Scyllium, appear to me as conclusive against Huxley's view as the character of the embryonic fin is against that of Gegenbaur; and I should be much more inclined to hold that the fin of Ceratodus has been derived from a fin like that of the Elasmobranchii by a series of steps similar to those which Huxley supposes to have led to the establishment of the Elasmobranch fin, but in exactly the reverse order.
There is one statement of Davidoff's which I cannot allow to pass without challenge. In comparing the skeletons of the paired and unpaired fins he is anxious to prove that the former are independent of the axial skeleton in their origin and that the latter have been segmented from the axial skeleton, and thus to shew that an homology between the two is impossible. In support of his view he states[495] that he has satisfied himself, from embryos of Acanthias and Scyllium, that the rays of the unpaired fins are undoubtedly products of the segmentation of the dorsal and ventral spinous processes.
This statement is wholly unintelligible to me. From my examination of the development of the first dorsal and the anal fins of Scyllium I find that their rays develop at a considerable distance from, and quite independently of, the neural and hæmal arches, and that they are at an early stage of development distinctly in a more advanced state of histological differentiation than the neural and hæmal arches of the same region. I have also found exactly the same in the embryos of Lepidosteus.
I have, in fact, no doubt that the skeleton of both the paired and the unpaired fins of Elasmobranchii and Lepidosteus is in its development independent of the axial skeleton. The phylogenetic mode of origin of the skeleton both of the paired and of the unpaired fins cannot, however, be made out without further investigation.
[Pg 733] EXPLANATION OF PLATE 33.[496]
Fig. 1. Transverse section through the pelvic fin of an embryo of Scyllium belonging to stage P1, magnified 50 diameters. bp. basipterygium. br. fin ray. m. muscle. hf. horny fibres supporting the peripheral part of the fin.
Fig. 2. Pelvic fin of a very young female embryo of Scyllium stellare, magnified 16 diameters. bp. basipterygium. pu. pubic process of pelvic girdle (cut across below). il. iliac process of pelvic girdle. fo. foramen.
Fig. 3. Pelvic fin of a young male embryo of Scyllium stellare, magnified 16 diameters. bp. basipterygium. mo. process of basipterygium continued into clasper. il. iliac process of pelvic girdle. pu. pubic section of pelvic girdle.
Fig. 4. Transverse section through the ventral part of the trunk of an embryo Scyllium of stage P, in the region of the pectoral fins, to shew how the fins are attached to the body, magnified 18 diameters. br. cartilaginous fin-ray. bp. basipterygium. m. muscle of fin. mp. muscle-plate.
Fig. 5. Transverse section through the ventral part of the trunk of an embryo Scyllium of stage P, in the region of the pelvic fin, on the same scale as fig. 4. bp. basipterygium. br. cartilaginous fin-rays. m. muscle of the fins. mp. muscle-plate.
Fig. 6. Pectoral fin of an embryo of Scyllium canicula, of a stage between O and P, in longitudinal and horizontal section (the skeleton of the fin was still in the condition of embryonic cartilage), magnified 36 diameters. bp. basipterygium (eventual metapterygium). fr. cartilaginous fin-rays. pg. pectoral girdle in transverse section. fo. foramen in pectoral girdle. pe. epithelium of peritoneal cavity.
Fig. 7. Transverse section through the pectoral fin of a Scyllium embryo of stage P, magnified 50 diameters. bp. basipterygium. br. cartilaginous fin-ray. m. muscle. hf. horny fibres.
Fig. 8. Pectoral fin of an embryo of Scyllium stellare, magnified 16 diameters. mp. metapterygium (basipterygium of earlier stage). me.p. rudiment of future pro- and mesopterygium. sc. cut surface of a scapular process. cr. coracoid process. fr. foramen. hf. horny fibres.
Fig. 9. Skeleton of the pectoral fin and part of pectoral girdle of a nearly ripe embryo of Scyllium stellare, magnified 10 diameters. mp. metapterygium. mes. mesopterygium. pp. propterygium. cr. coracoid process.
[479] From the Proceedings of the Zoological Society of London, 1881.
[480]
Monograph on the Development of Elasmobranch Fishes,
pp. 319, 320.
[481]
J. K. Thacker, Median and Paired Fins; a Contribution to the History of
the Vertebrate Limbs,
Trans.
of the Connecticut Acad. Vol. III. 1877. Ventral Fins of Ganoids,
Trans. of the Connecticut Acad. Vol. IV. 1877.
[482] Loc. cit. p. 298.
[483]
St George Mivart, On the Fins of Elasmobranchii,
Zoological
Trans. Vol. X.
[484] Mivart used the term exoskeletal in an unusual and (as it appears to me) inconvenient manner. The term is usually applied to dermal skeletal structures; but the skeleton of the limbs, with which we are here concerned, is undoubtedly not of this nature.
[485] Loc. cit. p. 480.
[486]
Description of Ceratodus,
Phil. Trans. 1871.
[487] Loc. cit. p. 534.
[488]
T. H. Huxley, On Ceratodus Fosteri, with some Observations
on the Classification of Fishes,
Proc. Zool. Soc. 1876.
[489]
C. Gegenbaur, Untersuchungen z. vergleich. Anat. d.
Wirbelthiere (Leipzig 1864-5): erstes Heft, Carpus u.
Tarsus;
zweites Heft, Brustflosse d. Fische.
Ueb. d. Skelet
d. Gliedmaassen d. Wirbelthiere im Allgemeinen u. d. Hintergliedmaassen d.
Selachier insbesondere,
Jenaische Zeitschrift,
Vol. V.
1870. Ueb. d. Archipterygium,
Jenaische
Zeitschrift, Vol. VII. 1873. Zur Morphologie d. Gliedmaassen d. Wirbelthiere,
Morphologisches Jahrbuch, Vol. II. 1876.
[490]
M. v. Davidoff, Beiträge z. vergleich. Anat. d. hinteren
Gliedmaassen d. Fische, I.,
Morphol. Jahrbuch,
Vol. V.
1879.
[491]
Zur Gliedmaassenfrage. An die Untersuchungen von Davidoff's
angeknüpfte Bemerkungen,
Morphol. Jahrbuch,
Vol. V. 1879.
[492] G. 'Swirski, Untersuch. üb. d. Entwick. d. Schultergürtels u. d. Skelets d. Brustflosse d. Hechts. Inaug. Diss. Dorpat, 1880.
[493] The horny fibres are mesoblastic products; they are formed, in the first instance, as extremely delicate fibrils on the inner side of the membrane separating the epiblast from the mesoblast.
[494] In one example where the metapterygium had 13 rays the mesopterygium had only 2 rays.
[495] Loc. cit. p. 514.
[496]
I employ here the same letters to indicate the stages as in my
Monograph on Elasmobranch Fishes.
From Owen's observations on the Marsupials it is clear that the yolk-sack in this group plays an important (if not the most important) part, in absorbing the maternal nutriment destined for the fœtus. The fact that in Marsupials both the yolk-sack and the allantois are concerned in rendering the chorion vascular, makes it à priori probable that this was also the case in the primitive types of the Placentalia; and this deduction is supported by the fact that in the Rodentia, Insectivora, and Cheiroptera this peculiarity of the fœtal membranes is actually found. In the primitive Placentalia it is also probable that from the discoidal allantoic region of the chorion simple fœtal villi, like those of the Pig, projected into uterine crypts; but it is not certain how far the umbilical region of the chorion, which was no doubt vascular, may also have been villous. From such a primitive type of fœtal membranes divergencies in various directions have given rise to the types of fœtal membranes found at the present day.
In a general way it may be laid down that variations in any direction which tended to increase the absorbing capacities of the chorion would be advantageous. There are two obvious ways in which this might be done, viz. (1) by increasing the complexity of the fœtal villi and maternal crypts over a limited area, (2) by increasing the area of the part of the chorion covered by the placental villi. Various combinations of the two processes would also, of course, be advantageous.
[Pg 735] The most fundamental change which has taken place in all the existing Placentalia is the exclusion of the umbilical vesicle from any important function in the nutrition of the fœtus.
The arrangement of the fœtal parts in the Rodentia, Insectivora, and Cheiroptera may be directly derived from the primitive form by supposing the villi of the discoidal placental area to have become more complex, so as to form a deciduate discoidal placenta, while the yolk-sack still plays a part, though physiologically an unimportant part, in rendering the chorion vascular.
In the Carnivora, again, we have to start from the discoidal placenta, as evinced by the fact that in the growth of the placenta the allantoic region of the placenta is at first discoidal, and only becomes zonary at a later stage. A zonary deciduate placenta indicates an increase both in area and in complexity. The relative diminution of the breadth of the placental zone in late fœtal life in the zonary placenta of the Carnivora is probably due to its being on the whole advantageous to secure the nutrition of the fœtus by insuring a more intimate relation between the fœtal and maternal parts, than by increasing their area of contact. The reason of this is not obvious, but, as shewn below, there are other cases where it is clear that a diminution in the area of the placenta has taken place, accompanied by an increase in the complexity of its villi.
The second type of differentiation from the primitive form of placenta is illustrated by the Lemuridæ, the Suidæ, and Manis. In all these cases the area of the placental villi appears to have increased so as to cover nearly the whole subzonal membrane, without the villi increasing to any great extent in complexity. From the diffused placenta covering the whole surface of the chorion, differentiations appear to have taken place in various directions. The placenta of Man and Apes, from its mode of ontogeny, is clearly derived from a diffused placenta (very probably similar to that of Lemurs) by a concentration of the fœtal villi, which are originally spread over the whole chorion, to a disk-shaped area, and by an increase in their arborescence. Thus the discoidal placenta of Man has no connexion with, and ought not to be placed in, the same class as those of the Rodentia, Cheiroptera, and Insectivora.
[Pg 736] The polycotyledonary forms of placenta are due to similar concentrations of the fœtal villi of an originally diffused placenta.
In the Edentata we have a group with very varying types of placenta. Very probably these may all be differentiations within the group itself from a diffused placenta such as that found in Manis. The zonary placenta of Orycteropus is capable of being easily derived from that of Manis by the disappearance of the fœtal villi at the two poles of the ovum. The small size of the umbilical vesicle in Orycteropus indicates that its discoidal placenta is not, like that of the Carnivora, directly derived from a type with both allantoic and umbilical vascularization of the chorion. The discoidal and dome-shaped placentæ of the Armadillos, Myrmecophaga, and the Sloths may easily have been formed from a diffused placenta, just as the discoidal placenta of the Simiidæ and Hominidæ appears to have been formed from a diffused placenta like that of the Lemuridæ.
The presence of zonary placenta in Hyrax and Elephas does not necessarily afford any proof of affinity of these types with the Carnivora. A zonary placenta may be quite as easily derived from a diffused placenta as from a discoidal placenta; and the presence of two villous patches at the poles of the chorion in Elephas very probably indicates that its placenta has been evolved from a diffused placenta.
Although it would not be wise to attempt to found a classification upon the placental characters alone, it may be worth while to make a few suggestions as to the affinities of the orders of Mammalia indicated by the structure of the placenta. We clearly, of course, have to start with forms which could not be grouped with any of the existing orders, but which might be called the Protoplacentalia. They probably had the primitive type of placenta described above: the nearest living representatives of the group are the Rodentia, Insectivora, and Cheiroptera. Before, however, these three groups had become distinctly differentiated, there must have branched off from the primitive stock the ancestors of the Lemuridæ, the Ungulata, and the Edentata.
It is obvious on general anatomical grounds that the Monkeys and Man are to be derived from a primitive Lemurian type; and [Pg 737] with this conclusion the form of the placenta completely tallies. The primitive Edentata and Ungulata had no doubt a diffused placenta which was probably not very different from that of the primitive Lemurs; but how far these groups arose quite independently from the primitive stock, or whether they may have had a nearer common ancestor, cannot be decided from the structure of the placenta. The Carnivora were certainly an offshoot from the primitive placental type which was quite independent of the three groups just mentioned; but the character of the placenta of the Carnivora does not indicate at what stage in the evolution of the placental Mammalia a primitive type of Carnivora was first differentiated.
No important light is thrown by the placenta on the affinities of the Proboscidea, the Cetacea, or the Sirenia; but the character of the placenta in the latter group favours the view of their being related to the Ungulata.
[497] From the Proceedings of the Zoological Society of London, 1881.
(With Plates 34-42.)
[498] From the Philosophical Transactions of the Royal Society, 1882.
| TABLE OF CONTENTS. | |
|---|---|
| PAGE | |
| Introduction | 739 |
| General Development | 740 |
| Brain— | |
| Adult brain | 759 |
| Development of the brain | 764 |
| Comparison of the larval and adult brain of Lepidosteus, together with some observations on the systematic value of the characters of the Ganoid brain | 767 |
| Sense Organs— | |
| Olfactory organ | 771 |
| Anatomy of the eye | ib. |
| Development of the eye | 772 |
| Suctorial Disc | 774 |
| Muscular System | 775 |
| Skeleton— | |
| Vertebral column and ribs of the adult | 776 |
| Development of the vertebral column and ribs. | 778 |
| Comparison of the vertebral column of Lepidosteus with that of other forms | 792 |
| The ribs of Fishes | 793 |
| The skeleton of the ventral lobe of the tail fin, and its bearing on the nature of the tail fin of the various types of Pisces | 801 |
| Excretory and Generative Organs— | |
| Anatomy of the excretory and generative organs of the female | 810 |
| Anatomy of the excretory and generative organs of the male | 813 |
| Development of the excretory and generative organs | 815 |
| Theoretical considerations | 822 |
| The Alimentary Canal and its Appendages—[739] | |
| Topographical anatomy of the alimentary canal | 828 |
| Development of the alimentary canal and its appendages | 831 |
| The Gill on the Hyoid Arch | 835 |
| The systematic position of Lepidosteus | 836 |
| List of memoirs on the Anatomy and Development of Lepidosteus | 840 |
| List of Reference Letters | 841 |
| Explanation of Plates | 842 |
The following paper is the outcome of the very valuable gift of a series of embryos and larvæ of Lepidosteus by Professor Alex. Agassiz, to whom we take this opportunity of expressing our most sincere thanks. The skull of these embryos and larvæ has been studied by Professor Parker, and forms the subject of a memoir already presented to the Royal Society.
Considering that Lepidosteus is one of the most interesting of existing Ganoids, and that it is very closely related to species of Ganoids which flourished during the Triassic period, we naturally felt keenly anxious to make the most of the opportunity of working at its development offered to us by Professor Agassiz' gift. Professor Agassiz, moreover, most kindly furnished us with four examples of the adult Fish, which have enabled us to make this paper a study of the adult anatomy as well as of the development.
The first part of our paper is devoted to the segmentation, formation of the germinal layers, and general development of the embryo and larva. The next part consists of a series of sections on the organs, in which both their structure in the adult and their development are dealt with. This part is not, however, in any sense a monograph, and where already known, the anatomy is described with the greatest possible brevity. In this part of the paper considerable space is devoted to a comparison of the organs of Lepidosteus with those of other Fishes, and to a statement of the conclusions which follow from such comparison.
The last part of the paper deals with the systematic position of Lepidosteus and of the Ganoids generally.
The spawning of Lepidosteus takes place in the neighbourhood of New York about May 20th. Agassiz (No. 1)[499] gives an account of the process from Mr S. W. Garman's notes, which we venture to quote in full.
"Black Lake is well stocked with Bill-fish. When they appear, they are said to come in countless numbers. This is only for a few days in the spring, in the spawning season, between the 15th of May and the 8th of June. During the balance of the season they are seldom seen. They remain in the deeper parts of the lake, away from the shore, and, probably, are more or less nocturnal in habits. Out of season, an occasional one is caught on a hook baited with a minnow. Commencing with the 20th of April, until the 14th of May we were unable to find the Fish, or to find persons who had seen them during this time. Then a fisherman reported having seen one rise to the surface. Later, others were seen. On the afternoon of the 18th, a few were found on the points, depositing the spawn. The temperature at the time was 68° to 69° on the shoals, while out in the lake the mercury stood at 62° to 63°. The points on which the eggs were laid were of naked granite, which had been broken by the frost and heat into angular blocks of 3 to 8 inches in diameter. The blocks were tumbled upon each other like loose heaps of brick-bats, and upon and between them the eggs were dropped. The points are the extremities of small capes that make out into the lake. The eggs were laid in water varying in depth from 2 to 14 inches. At the time of approaching the shoals, the Fish might be seen to rise quite often to the surface to take air. This they did by thrusting the bill out of the water as far as the corners of the mouth, which was then opened widely and closed with a snap. After taking the air, they seemed more able to remain at the surface. Out in the lake they are very timid, but once buried upon the shoals they become quite reckless as to what is going on about them. A few moments after being driven [Pg 741] off, one or more of the males would return as if scouting. If frightened, he would retire for some time; then another scout would appear. If all promised well, the females, with the attendant males, would come back. Each female was accompanied by from one to four males. Most often, a male rested against each side, with their bills reaching up toward the back of her head. Closely crowded together, the little party would pass back and forth over the rocky bed they had selected, sometimes passing the same spot half-a-dozen times without dropping an egg, then suddenly would indulge in an orgasm; and, lashing and plashing the water in all directions with their convulsive movements, would scatter at the same instant the eggs and the sperm. This ended, another season of moving slowly back and forth was observed, to be in turn followed by another of excitement. The eggs were excessively sticky. To whatever they happened to touch, they stuck, and so tenaciously that it was next to impossible to release them without tearing away a portion of their envelopes. It is doubtful whether the eggs would hatch if removed. As far as could be seen at the time, upon or under the rocks to which the eggs were fastened there was an utter absence of anything that might serve as food for the young Fishes.
Other Fishes, Bull-heads, &c., are said to follow the Bill-fish to eat the spawn.
It may be so. It was not verified. Certainly the points under observations
were unmolested. During the afternoon of the 18th of May a few eggs were
scattered on several of the beds. On the 19th there were more. With the
spear and the snare, several dozens of both sexes of the Fish were taken.
Taking one out did not seem greatly to startle the others. They returned
very soon. The males are much smaller than the average size of the females;
and, judging from those taken, would seem to have as adults greater
uniformity in size. The largest taken was a female, of 4 feet 1½ inch
in length. Others of 2 feet 6 inches contained ripe ova. With the 19th of
May all disappeared, and for a time—the weather being meanwhile cold
and stormy—there were no signs of their continued existence to be met
with. Nearly two weeks later, on the 31st of May, as stated by Mr Henry J.
Perry, they again came up, not in small detachments on scattered points as
before, but in [Pg 742] multitudes, on every shoal at all according
with their ideas of spawning beds. They remained but two days. During the
summer it happens now and then that one is seen to come up for his mouthful
of air; beyond this there will be nothing to suggest the ravenous masses
hidden by the darkness of the waters.
Egg membranes.—The ova of Lepidosteus are spherical bodies of about 3 millims. in diameter. They have a double investment consisting of (1) an outer covering formed of elongated, highly refractive bodies, somewhat pyriform at their outer ends (Plate 34, fig. 17, f.e.), which are probably metamorphosed follicular cells[500], and (2) of an inner membrane, divided into two zones, viz.: an outer and thicker zone, which is radially striated, and constitutes the zona radiata (z.r.), and an inner and narrow homogeneous zone (z.r´.).
Segmentation.—We have observed several stages in the segmentation, which shew that it is complete, but that it approaches the meroblastic type more nearly than in the case of any other known holoblastic ovum.
Our earliest stage shewed a vertical furrow at the upper or animal pole, extending through about one-fifth of the circumference (Plate 34, fig. 1), and in a slightly later stage we found a second similar furrow at right angles to the first (Plate 34, fig. 2). We have not been fortunate enough to observe the next phases of the segmentation, but on the second day after impregnation (Plate 34, fig. 3), the animal pole is completely divided into small segments, which form a disc, homologous to the blastoderm of meroblastic ova; while the vegetative pole, which subsequently forms a large yolk-sack, is divided by a few vertical furrows, four of which nearly meet at the pole opposite the blastoderm (Plate 34, fig. 4). The majority of the vertical furrows extend only a short way from the edge of the small spheres, and are partially intercepted by imperfect equatorial furrows.
[Pg 743] Development of the embryo.—We have not been able to work out the stages immediately following the segmentation, owing to want of material; and in the next stage satisfactorily observed, on the third day after impregnation, the body of the embryo is distinctly differentiated. The lower pole of the ovum is then formed of a mass in which no traces of the previous segments or segmentation furrows could any longer be detected.
Some of the dates of the specimens sent to us appear to have been transposed; so that our statements as to ages must only be taken as approximately correct.
Third day after impregnation.—In this stage the embryo is about 3.5 millims. in length, and has a somewhat dumb-bell shaped outline (Plate 34, fig. 5). It consists of (1) an outer area (p.z) with some resemblance to the area pellucida of the Avian embryo, forming the parietal part of the body; and (2) a central portion consisting of the vertebral and medullary plates and the axial portions of the embryo. In hardened specimens the peripheral part forms a shallow depression surrounding the central part of the embryo.
The central part constitutes a somewhat prominent ridge, the axial part of it being the medullary plate. Along the anterior half of this part a dark line could be observed in all our specimens, which we at first imagined to be caused by a shallow groove. We have, however, failed to find in our sections a groove in this situation except in a single instance (Plate 35, fig. 20, x), and are inclined to attribute the appearance above-mentioned to the presence of somewhat irregular ridges of the outer layer of the epiblast, which have probably been artificially produced in the process of hardening.
The anterior end of the central part is slightly dilated to form the brain (b); and there is present a pair of lateral swellings near the anterior end of the brain which we believe to be the commencing optic vesicles. We could not trace any other clear indications of the differentiation of the brain into distinct lobes.
At the hinder end of the central part of the embryo a very distinct dilatation may also be observed, which is probably homologous with the tail swelling of Teleostei. Its structure is more particularly dealt with in the description of our sections of this stage.
[Pg 744] After the removal of the egg-membranes described above we find that there remains a delicate membrane closely attached, to the epiblast. This membrane can be isolated in distinct portions, and appears to be too definite to be regarded as an artificial product.
We have been able to prepare several more or less complete series of sections of embryos of this stage (Plate 35, figs. 18-22). These sections present as a whole a most striking resemblance to those of Teleostean embryos at a corresponding stage of development.
Three germinal layers are already fully established. The epiblast (ep.) is formed of the same parts as in Teleostei, viz.:—of an outer epidermic and an inner nervous or mucous stratum. In the parietal region of the embryo these strata are each formed of a single row of cells only. The cells of both strata are somewhat flattened, but those of the epidermic stratum are decidedly the more flattened of the two.
Along the axial line there is placed, as we have stated above, the medullary plate. The epidermic stratum passes over this plate without undergoing any change of character, and the plate is entirely constituted of the nervous stratum of the epidermis.
The medullary plate has, roughly speaking, the form of a solid keel, projecting inwards towards the yolk. There is no trace, at this stage at any rate, of a medullary groove; and as, we shall afterwards shew, the central canal of the cerebro-spinal cord is formed in the middle of the solid keel. The shape of this keel varies according to the region of the body. In the head (Plate 35, fig. 18, m.c.), it is very prominent, and forming, as it does, the major part of the axial tissue of the body, impresses its own shape on the other parts of the head and gives rise to a marked ridge on the surface of the head directed towards the yolk. In the trunk (Plate 35, figs. 19, 20) the keel is much less prominent, but still projects sufficiently to give a convex form to the surface of the body turned towards the yolk.
In the head, and also near the hind end of the trunk, the nervous layer
of the epiblast continuous with the keel on each side is considerably
thicker than the lateral parts of the layer. The thickening of the nervous
layer in the head gives rise to [Pg 745] what has been called by Götte[501] the special sense plate,
owing to its being
subsequently concerned in the formation of parts of the organs of special
sense. We cannot agree with Götte in regarding it as part of the brain.
In the keel itself two parts may be distinguished, viz.: a superficial part, best marked in the region of the brain, formed of more or less irregularly arranged polygonal cells, and a deeper part of horizontally placed flatter cells. The upper part is mainly concerned in the formation of the cranial nerves, and of the dorsal roots of the spinal nerves.
The mesoblast (ms.) in the trunk consists of a pair of independent plates which are continued forwards into the head, and in the prechordal region of the latter, unite below the medullary keel.
The mesoblastic plates of the trunk are imperfectly divided into vertebral and lateral regions. Neither longitudinal sections nor surface views shew at this stage any trace of a division of the mesoblast into somites. The mesoblast cells are polygonal, and no indication is as yet present of a division into splanchnic and somatic layers.
The notochord (nc.) is well established, so that its origin could not be made out. It is, however, much more sharply separated from the mesoblastic plates than from the hypoblast, though the ventral and inner corners of the mesoblastic plates which run in underneath it on either side, are often imperfectly separated from it. It is formed of polygonal cells, of which between 40 and 50 may as a rule be seen in a single section. No sheath is present around it. It has the usual extension in front.
The hypoblast (hy.) has the form of a membrane, composed of a single row of oval cells, bounding the embryo on the side adjoining the yolk.
In the region of the caudal swelling the relations of the germinal layers undergo some changes. This region may, from the analogy of other Vertebrates, be assumed to constitute the lip of the blastopore. We find accordingly that the layers become more or less fused. In the anterior part of the tail [Pg 746] swelling, the boundary between the notochord and hypoblast becomes indistinct. A short way behind this point (Plate 35, fig. 21), the notochord unites with the medullary keel, and a neurenteric cord, homologous with the neurenteric canal of other Ichthyopsida, is thus established. In the same region the boundary between the lateral plates of mesoblast and the notochord, and further back (Plate 35, fig. 22), that between the mesoblast and the medullary keel, becomes obliterated.
Fifth day after impregnation.—Between the stage last described and the next stage of which we have specimens, a considerable progress has been made. The embryo (Plate 34, figs. 6 and 7) has grown markedly in length and embraces more than half the circumference of the ovum. Its general appearance is, however, much the same as in the earlier stage, but in the cephalic region the medullary plate is divided by constrictions into three distinct lobes, constituting the regions of the fore-brain, the mid-brain, and the hind-brain. The fore-brain (Plate 34, fig. 6, f.b.) is considerably the largest of the three lobes, and a pair of lateral projections forming the optic vesicles are decidedly more conspicuous than in the previous stage. The mid-brain (m.b.) is the smallest of the three lobes, while the hind-brain (h.b.) is decidedly longer, and passes insensibly into the spinal cord behind.
The medullary keel, though retaining to a great extent the shape it had in the last stage, is no longer completely solid. Throughout the whole region of the brain and in the anterior part of the trunk (Plate 35, figs. 23, 24, 25) a slit-like lumen has become formed. We are inclined to hold that this is due to the appearance of a space between the cells, and not, as supposed by Oellacher for Teleostei, to an actual absorption of cells, though we must admit that our sections are hardly sufficiently well preserved to be conclusive in settling this point. Various stages in its growth may be observed in different regions of the cerebro-spinal cord. When first formed, it is a very imperfectly defined cavity, and a few cells may be seen passing right across from one side of it to the other. It gradually becomes more definite, and its wall then acquires a regular outline.
The optic vesicles are now to be seen in section (Plate 35, fig. 23, op.) as flattish outgrowths of the wall of the fore-brain, [Pg 747] into which the lumen of the third ventricle is prolonged for a short distance.
The brain has become to some extent separate from the superjacent epiblast, but the exact mode in which this is effected is not clear to us. In some sections it appears that the separation takes place in such a way that the nervous keel is only covered above by the epidermic layer of the epiblast, and that the nervous layer, subsequently interposed between the two, grows in from the two sides. Such a section is represented in Plate 35, fig. 24. Other sections again favour the view that in the isolation of the nervous keel, a superficial layer of it remains attached to the nervous layer of the epidermis at the two sides, and so, from the first, forms a continuous layer between the nervous keel and the epidermic layer of the epiblast (Plate 35, fig. 25). In the absence of a better series of sections we do not feel able to determine this point. The posterior part of the nervous keel retains the characters of the previous stage.
At the sides of the hind-brain very distinct commencements of the auditory vesicles are apparent. They form shallow pits (Plate 35, fig. 24, au.) of the thickened part of the nervous layer adjoining the brain in this region. Each pit is covered over by the epidermic layer above, which has no share in its formation.
In many parts of the lateral regions of the body the nervous layer of the epidermis is more than one cell deep.
The mesoblastic plates are now divided in the anterior part of the trunk into a somatic and a splanchnic layer (Plate 35, fig. 25, so., sp.), though no distinct cavity is as yet present between these two layers. Their vertebral extremities are somewhat wedge-shaped in section, the base of the wedge being placed at the sides of the medullary keel. The wedge-shaped portions are formed of a superficial layer of palisade-like cells and an inner kernel of polygonal cells. The superficial layer on the dorsal side is continuous with the somatic mesoblast, while the remainder pertains to the splanchnic layer.
The diameter of the notochord has diminished, and the cells have assumed a flattened form, the protoplasm being confined to an axial region. In consequence of this, the peripheral layer appears clear in transverse sections. A delicate cuticular sheath [Pg 748] is formed around it. This sheath is probably the commencement of the permanent sheath of later stages, but at this stage it cannot be distinguished in structure from a delicate cuticle which surrounds the greater part of the medullary cord.
The hypoblast has undergone no changes of importance.
The layers at the posterior end of the embryo retain the characters of the last stage.
Sixth day after impregnation.—At this stage (Plate 34, fig. 8) the embryo is considerably more advanced than at the last stage. The trunk has decidedly increased in length, and the head forms a relatively smaller portion of the whole. The regions of the brain are more distinct. The optic vesicles (op.) have grown outwards so as to nearly reach the edges of the area which forms the parietal part of the body. The fore-brain projects slightly in front, and the mid-brain is seen as a distinct rounded prominence. Behind the latter is placed the hind-brain, which passes insensibly into the spinal cord. On either side of the mid- and hind-brain a small region is slightly marked off from the rest of the parietal part, and on this are seen two more or less transversely directed streaks, which, by comparison with the Sturgeon[502], we are inclined to regard as the two first visceral clefts (br.c.). We have, however, failed to make them out in sections, and owing to the insufficiency of our material, we have not even studied them in surface views as completely as we could have wished.
The body is now laterally compressed, and more decidedly raised from the yolk than in the previous stages. In the lateral regions of the trunk the two segmental or archinephric ducts (sg.) are visible in surface views: the front end of each is placed at the level of the hinder border of the head, and is marked by a flexure inwards towards the middle line. The remainder of each duct is straight, and extends backwards for about half the length of the embryo. The tail has much the same appearance as in the last stage.
The vertebral regions of the mesoblastic plates are now segmented for the greater part of the length of the trunk, and the [Pg 749] somites of which they are composed (Plate 36, fig. 30, pr.) are very conspicuous in surface views.
Our sections of this stage are not so complete as could be desired: they shew, however, several points of interest.
The central canal of the nervous system is large, with well-defined walls, and in hardened specimens is filled with a coagulum. It extends nearly to the region of the tail.
The optic vesicles, which are so conspicuous in surface views, appear in section (Plate 35, fig. 26, op.) as knob-like outgrowths of the fore-brain, and very closely resemble the figures given by Oellacher of these vesicles in Teleostei[503].
From the analogy of the previous stage, we are inclined to think that they have a lumen continuous with that of the fore-brain. In our only section through them, however, they are solid, but this is probably due to the section merely passing through them to one side.
The auditory pits (Plate 35, fig. 27, au.) are now well marked, and have the form of somewhat elongated grooves, the walls of which are formed of a single layer of columnar cells belonging to the nervous layer of the epidermis, and extending inwards so as nearly to touch the brain.
In an earlier stage it was pointed out that the dorsal part of the medullary keel was different in its structure from the remainder, and that it was destined to give rise to the nerves. The process of differentiation is now to a great extent completed, and may best be seen in the auditory region (Plate 35, fig. 27, VIII.). In this region there was present during the last stage a great rhomboidal mass of cells at the dorsal region of the brain (Plate 35, fig. 24, VIII.). In the present stage, this, which is the rudiment of the seventh and auditory nerves, is seen growing down on each side from the roof of the hind-brain, between the brain and the auditory involution, and abutting against the wall of the latter.
Rudiments of the spinal nerves are also seen at intervals as projections from the dorsal angles of the spinal cord (Plate 36, fig. 29, sp.n.). They extend only for a short distance outwards, gradually tapering off to a point, and situated [Pg 750] between the epiblast and the dorsal angles of the mesoblastic somites.
The process of formation of the cranial nerves and dorsal roots of the spinal nerves is, it will be seen, essentially the same as that already known in the case of Elasmobranchii, Aves, &c. The nerves arise as outgrowths of a special crest of cells, the neural crest of Marshall, which is placed along the dorsal angle of the cord. The peculiar position of the dorsal roots of the spinal nerves is also very similar to what has been met with in the early stages of these structures by Marshall in Birds[504], and by one of us in Elasmobranchii[505].
In the parietal region a cavity has now appeared in part of the trunk between the splanchnic and somatic layers of the mesoblast (Plate 36, fig. 29, b.c.), the somatic layer (so.) consisting of a single row of columnar cells on the dorsal side, while the remainder of each somite is formed of the splanchnic layer (sp.). In many of the sections the somatic layer is separated by a considerable interval from the epiblast.
We have been able to some extent to follow the development of the segmental duct. The imperfect preservation of our specimens has, as in other instances, rendered the study of the point somewhat difficult, but we believe that the figure representing the development of the duct some way behind its front end (Plate 36, fig. 29) is an accurate representation of what may be seen in a good many of our sections.
It appears from these sections that the duct (Plate 36, fig. 29, sg.) is developed as a hollow ridge-like outgrowth of the somatic layer of mesoblast, directed towards the epiblast, in which it causes a slight bulging. The cavity of the ridge freely communicates with the body-cavity. The anterior part of this ridge appears to be formed first. Very soon, in fact, in an older embryo belonging to this stage, the greater part of the groove becomes segmented off as a duct lying between the epiblast and somatic mesoblast (Plate 36, fig. 28, sg.), while the front end still remains, as we believe, in communication with the body-cavity by an anterior pore.
[Pg 751] This mode of development corresponds in every particular with that observed in Teleostei by Rosenberg and Oellacher.
The structure of the notochord (nc.) at this stage is very similar to that observed by one of us in Elasmobranchii[506]. The cord is formed of transversely arranged flattened cells, the outer parts of which are vacuolated, while the inner parts are granular, and contain the nuclei. This structure gives rise to the appearance in transverse sections of an axial darker area and a peripheral lighter portion.
The hypoblast retains for the most part its earlier constitution, but underneath the notochord, in the trunk, it is somewhat thickened, and the cells at the two sides spread in to some extent under the thickened portion (Plate 36, fig. 29, s.nc.). This thickening, as is shewn in transverse sections at the stage when the segmental duct becomes separated from the somatic mesoblast (Plate 36, fig. 28, s.nc.), is the commencement of the subnotochordal rod.
The tail end of the embryo still retains its earlier characters.
Seventh day after impregnation.—Our series of specimens of this stage is very imperfect, and we are only able to call attention to the development of a certain number of organs.
Our sections clearly establish the fact that the optic vesicles are now hollow processes of the fore-brain. Their outer ends are dilated, and are in contact with the external skin. The formation of the optic cup has not, however, commenced. The nervous layer of the skin adjoining the outer wall of the optic cup is very slightly thickened, constituting the earliest rudiment of the lens.
In one of our embryos of this day the developing auditory vesicle still has the form of a pit, but in the other it is a closed vesicle, already constricted off from the nervous layer of the epidermis.
With reference to the development of the excretory duct we cannot add much to what we have already stated in describing the last stage.
The duct is considerably dilated anteriorly (Plate 36, fig. 31, sg.); but our sections throw no light on the nature of the abdominal pore. The posterior part of the duct has still the form [Pg 752] of a hollow ridge united with somatic mesoblast (Plate 36, fig. 32, sg.).
During this stage, the embryo becomes to a small extent folded off from the yolk-sack both in front and behind, and in the course of this process the anterior and posterior extremities of the alimentary tract become definitely established.
We have not got as clear a view of the process of formation of these two sections of the alimentary tract as we could desire, but our observations appear to shew that the process is in many respects similar to that which takes place in the formation of the anterior part of the alimentary tract in Elasmobranchii[507]. One of us has shewn that in Elasmobranchii the ventral wall of the throat is formed not by a process of folding in of the hypoblastic sheet as in Birds, but by a growth of the ventral face of the hypoblastic sheet on each side of and at some little distance from the middle line. Each growth is directed inwards, and the two eventually meet and unite, thus forming a complete ventral wall for the gut. Exactly the same process would seem to take place in Lepidosteus, and after the lumen of the gut is in this way established, a process of mesoblast on each side also makes its appearance, forming a mesoblastic investment on the ventral side of the alimentary tract. Some time after the alimentary tract has been thus formed, the epiblast becomes folded in, in exactly the same manner as in the Chick, the embryo becoming thereby partially constricted off from the yolk (Plate 36, figs. 33, 34).
The form of the lumen of the alimentary tract differs somewhat in front and behind. In front, the hypoblastic sheet remains perfectly flat during the formation of the throat, and thus the lumen of the latter has merely the form of a slit. The lumen of the posterior end of the alimentary tract is, however, narrower and deeper (Plate 36, figs. 33, 34, al.). Both in front and behind, the lateral parts of the hypoblastic sheet become separated from the true alimentary tract as soon as the lumen of the latter is established.
It is quite possible that at the extreme posterior end of the embryo a modification of the above process may take place, for [Pg 753] in this region the hypoblast appears to us to have the form of a solid cord.
We could detect no true neurenteric canal, although a more or less complete fusion of the germinal layers at the tail end of the embryo may still be traced.
During this stage the protoplasm of the notochordal cells, which in the last stage formed a kind of axial rod in the centre of the notochord, begins to spread outwards toward the sheath of the notochord.
Eighth day after impregnation.—The external form of the embryo (Plate 34, fig. 9) shews a great advance upon the stage last figured. Both head and body are much more compressed laterally and raised from the yolk, and the head end is folded off for some distance. The optic vesicles are much less prominent externally. A commencing opercular fold is distinctly seen. Our figure of this stage is not, however, so satisfactory as we could wish.
A thickening of the nervous layer of the external epiblast which will form the lens (Plate 36, fig. 35, l.) is more marked than in the last stage, and presses against the slightly concave exterior wall of the optic vesicle (op.). The latter has now a large cavity, and its stalk is considerably narrowed.
The auditory vesicles (Plate 36, fig. 36, au.) are closed, appearing as hollow sacks one on each side of the brain, and are no longer attached to the epiblast.
The anterior opening of the segmental duct can be plainly seen close behind the head. The lumen of the duct is considerably larger.
The two vertebral portions of the mesoblast are now separated by a considerable space from the epiblast on one side and from the notochord on the other, and the cells composing them have become considerably elongated from side to side (Plate 36, fig. 37, ms.).
In some sections the aorta can be seen (Plate 36, fig. 37, ao.) lying close under the subnotochordal rod, between it and the hypoblast, and on either side of it a slightly larger cardinal vein (cd.v.).
The protoplasm of the notochord has now again retreated towards the centre, shewing a clear space all round. This is [Pg 754] most marked in the region of the trunk (Plate 36, fig. 37). The subnotochordal rod (s.nc.) lies close under it.
A completely closed fore-gut, lined by thickened hypoblast, extends about as far back as the auditory sacks (Plate 36, figs. 35 and 36, al.). In the trunk the hypoblast, which will form the walls of the alimentary tract, is separated from the notochord by a considerable interval.
Ninth day after impregnation: External characters.—Very considerable changes have taken place in the external characters of the embryo. It is about 8 millims. in length, and has assumed a completely piscine form. The tail especially has grown in length, and is greatly flattened from side to side: it is wholly detached from the yolk, and bends round towards the head, usually with its left side in contact with the yolk. It is provided with well-developed dorsal and ventral fin-folds, which meet each other round the end of the tail, the tail fin so formed being nearly symmetrical. The head is not nearly so much folded off from the yolk as the tail. At its front end is placed a disc with numerous papillæ, of which we shall say more hereafter. This disc is somewhat bifid, and is marked in the centre by a deep depression.
Dorsal to it, on the top of the head, are two widely separated nasal pits. On the surface of the yolk, in front of the head, is to be seen the heart, just as in Sturgeon embryos. Immediately below the suctorial disc is a slit-like space, forming the mouth. It is bounded below by the two mandibular arches, which meet ventrally in the median line. A shallow but well-marked depression on each side of the head indicates the posterior boundary of the mandibular arch. Behind this is placed the very conspicuous hyoid arch with its rudimentary opercular flap; and in the depression, partly covered over by the latter, may be seen a ridge, the external indication of the first branchial arch.
Eleventh day after impregnation: External characters.—The embryo (Plate 34, fig. 10) is now about 10 millims. in length, and in several features exhibits an advance upon the embryo of the previous stage.
The tail fin is now obviously not quite symmetrical, and the dorsal fin-fold is continued for nearly the whole length of the trunk. The suctorial disc (Plate 34, fig. 11, s.d.) is much more [Pg 755] prominent, and the papillæ (about 30 in number) covering it are more conspicuous from the surface. It is not obviously composed of two symmetrical halves. The opercular flap is larger, and the branchial arches behind it (two of which may be made out without dissection) are more prominent.
The anterior pair of limbs is now visible in the form of two longitudinal folds projecting in a vertical direction from the surface of the yolk-sack at the sides of the body.
The stages subsequent to hatching have been investigated with reference to the external features and to the habits by Agassiz, and we shall enrich our own account by copious quotations from his memoir.
He states that the first batch were hatched on the eighth[508] day after being laid. The young Fish possessed a
gigantic yolk-bag, and the posterior part of the body presented nothing
specially different from the general appearance of a Teleostean embryo,
with the exception of the great size of the chorda. The anterior part,
however, was most remarkable; and at first, on seeing the head of this
young Lepidosteus, with its huge mouth-cavity extending nearly to
the gill-opening, and surmounted by a hoof-shaped depression edged with a
row of protuberances acting as suckers, I could not help comparing this
remarkable structure, so utterly unlike anything in Fishes or Ganoids, to
the Cyclostomes, with which it has a striking analogy. This organ is also
used by Lepidosteus as a sucker, and the moment the young Fish is
hatched he attaches himself to the sides of the disc, and there remains
hanging immovable; so firmly attached, indeed, that it requires
considerable commotion in the water to make him loose his hold. Aërating
the water by pouring it from a height did not always produce sufficient
disturbance to loosen the young Fishes. The eye, in this stage, is rather
less advanced than in corresponding stages in bony Fishes; the brain is
also comparatively smaller, the otolith ellipsoidal, placed obliquely in
the rear above the gill-opening.... Usually the gill-cover is pressed
closely against the sides of the body, but in breathing an opening is seen
through which water is constantly passing, a [Pg 756] strong current
being made by the rapid movement of the pectorals, against the base of
which the extremity of the gill-cover is closely pressed. The large
yolk-bag is opaque, of a bluish-gray colour. The body of the young
Lepidosteus is quite colourless and transparent. The embryonic fin
is narrow, the dorsal part commencing above the posterior end of the
yolk-bag; the tail is slightly rounded, the anal opening nearer the
extremity of the tail than the bag. The intestine is narrow, and the
embryonic fin extending from the vent to the yolk-bag is quite narrow. In a
somewhat more advanced stage,—hatched a few hours earlier,—the
upper edge of the yolk-bag is covered with black pigment cells, and minute
black pigment cells appear on the surface of the alimentary canal. There
are no traces of embryonic fin-rays either in this stage or the one
preceding; the structure of the embryonic fin is as in bony
Fishes—previous to the appearance of these embryonic
fin-rays—finely granular. Seen in profile, the yolk-bag is ovoid; as
seen from above, it is flattened, rectangular in front, with rounded
corners, tapering to a rounded point towards the posterior extremity, with
re-entering sides.
We have figured an embryo of 11 millims. in length, shortly after hatching (Plate 34, fig. 12), the most important characters of which are as follows:—The yolk-sack, which has now become much reduced, forms an appendage attached to the ventral surface of the body, and has a very elongated form as compared with its shape just before hatching. The mouth, as also noticed by Agassiz, has a very open form. It is (Plate 34, fig. 13, m.) more or less rhomboidal, and is bounded behind by the mandibular arch (mn.) and laterally by the superior maxillary processes (s.mx). In front of the mouth is placed the suctorial disc (s.d.), the central papillæ of which are arranged in groups. The opercular fold (h.op.) is very large, covering the arches behind. A well-marked groove is present between the mandibular and opercular arches, but so far as we can make out it is not a remnant of the hyomandibular cleft.
The pectoral fins (Plate 34, fig. 12, pc.f.) are very prominent longitudinal ridges, which, owing to their being placed on the surface of the yolk-sack, project in a nearly vertical direction: a feature which is also found in many Teleostean embryos with large yolk-sacks.
[Pg 757] No traces of the pelvic fins have yet become developed.
The positions of the permanent dorsal, anal, and caudal fins, as pointed out by Agassiz, are now indicated by a deposit of pigment in the embryonic fin.
In an embryo on the sixth day after hatching, of about 15 millims. in length, of which we have also given a figure (Plate 34, fig. 14), the following fresh features deserve special notice.
In the region of the head there is a considerable elongation of the pre-oral part, forming a short snout, at the end of which is placed the suctorial disc. At the sides of the snout are placed the nasal pits, which have become somewhat elongated anteriorly.
The mouth has lost its open rhomboidal shape, and has become greatly narrowed in an antero-posterior direction, so that its opening is reduced to a slit. The mandibles and maxillary processes are nearly parallel, though both of them are very much shorter than in the adult. The operculum is now a very large flap, and has extended so far backwards as to cover the insertion of the pectoral fin. The two opercular folds nearly meet ventrally.
The yolk-sack is still more reduced in size, one important consequence of which is that the pectoral fins (pc.f.) appear to spring out more or less horizontally from the sides of the body, and at the same time their primitive line of attachment to the body becomes transformed from a longitudinal to a more or less transverse one.
The first traces of the pelvic fins are now visible as slight longitudinal projections near the hinder end of the yolk-sack (pl.f.).
The pigmentation marking the regions of the permanent fins has become more pronounced, and it is to be specially noted that the ventral part of the caudal fin (the permanent caudal) is considerably more prominent than the dorsal fin opposite to it.
The next changes, as Agassiz points out, are mainly in the lengthening
of the snout; the increase in length both of the lower and upper jaw; the
concentration of the sucker of the sucking disc; and the adoption of the
general colouring of somewhat older Fish. The lobe of the pectoral has
become specially prominent, and the outline of the fins is now indicated by
a fine milky granulation. Seen from above, the gill-cover is [Pg 758] seen to
leave a large circular opening leading to the gill-arches, into which a
current of water is constantly passing, by the lateral expansion and
contraction of the gill-cover; the outer extremity of the gill-cover covers
the base of the pectorals. In a somewhat older stage the snout has become
more elongated, the sucker more concentrated, and the disproportionate size
of the terminal sucking-disc is reduced; the head, when seen from above,
becoming slightly elongated and pointed.
In a larva of about 18 days old and 21 millims. in length, of which we have not given a figure, the snout has grown greatly in length, carrying with it the nasal organs, the openings of which now appear to be divided into two parts. The suctorial disc is still a prominent structure at the end of the snout. The lower jaw has elongated correspondingly with the upper, so that the gape is very considerable, though still very much less than in the adult.
The opercular flaps overlap ventrally, the left being superficial. They
still cover the bases of the pectoral fins. The latter are described by
Agassiz as being kept in constant rapid motion, so that the fleshy edge is
invisible, and the vibration seems almost involuntary, producing a constant
current round the opening leading into the cavity of the gills.
The pelvic fins are somewhat more prominent.
The yolk-sack, as pointed out by Agassiz, has now disappeared as an external appendage.
After the stage last described the young Fish rapidly approaches the adult form. To shew the changes effected we have figured the head of a larva of about a month old and 23 millims. in length (Plate 34, fig. 15). The suctorial disc, though much reduced, is still prominent at the end of the snout. Eventually, as shewn by Agassiz, it forms the fleshy globular termination of the upper jaw.
The most notable feature in which the larva now differs in its external form from the adult is in the presence of an externally heterocercal tail, caused by the persistence of the primitive caudal fin as an elongated filament projecting beyond the permanent caudal (Plate 41, fig. 68).
Delicate dermal fin-rays are now conspicuous in the peripheral parts of all the permanent fins. These rays closely [Pg 759] resemble the horny fin-rays in the fins of embryo Elasmobranchii in their development and structure. They appear gradually to enlarge to form the permanent rays, and we have followed out some of the stages of their growth, which is in many respects interesting. Our observations are not, however, complete enough to publish, and we can only say here that their early development and structure proves their homology with the horny fibres or rays in fins of Elasmobranchii. The skin is still, however, entirely naked, and without a trace of its future armour of enamelled scales.
The tail of a much older larva, 11 centims. in length, in which the scales have begun to be formed, is shewn in Plate 34, fig. 16.
We complete this section of our memoir by quoting the following passages from Agassiz as to the habits of the young fish at the stages last described:—
In the stages intervening between plate iii, fig.
19, and plate iii, fig. 30, the young Lepidosteus frequently swim
about, and become readily separated from their point of attachment. In the
stage of plate iii, fig. 30, they remain often perfectly quiet close to the
surface of the water; but, when disturbed, move very rapidly about through
the water.... The young already have also the peculiar habit of the adult
of coming to the surface to swallow air. When they go through the process
under water of discharging air again they open their jaws wide, and spread
their gill-covers, and swallow as if they were choking, making violent
efforts, until a minute bubble of air has become liberated, when they
remain quiet again. The resemblance to a Sturgeon in the general appearance
of this stage of the young Lepidosteus is quite marked.
[499] The numbers refer to the list of memoirs of the anatomy and development given at the end of this memoir.
[500] We have examined the structure of the ovarian ova in order to throw light on the nature of these peculiar pyriform bodies. Unfortunately, the ovaries of our adult examples of Lepidosteus were so badly preserved, that we could not ascertain anything on this subject. The ripe ova in the ovary have an investment of pyriform bodies similar to those of the just laid ova. With reference to the structure of the ovarian ova we may state that the germinal vesicles are provided with numerous nucleoli arranged in close proximity with the membrane of the vesicle.
[501]
Ueb. d. Entwick. d. Central Nerven Systems d. Teleostier,
Archiv für mikr. Anat. Vol. XV. 1878.
[502]
Salensky, Recherches s. le Développement du Sterlet.
Archives de Biol. Vol. II. 1881, pl. XVII. fig. 27.
[503]
Beiträge zur Entwick. d. Knochenfische,
Zeit. f. wiss. Zool. Vol.
XXIII. 1873,
taf. III. fig. IX. 2.
[504] Journal of Anat. and Physiol. Vol. XI. p. 491, plates XX. and XXI.
[505]
Elasmobranch Fishes,
p. 156, plates 10 and 13. [This edition,
p. 378, pl. 11, 14.]
[506]
Elasmobranch Fishes,
p. 136, plate 11, fig. 10. [This edition,
p. 354, pl. 12.]
[507]
F. M. Balfour, Monograph on the Development of Elasmobranch Fishes,
p. 87, plate 9, fig. 2. [This edition, p. 303, pl. 10.]
[508] This statement of Agassiz does not correspond with the dates on the specimens sent to us—a fact no doubt due to the hatching not taking place at the same time for all the larvæ.
I. Anatomy.
The brain of Lepidosteus has been figured by Busch (whose figure has been copied by Miklucho-Maclay, and apparently by Huxley), by Owen and by Wilder (No. 15). The figure of the latter author, representing a longitudinal section through the [Pg 760] brain, is the most satisfactory, the other figures being in many respects inaccurate; but even Wilder's figure and description, though taken from the fresh object, appear to us in some respects inadequate. He offers, moreover, fresh interpretations of certain parts of the brain which we shall discuss in the sequel.
We have examined two brains which, though extremely soft, were, nevertheless, sufficiently well preserved to enable us to study the external form. We have, moreover, made a complete series of transverse sections through one of the brains, and our sections, though utterly valueless from a histological point of view, have thrown some light on the topographical anatomy of the brain.
Plate 38, figs. 47A, B, and C, represent three views of the brain, viz.: from the side, from above, and from below. We will follow in our description the usual division of the brain into fore-brain, mid-brain, and hind-brain.
The fore-brain consists of an anterior portion forming the cerebrum, and a posterior portion constituting the thalamencephalon.
The cerebrum at first sight appears to be composed of (a) a pair of posterior and somewhat dorsal lobes, forming what have usually been regarded as the true cerebral hemispheres, but called by Wilder the prothalami, and (b) a pair of anterior and ventral lobes, usually regarded as the olfactory lobes, from which the olfactory nerves spring. Mainly from a comparison with our embryonic brains described in the sequel, we are inclined to think that the usual interpretations are not wholly correct, but that the true olfactory lobes are to be sought for in small enlargements (Plate 38. figs. 47A, B, and C, olf.) at the front end of the brain[509] from which the olfactory nerves spring. The cerebrum proper would then consist of a pair of anterior and ventral lobes (ce.), and of a pair of posterior lobes (ce´.), both pairs uniting to form a basal portion behind.
The two pairs of lobes probably correspond with the two parts of the cerebrum of the Frog, the anterior of which, like that of Lepidosteus, was held to be the olfactory lobe, till Götte's researches shewed that this view was not tenable.
[Pg 761] The anterior lobes of the cerebrum have a conical form, tapering anteriorly, and are completely separated from each other. The posterior lobes, as is best shewn in side views, have a semicircular form. Viewed from above they appear as rounded prominences, and their dorsal surface is marked by two conspicuous furrows (Plate 38, fig. 47B, ce´.), which have been noticed by Wilder, and are similar to those present in many Teleostei. Their front ends overhang the base of the anterior cerebral lobes. The basal portion of the cerebrum is an undivided lobe, the anterior wall of which forms the lamina terminalis.
What we have above described as the posterior cerebral lobes have been described by Wilder as constituting the everted dorsal border of the basal portion of the cerebrum.
The portion of the cerebro-spinal canal within the cerebrum presents
certain primitive characters, which are in some respects dissimilar to
those of higher types, and have led Wilder to hold the posterior cerebral
lobes, together with what we have called the basal portion of the cerebrum,
to be structures peculiar to Fishes, for which he has proposed the name
prothalami.
In the basal portion of the cerebrum there is an unpaired slit-shaped ventricle, the outer walls of which are very thick. It is provided with a floor formed of nervous matter, in part of which, judging from Wilder's description, a well-marked commissure is placed. We have found in the larva a large commissure in this situation (Plate 37, figs. 44 and 45, a.c.); and it may be regarded as the homologue of the anterior commissure of higher types. This part of the ventricle is stated by Wilder to be without a roof. This appears to us highly improbable. We could not, however, determine the nature of the roof from our badly preserved specimens, but if present, there is no doubt that it is extremely thin, as indeed it is in the larva (Plate 37, fig. 46B). In a dorsal direction the unpaired ventricle extends so as to separate the two posterior cerebral lobes. Anteriorly the ventricle is prolonged into two horns, which penetrate for a short distance, as the lateral ventricles, into the base of the anterior cerebral lobes. The front part of each anterior cerebral lobe, as well as of the whole of the posterior lobes, appears solid in our sections; but Wilder describes the anterior horns of the [Pg 762] ventricle as being prolonged for the whole length of the anterior lobes.
In the embryos of all Vertebrates the cerebrum is not at first divided into two lobes, so that the fact of the posterior part of the cerebrum in Lepidosteus and probably other Ganoids remaining permanently in the undivided condition does not appear to us a sufficient ground for giving to the lobes of this part of the cerebrum the special name of prothalami, as proposed by Wilder, or for regarding them as a section of the brain peculiar to Fishes.
The thalamencephalon (th.) contains the usual parts, but is in[TN17] some respects peculiar. Its lateral walls, forming the optic thalami, are thick, and are not sharply separated in front from the basal part of the cerebrum; between them is placed the third ventricle. The thalami are of considerable extent, though partially covered by the optic lobes and the posterior lobes of the cerebrum. They are not, however, relatively so large as in other Ganoid forms, more especially the Chondrostei and Polypterus.
On the roof of the thalamencephalon is placed a large thin-walled vesicle (Plate 38, figs. 47A and B, v.th.), which undoubtedly forms the most characteristic structure connected with this part of the brain. Owing to the wretched state of preservation of the specimens, we have found it impossible to determine the exact relations of this body to the remainder of the thalamencephalon; but it appears to be attached to the roof of the thalamencephalon by a narrow stalk only. It extends forwards so as to overlap part of the cerebrum in front, and is closely invested by a highly vascular layer of the pia mater.
No mention is made by Wilder of this body; nor is it represented in his figures or in those of the other anatomists who have given drawings of the brain of Lepidosteus. It might at first be interpreted as a highly-developed pineal gland, but a comparison with the brain of the larva (vide p. 764) shews that this is not the case, but that the body in question is represented in the larva by a special outgrowth of the roof of the thalamencephalon. The vesicle of the roof of the thalamencephalon is therefore to be regarded as a peculiar development of the tela choroidea of the third ventricle.
[Pg 763] How far this vesicle has a homologue in the brains of other Ganoids is not certain, since negative evidence on this subject is all but valueless. It is possible that a vesicular sack covering over the third ventricle of the Sturgeon described by Stannius[510], and stated by him to be wholly formed of the membranes of the brain, is really the homologue of our vesicle.
Wiedersheim[511] has recently described in Protopterus a body which is undoubtedly homologous with our vesicle, which he describes in the following way:—
Dorsalwärts ist das Zwischenhirn durch
ein tiefes, von Hirnschlitz eingenommenes Thal von Vorderhirn abgesetzt;
dasselbe ist jedoch durch eine häutige, mit der Pia mater zusammenhängende
Kuppel oder Kapsel überbrückt.
This Kuppel
has precisely the same relations and a very similar
appearance to our vesicle. The true pineal gland is placed behind it. It
appears to us possible that the body found by Huxley[512]
in Ceratodus, which he holds to be the pineal gland, is in reality
this vesicle. It is moreover possible that what has usually been regarded
as the pineal gland in Petromyzon may in reality be the homologue of
the vesicle we have found in Lepidosteus.
We have no observations on the pineal gland of the adult, but must refer the reader for the structure and relations of this body to the embryological section.
The infundibulum (Plate 38, fig. 47A, in.) is very elongated. Immediately in front of it is placed the optic chiasma (Plate 38, figs. 47A and C, op.ch.) from which the optic fibres can be traced passing along the sides of the optic thalami and to the optic lobes, very much as in Müller's figure of the brain of Polypterus.
On the sides of the infundibulum are placed two prominent bodies, the lobi inferiores (l.in.), each of which contains a cavity continuous with the prolongation of the third ventricle [Pg 764] into the infundibulum. The apex of the infundibulum is enlarged, and to it is attached a pituitary body (pt.).
The mid-brain is of considerable size, and consists of a basal portion connecting the optic thalami with the medulla, and a pair of large optic lobes (op.l.). The iter a tertio ad quartum ventriculum, which forms the ventricle of this part of the brain, is prolonged into each optic lobe, and the floor of each prolongation is taken up by a dome-shaped projection, the homologue of the torus semicircularis of Teleostei.
The hind-brain consists of the usual parts, the medulla oblongata and the cerebellum. The medulla presents no peculiar features. The sides of the fourth ventricle are thickened and everted, and marked with peculiar folds (Plate 38, figs. 47A and B, m.o.).
The cerebellum is much larger than in the majority of Ganoids, and resembles in all essential features the cerebellum of Teleostei. In side views it has a somewhat S-shaped form, from the presence of a peculiar lateral sulcus (Plate 38, fig. 47A, cb.). As shewn by Wilder, its wall actually has in longitudinal section this form of curvature, owing to its anterior part projecting forwards into the cavity of the iter[513]. This forward projection is not, however, so conspicuous as in most Teleostei. The cerebellum contains a large unpaired prolongation of the fourth ventricle.
II. Development.
The early development of the brain has already been described; and, although we do not propose to give any detailed account of the later stages of its growth, we have thought it worth while calling attention to certain developmental features which may probably be regarded as to some extent characteristic of the Ganoids. With this view we have figured (Plate 37, figs. 44, 45) longitudinal sections of the brain at two stages, viz.: of larvæ of 15 and 26 millims., and transverse sections (Plate 37, figs. 46A-G) of the brain of a larva at about the latter stage (25 millims.).
[Pg 765] The original embryonic fore-brain is divided in both embryos into a cerebrum (ce.) in front and a thalamencephalon (th.) behind. In the younger embryo the cerebrum is a single lobe, as it is in the brains of all Vertebrate embryos; but in the older larva it is anteriorly (Plate 37, fig. 46A) completely divided into two hemispheres. The roof of the undivided posterior part of the cerebrum is extremely thin (Plate 37, fig. 46B). Near the posterior border of the base of the cerebrum there is a great development of nervous fibres, which may probably be regarded as in part equivalent to the anterior commissure (Plate 37, figs. 44, 45 a.c.).
Even in the oldest of the two brains the olfactory lobes are very slightly developed, constituting, however, small lateral and ventral prominences of the front end of the hemispheres. From each of them there springs a long olfactory nerve, extending for the whole length of the rostrum to the olfactory sack.
The thalamencephalon presents a very curious structure, and is relatively a more important part of the brain than in the embryo of any other form which we know of. Its roof, instead of being, as usual, compressed antero-posteriorly[514], so as to be almost concealed between the cerebral hemispheres and the optic lobes (mid-brain), projects on the surface for a length quite equal to that of the cerebral hemispheres (Plate 37, figs. 44 and 45, th.). In the median line the roof of the thalamencephalon is thin and folded; at its posterior border is placed the opening of the small pineal gland. This body is a papilliform process of the nervous matter of the roof of this part of the brain, and instead of being directed forwards, as in most Vertebrate types, tends somewhat backwards, and rests on the mid-brain behind (Plate 37, figs. 44, 45, and 46C and D, pn.). The roof of the thalamencephalon immediately in front of the pineal gland forms a sort of vesicle, the sides of which extend laterally as a pair of lobes, shewn in transverse sections in Plate 37, figs. 46C and D, as th.l. This vesicle becomes, we cannot doubt, the vesicle on the roof of the thalamencephalon which we have described in the adult brain. Immediately in front of the pineal gland the roof of the thalamencephalon contains a transverse commissure [Pg 766] (Plate 37, fig. 46C, z.), which is the homologue of a similarly situated commissure present in the Elasmobranch brain[515], while behind the pineal gland is placed the posterior commissure. The sides of the thalamencephalon are greatly thickened, forming the optic thalami (Plate 37, figs. 46C and D, op.th.), which are continuous in front with the thickened outer walls of the hemispheres. Below, the thalamencephalon is produced into a very elongated infundibulum (Plate 37, figs. 44, 45, 46E, in.), the apex of which is trilobed as in Elasmobranchii and Teleostei. The sides of the infundibulum exhibit two lobes, the lobi inferiores (Plate 37, fig. 46E, l.in.), which are continued posteriorly into the crura cerebri.
The pituitary body[516] (Plate 37, figs. 44, 45, 46E, pt.) is small, not divided into lobes, and provided with a very minute lumen.
In front of the infundibulum is the optic chiasma (Plate 37, fig. 46D, op.ch.), which is developed very early. It is, as stated by Müller, a true chiasma.
The mid-brain (Plate 37, figs. 44 and 45, m.b.) is large, and consists in both stages of (1) a thickened floor forming the crura cerebri, the central canal of which constitutes the iter a tertio ad quartum ventriculum; and (2) the optic lobes (Plate 37, figs. 46E, F, G, op.l.) above, each of which is provided with a cavity continuous with the median iter. The optic lobes are separated dorsally and in front by a well-marked median longitudinal groove. Posteriorly they largely overlap the cerebellum. In the anterior part of the optic lobes, at the point where the iter joins the third ventricle, there may be seen slight projections of the floor into the lumen of the optic lobes (Plate 37, fig. 46E). These masses probably become in the adult the more conspicuous [Pg 767] prominences of the floor of the ventricles of the optic lobes, which we regard as homologous with the tori semicirculares of the brain of the Teleostei.
The hind-brain is formed of the usual divisions, viz.: cerebellum and medulla oblongata (Plate 37, figs. 44 and 45, cb., md.). The former constitutes a bilobed projection of the roof of the hind-brain. Only a small portion of it is during these stages left uncovered by the optic lobes, but the major part extends forwards for a considerable distance under the optic lobes, as shewn in the transverse sections (Plate 37, figs. 46F and G, cb.); and its two lobes, each with a prolongation of its cavity, are continued forwards beyond the opening of the iter into the fourth ventricle.
It is probable that the anterior horns of the cerebellum are equivalent to the prolongations of the cerebellum into the central cavity of the optic lobes of Teleostei, which are continuous with the so-called fornix of Göttsche.
III. Comparison of the larval and adult brain of Lepidosteus, together with some observations on the systematic value of the characters of the Ganoid brain.
The brain of the older of the two larvæ, which we have described, sufficiently resembles in most of its features that of the adult to render material assistance in the interpretation of certain of the parts of the latter. It will be remembered that in the adult brain the parts usually held to be olfactory lobes were described as the anterior cerebral lobes. The grounds for this will be apparent by a comparison of the cerebrum of the larva and adult. In the larva the cerebrum is formed of (1) an unpaired basal portion with a thin roof, and (2) of a pair of anterior lobes, with small olfactory bulbs at their free extremities.
The basal portion in the larva clearly corresponds in the adult with the basal portion, together with the two posterior cerebral lobes, which are merely special outgrowths of the dorsal edge of the primitive basal portion. The pair of anterior lobes have exactly the same relations in the larva as in the adult, except that in the former the ventricles are prolonged for their [Pg 768] whole length instead of being confined to their proximal portions. If, therefore, our identifications of the larval parts of the brain are correct, there can hardly be a question as to our identifications of the parts in the adult. As concerns these identifications, the comparison of the brain of our two larvæ appears conclusive in favour of regarding the anterior lobes as parts of the cerebrum, as distinguished from the olfactory lobes, in that they are clearly derived from the undivided anterior portion of the cerebrum of the younger larva.
The comparison of the larval brain with that of the adult again appears to us to leave no doubt that the vesicle attached to the roof of the thalamencephalon in the adult is the same structure as the bilobed outgrowth of this roof in the larva; and since there is in addition a well-developed pineal gland in the larva with the usual relations, there can be no ground for identifying the vesicle in the adult with the pineal gland.
Müller, in his often quoted memoir (No. 13), states that the brains of Ganoids are peculiar and distinct from those both of Teleostei and Elasmobranchii; but in addition to pointing out that the optic nerves form a chiasma he does not particularly mention the features, to which he alludes in general terms. More recently Wilder (No. 15) has returned to this subject; and though, as we have already had occasion to point out, we cannot accept all his identifications of the parts of the Ganoid brain, yet he has called attention to certain characteristic features of the cerebrum which have an undoubted systematic value.
The distinctive characters of the Ganoid brain are, in our opinion, (1) the great elongation of the region of the thalamencephalon; and (2) the unpaired condition of the posterior part of the cerebrum, and the presence of so thin a roof to the ventricle of this part as to cause it to appear open above.
The immense length of the region of the thalamencephalon is a feature in the Ganoid brain which must at once strike any one who examines figures of the brains of Chondrostei, Polypterus, or Amia. It is less striking in the adult Lepidosteus, though here also we have shewn that the thalamencephalon is really very greatly developed; but in the larva of Lepidosteus this feature is still better marked, so that the brain of the larva may be described as being more characteristically Ganoid than that of the adult.
[Pg 769] The presence of a largely developed thalamencephalon at once distinguishes a Ganoid brain from that of a Teleostean Fish, in which the optic thalami are very much reduced; but Lepidosteus shews its Teleostean affinities by a commencing reduction of this part of the brain.
The large size of the thalamencephalon is also characteristic of the Ganoid brain in comparison with the brain of the Dipnoi; but is not however so very much more marked in the Ganoids than it is in some Elasmobranchii.
On the whole, we may consider the retention of a large thalamencephalon as a primitive character.
The second feature which we have given as characteristic of the Ganoid brain is essentially that which has been insisted upon by Wilder, though somewhat differently expressed by him.
The simplest condition of the cerebrum is that found in the larva of Lepidosteus, where there is an anterior pair of lobes, and an undivided posterior portion with a simple prolongation of the third ventricle, and a very thin roof. The dorsal edges of the posterior portion, adjoining the thin roof, usually become somewhat everted (cf. Wilder), and in Lepidosteus these edges have in the adult a very great development, and form (vide Plate 38, fig. 47A-C, ce´.) two prominent lobes, which we have spoken of as the posterior cerebral lobes.
These characters of the cerebrum are perhaps even more distinctive than those of the thalamencephalon.
In Teleostei the cerebrum appears to be completely divided into two hemispheres, which are, however, all but solid, the lateral ventricles being only prolonged into their bases. In Dipnoi again there is either (Protopterus, Wiedersheim[517]) a completely separated pair of oval hemispheres, not unlike those of the lower Amphibia, or the oval hemispheres are not completely separated from each other (Ceratodus, Huxley[518], Lepidosiren, Hyrtl[519]); in either case the hemispheres are traversed for the whole length by lateral ventricles which are either completely or nearly completely separated from each other.
[Pg 770] In Elasmobranchii the cerebrum is an unpaired though bilobed body, but traversed by two completely separated lateral ventricles, and without a trace of the peculiar membranous roof found in Ganoids.
Not less interesting than the distinguishing characters of the Ganoid brain are those cerebral characters which indicate affinities between Lepidosteus and other groups. The most striking of these are, as might have been anticipated, in the direction of the Teleostei.
Although the foremost division of the brain is very dissimilar in the two groups, yet the hind-brain in many Ganoids and the mid-brain also in Lepidosteus approaches closely to the Teleostean type. The most essential feature of the cerebellum in Teleostei is its prolongation forwards into the ventricles of the optic vesicles as the valvula cerebelli. We have already seen that there is a homologous part of the cerebellum in Lepidosteus; Stannius also describes this part in the Sturgeon, but no such part is represented in Müller's figure of the brain of Polypterus, or described by him in the text.
The cerebellum is in most Ganoids relatively smaller, and this is even the case with Amia; but the cerebellum of Lepidosteus is hardly less bulky than that of most Teleostei.
The presence of tori semicirculares on the floor of the mid-brain of Lepidosteus again undoubtedly indicates its affinities with the Teleostei, and such processes are stated by Stannius to be absent in the Sturgeon, and have not, so far as we are aware, been described in other Ganoids. Lastly we may point to the presence of well-developed lobi inferiores in the brain of Lepidosteus as an undoubted Teleostean character.
On the whole, the brain of Lepidosteus, though preserving its true Ganoid characters, approaches more closely to the brain of the Teleostei than that of any other Ganoid, including even Amia.
It is not easy to point elsewhere to such marked resemblances of the Ganoid brain, as to the brain of the Teleostei.
The division of the cerebrum into anterior and posterior lobes, which is found in Lepidosteus, probably reappears again, as already indicated, in the higher Amphibia. The presence of the peculiar vesicle attached to the roof of the thalamencephalon [Pg 771] has its parallel in the brain of Protopterus, and as pointing in the same direction a general similarity in the appearance of the brain of Polypterus to that of the Dipnoi may be mentioned.
There appears to us to be in no points a close resemblance between the brain of Ganoids and that of Elasmobranchii.
[509] The homologies of the olfactory lobes throughout the group of Fishes require further investigation.
[510]
Ueb. d. Gehirn des Störs,
Müller's Archiv, 1843, and Lehrbuch d. vergl. Anat. d.
Wirbelthiere. Cattie, Archives de Biologie,
Vol. III. 1882, has recently described in Acipenser
sturio a vesicle on the roof of the thalamencephalon, whose cavity is
continuous with the third ventricle. This vesicle is clearly homologous
with that in Lepidosteus. (June 28, 1882.)
[511] R. Wiedersheim, Morphol. Studien, 1880, p. 71.
[512]
On Ceratodus Forsteri,
&c., Proc. Zool. Soc. 1876.
[513] In Wilder's figure the walls of the cerebellum are represented as much too thin.
[514] Vide F. M. Balfour, Comparative Embryology, Vol. II. figs. 248 and 250.
[515] Vide F. M. Balfour, Comparative Embryology, Vol. II. pp. 355-6 [the original edition], where it is suggested that this commissure is the homologue of the grey commissure of higher types.
[516] We have not been able to work out the early development of the pituitary body as satisfactorily as we could have wished. In Plate 37, fig. 40, there is shewn an invagination of the oral epithelium to form it; in Plate 37, figs. 41 and 42, it is represented in transverse section in two consecutive sections. Anteriorly it is still connected with the oral epithelium (fig. 41), while posteriorly it is free. It is possible that an earlier stage of it is shewn in Plate 36, fig. 35. Were it not for the evidence in other types of its being derived from the epiblast we should be inclined to regard it as hypoblastic in origin.
[517] Morphol. Studien, III. Jena, 1880.
[518]
On Ceratodus Forsteri,
Proc. Zool. Soc. 1876.
[519] Lepidosiren paradoxa. Prag. 1845.
Olfactory organ.
Development.—The nasal sacks first arise during the late embryonic period in the form of a pair of thickened patches of the nervous layer of the epiblast on the dorsal surface of the front end of the head (Plate 37, fig. 39, ol.). The patches very soon become partially invaginated; and a small cavity is developed between them and the epidermic layer of the epiblast (Plate 37, figs. 42 and 43, ol.). Subsequently, the roof of this space, formed by the epidermic layer of the epiblast, is either broken through or absorbed; and thus open pits, lined entirely by the nervous layer of the epidermis, are formed.
We are not acquainted with any description of an exactly similar mode of origin of the olfactory pits, though the process is almost identical with that of the other sense organs.
We have not worked out in detail the mode of formation of the double openings of the olfactory pits, but there can be but little doubt that it is caused by the division of the single opening into two.
The olfactory nerve is formed very early (Plate 37, fig. 39, I), and, as Marshall has found in Aves and Elasmobranchii, it arises at a stage prior to the first differentiation of an olfactory bulb as a special lobe of the brain.
The Eye.
Anatomy.—We have not made a careful histological examination of the eye of Lepidosteus, which in our specimens was not sufficiently well preserved for such a purpose; but we have [Pg 772] found a vascular membrane enveloping the vitreous humour on its retinal aspect, which, so far as we know, is unlike anything which has so far been met with in the eye of any other adult Vertebrate.
The membrane itself is placed immediately outside the hyaloid membrane, i.e. on the side of the hyaloid membrane bounding the vitreous humour. It is easily removed from the retina, to which it is only adherent at the entrance of the optic nerve. In both the eyes we examined it also adhered, at one point, to the capsule of the lens, but we could not make out whether this adhesion was natural, or artificially produced by the coagulation of a thin layer of albuminous matter. In one instance, at any rate, the adhesion appeared firmer than could easily be produced artificially.
The arrangement of the vessels in the membrane is shewn diagrammatically in Plate 38, fig. 49, while the characteristic form of the capillary plexus is represented in Plate 38, fig. 50.
The arterial supply appears to be derived from a vessel perforating the retina close to the optic nerve, and obviously homologous with the artery of the processus falciformis and pecten of Teleostei and Birds, and with the arteria centralis retinæ of Mammals. From this vessel branches diverge and pursue a course towards the periphery. They give off numerous branches, the blood from which enters a capillary plexus (Plate 38, figs. 49 and 50) and is collected again by veins, which pass outwards and finally bend over and fall into (Plate 38, fig. 49) a circular vein (cr.v.) placed at the outer edge of the retina along the insertion of the iris (ir). The terminal branches of some of the main arteries appear also to fall directly into this vein.
The membrane supporting the vessels just described is composed of a transparent matrix, in which numerous cells are embedded (Plate 38, fig. 50).
Development.—In the account of the first stages of development of Lepidosteus, the mode of formation of the optic cup, the lens, &c., have been described (vide Plates 35 and 36, figs. 23, 26, 35). With reference to the later stages in the development of the eye, the only subject with which we propose to deal is the growth of the mesoblastic processes which enter the cavity of the vitreous humour through the choroid slit.
[Pg 773] Lepidosteus is very remarkable for the great number of mesoblast cells which thus enter the cavity of the vitreous humour, and for the fact that these cells are at first unaccompanied by any vascular structures (Plate 37, fig. 43, v.h). The mesoblast cells are scattered through the vitreous humour, and there can be no doubt that during early larval life, at a period however when the larva is certainly able to see, every histologist would consider the vitreous humour to be a tissue formed of scattered cells, with a large amount of intercellular substance; and the fact that it is so appears to us to demonstrate that Kessler's view of the vitreous humour being a mere transudation is not tenable.
In the larva five or six days after hatching, and about 15 millims. in length, the choroid slit is open for its whole length. The edges of the slit near the lens are folded, so as to form a ridge projecting into the cavity of the vitreous humour, while nearer the insertion of the optic nerve they cease to exhibit any such structure. The mesoblast, though it projects between the lips of the ridge near the lens, only extends through the choroid slit into the cavity of the vitreous humour in the neighbourhood of the optic nerve. Here it forms a lamina with a thickened edge, from which scattered cells in the cavity of the vitreous humour seem to radiate.
At a slightly later stage than that just described, blood-vessels become developed within the cavity of the vitreous humour, and form the vascular membrane already described in the adult, placed close to the layer of nerve-fibres of the retina, but separated from this layer by the hyaloid membrane (Plate 38, fig. 48, v.sh.). The artery bringing the blood to the above vascular membrane is bound up in the same sheath as the optic nerve, and passes through the choroid slit very close to the optic nerve. Its entrance into the cavity of the vitreous humour is shewn in Plate 38, fig. 48 (vs.); its relation to the optic nerve in Plate 37, fig. 46, C and D (vs.).
The above sheath has, so far as we know, its nearest analogue in the eye of Alytes, where, however, it is only found in the larva.
The reader who will take the trouble to refer to the account of the imperfectly-developed processus falciformis of the Elasmobranch [Pg 774] eye in the treatise On Comparative Embryology, by one of us[520], will not fail to recognize that the folds of the retina at the sides of the choroid slit, and the mesoblastic process passing through this slit, are strikingly similar in Lepidosteus and Elasmobranchii; and that, if we are justified in holding them to be an imperfectly-developed processus falciformis in the one case, we are equally so in the other.
Johannes Müller mentions the absence of a processus falciformis as one of the features distinguishing Ganoids and Teleostei. So far as the systematic separation of the two groups is concerned, he is probably perfectly justified in this course; but it is interesting to notice that both in Ganoids and Elasmobranchii we have traces of a structure which undergoes a very special development in the Teleostei, and that the processus falciformis of Teleostei is therefore to be regarded, not as an organ peculiar to them, but as the peculiar modification within the group of a primitive Vertebrate organ.
[520] Vol. II. p. 414 [the original edition].
One of the most remarkable organs of the larval Lepidosteus is the suctorial disc, placed at the front end of the head, to which we have made numerous allusions in the first section of this memoir.
The external features of the disc have been fully dealt with by Agassiz, and he also explained its function by observations on the habits of the larva. We have already quoted (p. 755) a passage from Agassiz' memoir shewing how the young Fishes use the disc to attach themselves firmly to any convenient object. The discs appear in fact to be highly efficient organs of attachment, in that the young Fish can remain suspended by them to the sides of the jar, even after the water has been lowered below the level at which they are attached.
The disc is formed two or three days before hatching, and from Agassiz' statements, it appears to come into use immediately the young Fish is liberated from the egg membranes.
We have examined the histological structure of the disc at various ages of its growth, and may refer the reader to Plate 34, [Pg 775] figs. 11 and 13, and Plate 37, figs. 40 and 44. The result of our examination has been to shew that the disc is provided with a series of papillæ often exhibiting a bilateral arrangement. The papillæ are mainly constituted of highly modified cells of the mucous layer of the epidermis. These cells have the form of elongated columns, the nucleus being placed at the base, and the main mass of the cells being filled with a protoplasmic reticulum. They may probably be regarded as modified mucous cells. In the mesoblast adjoining the suctorial disc there are numerous sinus-like vascular channels.
It does not appear probable that the disc has a true sucking action. It is unprovided with muscular elements, and there appears to be no mechanism by which it could act as a sucking organ. We must suppose, therefore, that its adhesive power depends upon the capacity of the cells composing its papillæ to pour out a sticky secretion.
There is a peculiarity in the muscular system of Lepidosteus, which so far as we know has not been previously noticed. It is that the lateral muscles of each side are not divided, either in the region of the trunk or of the tail, into a dorso-lateral and ventro-lateral division.
This peculiarity is equally characteristic of the older larvæ as of the adult, and is shewn in Plate 41, figs. 67, 72, and 73, and Plate 42, figs. 74-76. In the Cyclostomata the lateral muscles are not divided into dorsal and ventral sections; but except in this group such a division has been hitherto considered as invariable amongst Fishes.
This character must, without doubt, be held to be the indication of a very primitive arrangement of the muscular system. In the embryos of all Fishes with the usual type of the lateral muscles, the undivided condition of the muscles precedes the divided condition; and in primitive forms such as the Cyclostomata and Amphioxus the embryonic condition is retained, as it is in Lepidosteus.
Part I.—Vertebral column and ribs of the adult.
A typical vertebra from the trunk of Lepidosteus has the following characters (Plate 42, figs. 80 and 81).
The centrum is slightly narrower in the middle than at its two extremities. It articulates with adjacent vertebræ by a convex face in front and a concave face behind, being thus, according to Owen's nomenclature, opisthocœlous. It presents on its under surface a well-marked longitudinal ridge, which in many vertebræ is only united at its two extremities with the main body of the vertebra.
From the lateral borders of the centrum there project, at a point slightly nearer the front than the hind end, a pair of prominent hæmal processes (h.a.), to the ends of which are articulated the ribs. These processes have a nearly horizontal direction in the greater part of the trunk, though bent downwards in the tail.
The neural arches (n.a.) have a somewhat complicated form. They are mainly composed of two vertical plates, the breadth of the basal parts of which is nearly as great as the length of the vertebræ, so that comparatively narrow spaces are left between the neural arches of successive vertebræ for the passage of the spinal nerves. Some little way from its dorsal extremity each neural arch sends a horizontal process inwards, which meets its fellow and so forms a roof for the spinal canal. These processes appear to be confined to the posterior parts of the vertebræ, so that at the front ends of the vertebræ, and in the spaces between them, the neural canal is without an osseous roof. Above the level of this osseous roof there is a narrow passage, bounded laterally by the dorsal extremities of the neural plates. This passage is mainly filled up by a series of cartilaginous elements (Plate 42, figs. 80 and 81, i.c.) (probably fibro-cartilage), which rest upon the roof of the neural canal. Each element is situated intervertebrally, its anterior end being wedged in between the two dorsal processes of the neural arch of the vertebra in front, and its posterior end extending for some [Pg 777] distance over the vertebra behind. The successive elements are connected by fibrous tissue, and are continuous dorsally with a fibrous band, known as the ligamentum longitudinale superius (Plate 42, figs. 80 and 81, l.l.), characteristic of Fishes generally, and running continuously for the whole length of the vertebral column. Each of the cartilaginous elements is, as will be afterwards shewn, developed as two independent pieces of cartilage, and might be compared with the dorsal element which usually forms the keystone of the neural arch in Elasmobranchii, were not the latter vertebral instead of intervertebral in position. More or less similar elements are described by Götte in the neural arches of many Teleostei, which also, however, appear to be vertebrally placed, and he has compared them and the corresponding elements in the Sturgeon with the Elasmobranch cartilages forming the keystone of the neural arch. Götte does not, however, appear to have distinguished between the cartilaginous elements, and the osseous elements forming the roof of the spinal canal, which are true membrane bones; it is probable that the two are not so clearly separated in other types as in Lepidosteus.
The posterior ends of the neural plates of the neural arches are continued into the dorsal processes directed obliquely upwards and backwards, which have been somewhat unfortunately described by Stannius as rib-like projections of the neural arch. The dorsal processes of the two sides do not meet, but between them is placed a median free spinous element, also directed obliquely upwards and backwards, which forms a kind of roof for the groove in which the cartilaginous elements and the ligamentum longitudinale are placed.
The vertebræ are wholly formed of a very cellular osseous tissue, in which a distinction between the bases of the neural and hæmal processes and the remainder of the vertebra is not recognizable. The bodies of the vertebræ are, moreover, directly continuous with the neural and hæmal arches.
The ribs in the region of the trunk are articulated to the ends of the long hæmal processes. They envelop the body-cavity, their proximal parts being placed immediately outside the peritoneal membrane, along the bases of the intermuscular septa. Their distal ends do not, however, remain close to the [Pg 778] peritoneal membrane, but pass outwards along the intermuscular septa till their free ends come into very close proximity with the skin. This peculiarity, which holds good in the adult for all the free ribs, is shewn in one of the anterior ribs of an advanced larva in Plate 41, fig. 72 (rb.). We are not aware that this has been previously noticed, but it appears to us to be a point not without interest in all questions which concern the homology of rib-like structures occupying different positions in relation to the muscles. Its bearings are fully dealt with in the section of this paper devoted to the consideration of the homologies of the ribs in Fishes.
As regards the behaviour of the ribs in the transitional region between
the trunk and the tail, we cannot do better than translate the description
given by Gegenbaur of this region (No. 6,
p. 411):—Up to the
34th vertebra the ribs borne by the laterally and posteriorly directed
processes present nothing remarkable, though they have gradually become
shorter. The ribs of the 35th vertebra exhibit a slight curvature outwards
of their free ends, a peculiarity still more marked in the 36th. The last
named pair of ribs converge somewhat in their descent backwards so that
both ribs decidedly approach before bending outwards. The 37th vertebra is
no longer provided with freely terminating ribs, but on the contrary, the
same pair of processes which in front was provided with ribs, bears a short
forked process as the hæmal arch. The two, up to this point separated
ribs, have here formed a hæmal arch by the fusion of their lower ends,
which arch is movable just like the ribs, and, like them, is attached to
the vertebral column.
In the region of the tail-fin the hæmal arches supporting the caudal fin-rays are very much enlarged.
Part II.—Development of the vertebral column and ribs.
The first development and early histological changes of the notochord have already been given, and we may take up the history of the vertebral column at a period when the notochord forms a large circular rod, whose cells are already highly vacuolated, while the septa between the vacuoles form a delicate [Pg 779] wide-meshed reticulum. Surrounding the notochord is the usual cuticular sheath, which is still thin.
The first indications of the future vertebral column are to be found in the formation of a distinct mesoblastic investment of the notochord. On the dorsal aspect of the notochord, the mesoblast forms two ridges, one on each side, which are prolonged upwards so as to meet above the neural canal, for which they form a kind of sheath. On the ventral side of the notochord there are also two ridges, which are, however, except on the tail, much less prominent than the dorsal ridges.
The changes which next ensue are practically identical with those which take place in Teleostei. Around the cuticular sheath of the notochord there is formed an elastic membrane—the membrana elastica externa. At the same time the basal parts of the dorsal, or as we may perhaps more conveniently call them, the neural ridges of the notochord become enlarged at each intermuscular septum, and the tissue of these enlargements soon becomes converted into cartilage, thus forming a series of independent paired neural processes riding on the membrana elastica externa surrounding the notochord, and extending about two-thirds of the way up the sides of the medullary cord. They are shewn in transverse section in Plate 41, fig. 67 (n.a.), and in a side view in fig. 68 (n.a.).
Simultaneously with the neural arches, the hæmal arches also become established, and arise by the formation of similar enlargements of the ventral or hæmal ridges. In the trunk they are very small, but in the region of the tail their condition is very different. At the front end of the anal fin the paired hæmal arches suddenly enlarge and extend ventralwards (Plate 41, fig. 67, h.a.).
Each succeeding pair of arches becomes larger than the one in front, and the two elements of each arch first nearly meet below the caudal vein (Plate 41, fig. 67) and finally actually do so, forming in this way a completely closed hæmal canal. At the point where they first meet the permanent caudal fin commences, and here (Plate 41, fig. 68) we find that not only do the hæmal arches meet and coalesce below the caudal vein, but they are actually produced into long spines supporting the fin-rays of the caudal fin, which thus differs from the other fins in being [Pg 780] supported by parts of the true vertebral column and not by independently formed elements of the skeleton.
Each of the large caudal hæmal arches, including the spine, forms a continuous [TN18] whole, and arises at an earlier period of larval life than any other part of the vertebral column. We noticed the first indications of the neural arches in the larva of about a week old, while they are converted into fully formed cartilage in the larva of three weeks.
The neural and hæmal arches, resting on the membrana elastica externa, do not at this early stage in the least constrict the notochord. They grow gradually more definite, till the larva is five or six weeks old and about 26 millims. in length, but otherwise for a long time undergo no important changes. During the same period, however, the true sheath of the notochord greatly increases in thickness, and the membrana elastica externa becomes more definite. So far it would be impossible to distinguish the development of the vertebral column of Lepidosteus from that of a Teleostean Fish.
Of the stages immediately following we have unfortunately had no examples, but we have been fortunate enough to obtain some young specimens of Lepidosteus[521], which have enabled us to work out with tolerable completeness the remainder of the developmental history of the vertebral column. In the next oldest larva, of about 5.5 centims., the changes which have taken place are already sufficient to differentiate the vertebral column of Lepidosteus from that of a Teleostean, and to shew how certain of the characteristic features of the adult take their origin.
In the notochord the most important and striking change consists in the appearance of a series of very well marked vertebral constrictions opposite the insertions of the neural and hæmal arches. The first constrictions of the notochord are thus, as in other Fishes, vertebral; and although, owing to the growth of the intervertebral cartilage, the vertebral constrictions are subsequently replaced by intervertebral constrictions, yet at the same time the primitive occurrence of vertebral constrictions demonstrates that the vertebral column of Lepidosteus is a modification of a type of vertebral column with biconcave vertebræ.
[Pg
781] The structure of the gelatinous body of the notochord has
undergone no important change. The sheath, however, exhibits certain
features which deserve careful description. In the first place the
attention of the observer is at once struck by the fact that, in the
vertebral regions, the sheath is much thicker (.014 millims.) than in the intervertebral (.005 millims.), and a careful examination of the
sheath in longitudinal sections shews that the thickening is due to the
special differentiation of a superficial part (Plate 41, fig. 69, sh.) of the sheath in each vertebral region.
This part is somewhat granular as compared to the remainder, especially in
longitudinal sections. It forms a cylinder (the wall of which is about .01
millim. thick) in each vertebral region,
immediately within the membrana elastica externa. Between it and the
gelatinous tissue of the notochord within there is a very thin unmodified
portion of the sheath, which is continuous with the thinner intervertebral
parts of the sheath. This part of the sheath is faintly, but at the same
time distinctly, concentrically striated—a probable indication of
concentric fibres. The inner unmodified layer of the sheath has the
appearance in transverse sections through the vertebral regions of an inner
membrane, and may perhaps be Kölliker's membrana elastica
interna.
We are not aware that any similar modification of the sheath has been described in other forms.
The whole sheath is still invested by a very distinct membrana elastica externa (m.el).
The changes which have taken place in the parts which form the permanent vertebræ will be best understood from Plate 41, figs. 69-71. From the transverse section (fig. 70) it will be seen that there are still neural and hæmal arches resting upon the membrana elastica externa; but longitudinal sections (fig. 69) shew that laterally these arches join a cartilaginous tube, embracing the intervertebral regions of the notochord, and continuous from one vertebra to the next.
It will be convenient to treat separately the neural arches, the hæmal arches with their appendages, and the intervertebral cartilaginous rings.
The neural arches, except in the fact of embracing a relatively smaller part of the neural tube than in the earlier stage, do not [Pg 782] at first sight appear to have undergone any changes. Viewed from the side, however, in dissected specimens, they are seen to be prolonged upwards so as to unite above with bars of cartilage directed obliquely backwards. An explanation of this appearance is easily found in the sections. The cartilaginous neural arches are invested by a delicate layer of homogeneous bone, developed in the perichondrium, and this bone is prolonged beyond the cartilage and joins a similar osseous investment of the dorsal bars above mentioned. The whole of these parts may, it appears to us, be certainly reckoned as parts of the neural arches, so that at this stage each neural arch consists of: (1) a pair of basal portions resting on the notochord consisting of cartilage invested by bone, (2) of a pair of dorsal cartilaginous bars invested in bone (n.a´.), and (3) of osseous bars connecting (1) and (2).
Though, in the absence of the immediately preceding stages, it is not perfectly certain that the dorsal pieces of cartilage are developed independently of the ventral, there appears to us every probability that this is so; and thus the cartilage of each neural arch is developed discontinuously, while the permanent bony neural arch, which commences as a deposit of bone partly in the perichondrium and partly in the intervening membrane, forms a continuous structure.
Analogous occurrences have been described by Götte in Teleostei.
The dorsal portion of each neural arch becomes what we have called the dorsal process of the adult arch.
Between the dorsal processes of the two sides there is placed a median rod of cartilage (Plate 41, fig. 70, i.s.), which in its development is wholly independent of the true neural arches, and which constitutes the median spinous element of the adult. In tracing these backwards it becomes obvious that they are homologous with the interspinous elements supporting the dorsal fin, in that they are replaced by these interspinous elements in the region of the dorsal fin, and that the interspinous bones occupy the same position as the median spinous processes. This homology was first pointed out by Götte in the case of the Teleostei.
Immediately beneath this rod is placed the longitudinal [Pg 783] ligament (Plate 41, fig. 70, l.l.), but there is as yet no trace of a junction between the neural arches of the two sides in the space between the longitudinal ligament and the spinal cord.
The basal parts of the neural arches of the two sides are united dorsally by a thin cartilaginous layer resting on the sheath of the notochord, but they are not united ventrally with the hæmal arches.
The hæmal processes in the trunk are much more prominent than in the preceding stage, and their bases are united ventrally by a tolerably thick layer of cartilage. In the trunk they are continuous with the so-called ribs of the adult (Plate 41, fig. 70); but in order to study the nature of these ribs it is necessary to trace the modifications undergone by the hæmal arches in passing from the tail to the trunk.
It will be remembered that at an earlier stage the hæmal arches in the region of the tail-fin were fully formed, and that through the anterior part of the caudal region the hæmal processes were far advanced in development, and just in front of the caudal fin had actually met below the caudal vein.
The mode of development of the hæmal arches in the tail as unjointed cartilaginous bars investing the caudal arteries and veins is so similar to that of the caudal hæmal arches of Elasmobranchii, that it appears to us impossible to doubt their identity in the two groups[522].
The changes which have taken place by this stage with reference to the hæmal arches of the tail are not very considerable.
In the case of a few more vertebræ the hæmal processes [Pg 784] have united into an arch, and the spinous processes of the arches in the region of the caudal fin have grown considerably in length. A more important change is perhaps the commencement of a segmentation of the distal parts of the hæmal arches from the proximal. This process has not, however, as yet resulted in a complete separation of the two, such as we find in the adult.
If the hæmal processes are traced forwards (Plate 42, figs. 75 and 76) from the anterior segment where they meet ventrally, it will be found that each hæmal process consists of a basal portion, adjoining the notochord, and a peripheral portion. These two parts are completely continuous, but the line of a future separation is indicated by the structure of the cartilage, though not shewn in our figures. As the true body-cavity of the trunk replaces the obliterated body-cavity of the caudal region, no break of continuity will be found in the structure of the hæmal processes (Plates 41 and 42, figs. 73 and 74), but while the basal portions grow somewhat larger, the peripheral portions gradually elongate and take the form of delicate rods of cartilage extending ventralwards, on each side of the body-cavity, immediately outside the peritoneal membrane, and along the lines of insertion of the intermuscular septa. These rods obviously become the ribs of the adult.
As one travels forwards the ribs become continually longer and more important, and though they are at this stage united with the hæmal processes in every part of the trunk, yet they are much more completely separated from these processes in front than behind (Plate 41, fig. 72).
In front (Plate 41, fig. 72), each rib (rb.), after continuing its ventral course for some distance, immediately outside the peritoneal membrane, turns outwards, and passes along one of the intermuscular septa till it reaches the epidermis. This feature in the position of the ribs is, as has been already pointed out in the anatomical part of this section, characteristic of all the ribs of the adult.
It is unfortunate that we have had no specimens shewing the ribs at an earlier stage of development; but it appears hardly open to doubt that the ribs are originally continuous with the hæmal processes, and that the indications of a separation between [Pg 785] those two parts at this stage are not due to a secondary fusion, but to a commencing segmentation.
It further appears, as Müller, Gegenbaur and others have stated, that the ribs and hæmal processes of the tail are serially homologous structures; but that the view maintained by Götte in his very valuable memoirs on the Vertebrate skeleton is also correct to the effect that the hæmal arches of the tail are homologous throughout the series of Fishes.
To this subject we shall return again at the end of the section.
Before leaving the hæmal arches it may be mentioned that behind the region of the ventral caudal fin the two hæmal processes merge into one, and form an unpaired knob resting on the ventral side of the notochord, and not perforated by a canal.
There are now present well-developed intervertebral rings of cartilage, each of which eventually becomes divided into two parts, and converted into the adjacent faces of the contiguous vertebræ. These rings are united with the neural and hæmal arches of the vertebræ in front and behind.
Each ring, as shewn by the transverse section (Plate 41, fig. 71), is not uniformly thick, but exhibits four projections, two dorsal and two ventral. These four projections are continuous with the bases of the neural and hæmal arches of the adjacent vertebræ, and afford presumptive evidence of the derivation of the intervertebral rings from the neural and hæmal arches; in that had they so originated, it would be natural to anticipate the presence of four thickenings indicating the four points from which the cartilage had spread, while if the rings had originated independently, it would not be easy to give any explanation of the presence of such thickenings. Gegenbaur (No. 6), from the investigation of a much older larva than that we are now describing, also arrived at the conclusion that the intervertebral cartilages were derived from the neural and hæmal arches; but as doubts have been thrown upon this conclusion by Götte, and as it obviously required further confirmation, we have considered it important to attempt to settle this point. From the description given above, it is clear that we have not, however, been able absolutely to trace the origin of this cartilage, but at the same [Pg 786] time we think that we have adduced weighty evidence in corroboration of Gegenbaur's view.
As shewn in longitudinal section (Plate 41, fig. 69, iv.r.), the intervertebral rings are thicker in the middle than at the two ends. In this thickened middle part the division of the cartilage into two parts to form the ends of two contiguous vertebræ is subsequently effected. The curved line which this segmentation will follow is, however, already marked out, and from surface views it might be supposed that this division had actually occurred.
The histological structure of the intervertebral cartilage is very distinct from that of the cartilage of the bases of the arches, the nuclei being much more closely packed. In parts, indeed, the intervertebral cartilage has almost the character of fibro-cartilage. On each side of the line of division separating two vertebræ it is invested by a superficial osseous deposit.
The next oldest larva we have had was 11 centims. in length. The filamentous dorsal lobe of the caudal fin still projected far beyond the permanent caudal fin (Plate 34, fig. 16).
The vertebral column was considerably less advanced in development than that dissected by Gegenbaur, though it shews a great advance on the previous stage. Its features are illustrated by two transverse sections, one through the median plane of a vertebral region (Plate 42, fig. 78) and the other through that of an intervertebral region (Plate 42, fig. 79), and by a horizontal section (Plate 42, fig. 77).
In the last stage the notochord was only constricted vertebrally. Now, however, by the great growth of intervertebral cartilage there have appeared (Plate 42, fig. 77) very well-marked intervertebral constrictions, by the completion of which the vertebræ of Lepidosteus acquire their unique character amongst Fishes.
These constrictions still, however, coexist with the earlier, though at this stage relatively less conspicuous, vertebral constrictions.
The gelatinous body of the notochord retains its earlier condition. The sheath has, however, undergone some changes. In the vertebral regions there is present in any section of the sheath—(1) externally, the membrana elastica externa (m.el.); [Pg 787] then (2) the external layer of the sheath (sh.), which is, however, less thick than before, and exhibits a very faint form of radial striation; and (3) internally, a fairly thick and concentrically striated layer. The whole thickness is, on an average, 0.18 millim.
In the intervertebral regions the membrana elastica externa is still present in most parts, but has become absorbed at the posterior border of each vertebra, as shewn in longitudinal section in Plate 42, fig. 77. It is considerably puckered transversely. The sheath of the notochord within the membrana elastica externa is formed of a concentrically striated layer, continuous with the innermost layer of the sheath in the vertebral regions. It is puckered longitudinally. Thus, curiously enough, the membrana elastica externa and the sheath of the notochord in the intervertebral regions are folded in different directions, the folds of the one being only visible in transverse sections (Plate 42, fig. 79), and those of the other in longitudinal sections (Plate 42, fig. 77).
The osseous and cartilaginous structures investing the notochord may conveniently be dealt with in the same order as before, viz.: the neural arches, the hæmal arches, and the intervertebral cartilages.
The cartilaginous portions of the neural arches are still unossified, and form (Plate 42, fig. 78, n.a.) small wedge-shaped masses resting on the sheath of the notochord. They are invested by a thick layer of bone prolonged upwards to meet the dorsal processes (n.a´.), which are still formed of cartilage invested by bone.
It will be remembered that in the last stage there was no key-stone closing in the neural arch above. This deficiency is now however supplied, and consists of (1) two bars of cartilage repeated for each vertebra, but intervertebrally placed, which are directly differentiated from the ligamentum longitudinale superius, into which they merge above; and (2) two osseous plates placed on the outer sides of these cartilages, which are continuous with the lateral osseous bars of the neural arch. The former of these elements gives rise to the cartilaginous elements above the osseous bridge of the neural arch in the adult. The two osseous plates supporting these cartilages clearly form what we [Pg 788] have called in our description of the adult the osseous roof of the spinal canal.
A comparison of the neural arch at this stage with the arch in the adult, and in the stage last described, shews that the greater part of the neural arch of the adult is formed of membrane-bone, there being preformed in cartilage only a small basal part, a dorsal process, and paired key-stones below the ligamentum longitudinale superius.
The hæmal arches (Plate 42, fig. 78) are still largely cartilaginous, and rest upon the sheath of the notochord. They are invested by a thick layer of bone. The bony layer investing the neural and hæmal arches is prolonged to form a continuous investment round the vertebral portions of the notochord (Plate 42, fig. 78). This investment is at the sides prolonged outwards into irregular processes (Plate 42, fig. 78), which form the commencement of the outer part of the thick but cellular osseous cylinder forming the middle part of the vertebral body.
The intervertebral cartilages are much larger than in the earlier stage (Plate 42, figs. 77 and 79), and it is by their growth that the intervertebral constrictions of the notochord are produced. They have ceased to be continuous with the cartilage of the arches, the intervening portion of the vertebral body between the two being only formed of bone. They are not yet divided into two masses to form the contiguous ends of adjacent vertebræ.
Externally, the part of each cartilage which will form the hinder end of a vertebral body is covered by a tube of bone, having the form of a truncated funnel, shewn in longitudinal section in Plate 42, fig. 77, and in transverse section in Plate 42, fig. 79.
At each end, the intervertebral cartilages are becoming penetrated and replaced by beautiful branched processes from the homogeneous bone which was first of all formed in the perichondrium (Plate 42, fig. 77).
This constitutes the latest stage which we have had.
Gegenbaur (No. 6) has described the vertebral column in a somewhat older larva of 18 centims.
The chief points in which the vertebral column of this larva differed from ours are: (1) the disappearance of all trace of the [Pg 789] primitive vertebral constriction of the notochord; (2) the nearly completed constriction of the notochord in the intervertebral regions; (3) the complete ossification of the vertebral portions of the bodies of the vertebræ, the terminal so-called intervertebral portions alone remaining cartilaginous; (4) the complete ossification of the basal portions of the hæmal and neural processes included within the bodies of the vertebræ, so that in the case of the neural arch all trace of the fact that the greater part was originally not formed in cartilage had become lost. The cartilage of the dorsal spinous processes was, however, still persistent.
The only points which remain obscure in the later history of the vertebral column are the history of the notochord and of its sheath. We do not know how far these are either simply absorbed or partially or wholly ossified.
Götte in his memoir on the formation of the vertebral bodies of the Teleostei attempts to prove (1) that the so-called membrana elastica externa of the Teleostei is not a homogeneous elastica, but is formed of cells, and (2) that in the vertebral regions ossification first occurs in it.
In Lepidosteus we have met with no indication that the membrana elastica externa is composed of cells; though it is fair to Götte to state that we have not examined such isolated portions of it as he states are necessary in order to make out its structure. But further than this we have satisfied ourselves that during the earlier stage of ossification this membrane is not ossified, and indeed in part becomes absorbed in proximity to the intervertebral cartilages; and Gegenbaur met with no ossification of this membrane in the later stage described by him.
Summary of the development of the vertebral column and ribs.
A mesoblastic investment is early formed round the notochord, which is produced into two dorsal and two ventral ridges, the former uniting above the neural canal. Around the cuticular sheath of the notochord an elastic membrane, the membrana elastica externa, is next developed. The neural ridges become enlarged at each inter-muscular septum, and these enlargements [Pg 790] soon become converted into cartilage, thus forming a series of neural processes riding on the membrana elastica externa, and extending about two-thirds of the way up the sides of the neural canal. The hæmal processes arise simultaneously with, and in the same manner as, the neural. They are small in the trunk, but at the front end of the anal fin they suddenly enlarge and extend ventralwards. Each succeeding pair of hæmal arches becomes larger than the one in front, each arch finally meeting its fellow below the caudal vein, thus forming a completely closed hæmal canal. These arches are moreover produced into long spines supporting the fin-rays of the caudal fin, which thus differs from the other unpaired fins in being supported by parts of the vertebral column, and not by separately formed skeletal elements.
In the next stage which we have had the opportunity of studying (larva of 5½ centims.), a series of very well-marked vertebral constrictions are to be seen in the notochord. The sheath is now much thicker in the vertebral than in the intervertebral regions: this is due to a special differentiation of a superficial part of the sheath, which appears more granular than the remainder. This granular part of the sheath thus forms a cylinder in each vertebral region. Between it and the gelatinous tissue of the notochord there remains a thin unmodified portion of the sheath, which is continuous with the intervertebral parts of the sheath. The neural and hæmal arches are seen to be continuous with a cartilaginous tube embracing the intervertebral regions of the notochord, and continuous from one vertebra to the next. A delicate layer of bone, developed in the perichondrium, invests the cartilaginous neural arches, and this bone grows upwards so as to unite above with the osseous investment of separately developed bars of cartilage, which are directed obliquely backwards. These bars, or dorsal processes, may be reckoned as parts of the neural arches. Between the dorsal processes of the two sides is placed a median rod of cartilage, which is developed separately from the true neural arches, and which constitutes the median spinous element of the adult. Immediately below this rod is placed the ligamentum longitudinale superius. There is now a commencement of separation between the dorsal and ventral parts of the hæmal arches, not only in the tail, but also [Pg 791] in the trunk, where they pass ventralwards on each side of the body-cavity, immediately outside the peritoneal membrane, along the lines of insertion of the intermuscular septa. These are obviously the ribs of the adult, and there is no break of continuity of structure between the hæmal processes of the tail and the ribs. In the anterior part of the trunk the ribs pass outwards along the intermuscular septa till they reach the epidermis. Thus the ribs are originally continuous with the hæmal processes. Behind the region of the ventral caudal fin the two hæmal processes merge into one, which is not perforated by a canal.
Each of the intervertebral rings of cartilage becomes eventually divided into two parts, and converted into the adjacent faces of contiguous vertebræ, the curved line where this will be effected being plainly marked out. These rings are united with the neural and hæmal arches of the vertebræ next in front and behind. As these rings are formed originally by the spreading of the cartilage from the primitive neural and hæmal processes, the intervertebral cartilages are clearly derived from the neural and hæmal arches. The intervertebral cartilages are thicker in the middle than at their two ends.
In our latest stage (11 centims.), the vertebral constrictions of the notochord are rendered much less conspicuous by the growth of the intervertebral cartilages giving rise to marked intervertebral constrictions. In the intervertebral regions the membrana elastica externa has become aborted at the posterior border of each vertebra, and the remaining part is considerably puckered transversely. The inner sheath of the notochord is puckered longitudinally in the intervertebral regions. The granular external layer of the sheath in the vertebral regions is less thick than in the last stage, and exhibits faint radial striations.
Two closely approximated cartilaginous elements now form a key-stone to the neural arch above: these are directly differentiated from the ligamentum longitudinale superius, into which they merge above. An osseous plate is formed on the outer side of each of these cartilages. These plates are continuous with the lateral osseous bars of the neural arches, and also give rise to the osseous roof of the spinal canal of the adult.
[Pg 792] Thus the greater part of the neural arches is formed of membrane bone. The hæmal arches are invested by a thick layer of bone, and there is also a continuous osseous investment round the vertebral portions of the notochord. The intervertebral cartilages become penetrated by branched processes of bone.
Comparison of the vertebral column of Lepidosteus with that of other forms.
The peculiar form of the articulatory faces of the vertebræ of Lepidosteus caused L. Agassiz (No. 2) to compare them with the vertebræ of Reptiles, and subsequent anatomists have suggested that they more nearly resemble the vertebræ of some Urodelous Amphibia than those of any other form.
If, however, Götte's account of the formation of the amphibian vertebræ is correct, there are serious objections to a comparison between the vertebræ of Lepidosteus and Amphibia on developmental grounds. The essential point of similarity supposed to exist between them consists in the fact that in both there is a great development of intervertebral cartilage which constricts the notochord intervertebrally, and forms the articular faces of contiguous vertebræ.
In Lepidosteus this cartilage is, as we have seen, derived from the bases of the arches; but in Amphibia it is held by Götte to be formed by a special thickening of a cellular sheath round the notochord which is probably homologous with the cartilaginous sheath of the notochord of Elasmobranchii, and therefore with part of the notochordal sheath placed within the membrana elastica externa.
If the above statements with reference to the origin of the intervertebral cartilage in the two types are true, it is clear that no homology can exist between structures so differently developed. Provisionally, therefore, we must look elsewhere than in Lepidosteus for the origin of the amphibian type of vertebræ.
The researches which we have recorded demonstrate, however, in a very conclusive manner that the vertebræ of Lepidosteus have very close affinities with those of Teleostei.
[Pg 793] In support of this statement we may point: (1) To the structure of the sheath of the notochord; (2) to the formation of the greater part of the bodies of the vertebræ from ossification in membrane around the notochord; (3) to the early biconcave form of the vertebræ, only masked at a later period by the development of intervertebral cartilages; (4) to the character of the neural arches.
This latter feature will be made very clear if the reader will compare our figures of the sections of later vertebræ (Plate 42, fig. 78) with Götte's[523] figure of the section of the vertebra of a Pike (Plate 7, fig. 1). In Götte's figure there are shewn similar intercalated pieces of cartilage to those which we have found, and similar cartilaginous dorsal processes of the vertebræ. Thus we are justified in holding that whether or no the opisthocœlous form of the vertebræ of Lepidosteus is a commencement of a type of vertebræ inherited by the higher forms, yet in any case the vertebræ are essentially built on the type which has become inherited by the Teleostei from the bony Ganoids.
Part III.—The ribs of Fishes.
The nature and homologies of the ribs of Fishes have long been a matter of controversy; but the subject has recently been brought forward in the important memoirs of Götte[524] on the Vertebrate skeleton. The alternatives usually adopted are, roughly speaking, these:—Either the hæmal arches of the tail are homologous throughout the piscine series, while the ribs of Ganoids and Teleostei are not homologous with those of Elasmobranchii; or the ribs are homologous in all the piscine groups, and the hæmal arches in the tail are differently formed in the different types. Götte has brought forward a great body of evidence in favour of the first view; while Gegenbaur[525] may [Pg 794] be regarded as more especially the champion of the second view.
One of us held in a recent publication[526] that the question was not yet settled, though the view that the ribs are homologous throughout the series was provisionally accepted.
It is admitted by both Gegenbaur and Götte that in Lepidosteus the ribs, in the transition from the trunk to the tail, bend inwards, and finally unite in the region of the tail to form the ventral parts of the hæmal arches, and our researches have abundantly confirmed this conclusion.
Are the hæmal arches, the ventral parts of which are thus formed by the coalescence of the ribs, homologous with the hæmal arches in Elasmobranchii? The researches recorded in the preceding pages appear to us to demonstrate in a conclusive manner that they are so.
The development of the hæmal arches in the tail in these two groups is practically identical; they are formed in both as simple elongations of the primitive hæmal processes, which meet below the caudal vein. In the adult there is an apparent difference between them, arising from the fact that in Lepidosteus the peripheral parts of the hæmal processes are only articulated with the basal portions, and not, as in Elasmobranchii, continuous with them. This difference does not, however, exist in the early larva, since in the larval Lepidosteus the hæmal arches of the tail are unsegmented cartilaginous arches, as they permanently are in Elasmobranchii. If, however, the homology between the hæmal arches of the two types should still be doubted, the fact that in both types the hæmal arches are similarly modified to support the fin-rays of the ventral lobe of the caudal fin, while in neither type are they modified to support the anal fin, may be pointed out as a very strong argument in confirmation of their homology.
The demonstration of the homology of the hæmal arches of the tail in Lepidosteus and Elasmobranchii might at first sight be taken as a conclusive argument in favour of Götte's view, that the ribs of Elasmobranchii are not homologous with those of Ganoidei. This view is mainly supported by two facts:—
[Pg 795] (1) In the first place, the ribs in Elasmobranchii do not at first sight appear to be serially homologous with the ventral parts of the hæmal arches of the tail, but would rather seem to be lateral offshoots of the hæmal processes, while the hæmal arches of the tail appear to be completed by the coalescence of independent ventral prolongations of the hæmal processes.
(2) In the second place, the position of the ribs is different in the two groups. In Elasmobranchii they are situated between the dorso-lateral and ventro-lateral muscles (woodcut, fig. 1, rb.), while in Lepidosteus and other Ganoids they immediately girth the body-cavity.
Fig. 1.
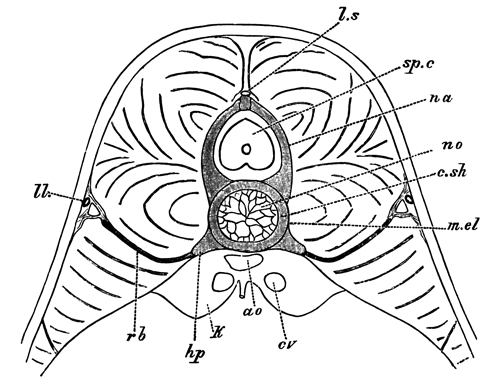
Diagrammatic section through the trunk of an advanced embryo of Scyllium, to shew the position of the ribs.
ao., aorta; c.sh., cartilaginous notochordal sheath; cv., cardinal vein; hp., hæmal process; k., kidney; l.s., ligamentum longitudinale superius; m.el., membrana elastica externa; na., neural arch; no., notochord; ll., lateral line; rb., rib; sp.c., spinal cord.
There is much, therefore, to be said in favour of Götte's view. At the same time, there is another possible interpretation of the facts which would admit the homology of the ribs as well as of the hæmal arches throughout the Pisces.
[Pg 796] Let us suppose, to start with, that the primitive arrangement of the parts is more or less nearly that found in Lepidosteus, where we have well-developed ribs in the region of the trunk, girthing the body-cavity, and uniting in the caudal region to form the ventral parts of the hæmal arches. It is easy to conceive that the ribs in the trunk might somewhat alter their position by passing into the muscles, along the inter-muscular septa, till they come to lie between the dorso-lateral and ventro-lateral muscles, as in Elasmobranchii. Lepidosteus itself affords a proof that such a change in the position of the ribs is not impossible, in that it differs from other Ganoids and from Teleostei in the fact that the free ends of the ribs leave the neighbourhood of the body-cavity and penetrate into the muscles.
If it be granted that the mere difference in position between the ribs of Ganoids and Elasmobranchii is not of itself sufficient to disprove their homology, let us attempt to picture what would take place at the junction of the trunk and tail in a type in which the ribs had undergone the above change in position. On nearing the tail it may be supposed that the ribs would gradually become shorter, and at the same time alter their position, till finally they shaded off into ordinary hæmal processes. If, however, the hæmal canal became prolonged forwards by the formation of some additional complete or nearly complete hæmal arches, an alteration in the relation of the parts would necessarily take place. Owing to the position of the ribs, these structures could hardly assist in the new formation of the anterior part of the hæmal canal, but the continuation forwards of the canal would be effected by prolongations of the hæmal processes supporting the ribs. The new arches so formed would naturally be held to be homologous with the hæmal arches of the tail, though really not so, while the true nature of the ribs would also be liable to be misinterpreted, in that the ribs would appear to be lateral outgrowths of the hæmal processes of a wholly different nature to the ventral parts of the hæmal arches of the tail.
In some Elasmobranchii, as shewn in the accompanying woodcut (fig. 2), in the transitional vertebræ between the trunk and the tail, the ribs are supported by lateral outgrowths of the hæmal processes, while the wholly independent prolongations of [Pg 797] the hæmal processes appear to be about to give rise to the hæmal arches of the tail.
This peculiar state of things led Götte, and subsequently one of us, to deny for Elasmobranchii all homology between the ribs and any part of the hæmal arches of the tail; but in view of the explanation just suggested, this denial was perhaps too hasty.
Fig. 2.
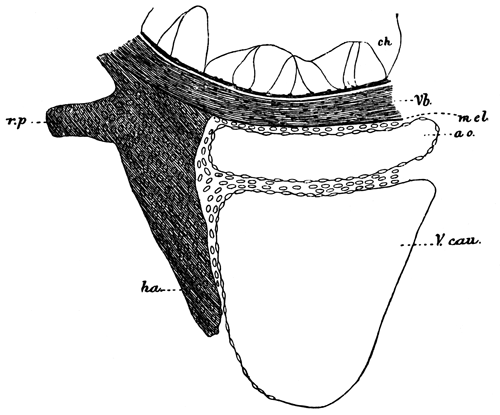
Transverse section through the ventral part of the notochord, and adjoining structures of an advanced Scyllium embryo at the root of the tail.
Vb., cartilaginous sheath of the notochord; ha., hæmal process; r.p., process to which the rib is articulated; m.el., membrana elastica externa; ch., notochord; ao., aorta; V.cau., caudal vein.
We are the more inclined to take this view because the researches of Götte appear to shew that an occurrence, in many respects analogous, has taken place in some Teleostei.
In Teleostei, Johannes Müller, and following him Gegenbaur, do not admit
that the hæmal arches of the tail are in any part formed by the ribs.
Gegenbaur (Elements of Comp.
Anat., translation, p. 431) says, In the Teleostei,
the costiferous transverse processes
(what we have called the hæmal
processes) gradually [Pg 798] converge in the caudal region, and form
inferior arches, which are not homologous with those of Selachii and
Ganoidei, although they also form spinous processes.
The opposite view, that the hæmal arches of the tail in Teleostei contain parts serially homologous with the basal parts of the hæmal processes as well as with the ribs, has been also maintained by many anatomists, e.g., Meckel, Aug. Müller, &c., and has recently found a powerful ally in Götte.
In many cases, the relations of the parts appear to be fundamentally those found in Lepidosteus and Amia, and Götte has shewn by his careful embryological investigations on Esox and Anguilla, that in these two forms there is practically conclusive evidence that the ribs as well as the hæmal costiferous processes of Gegenbaur, which support them, enter into the formation of the hæmal arches of the tail.
In a great number of Teleostei, e.g., the Salmon and most Cyprinoids, &c., the hæmal arches in the region of transition from the trunk to the tail have a structure which at first sight appears to support Johannes Müller's and Gegenbaur's view. The hæmal processes grow larger and meet each other ventrally; while the ribs articulated to them gradually grow smaller and disappear.
The Salmon is typical in this respect, and has been carefully studied by Götte, who attempts to shew (with, in our opinion, complete success) that the anterior hæmal arches are really not entirely homologous with the true hæmal arches behind, but that in the latter, the closure of the arch below is effected by the hæmal spine, which is serially homologous with a pair of coalesced ribs, while in the anterior hæmal arches, i.e., those of the trunk, the closure of the arch is effected by a bridge of bone uniting the hæmal processes.
The arrangement of the parts just described, as well as the view of Götte with reference to them, will be best understood from the accompanying woodcut (fig. 3), copied from Götte's memoir.
Götte sums up his own results on this point in the following words
(p. 138): It follows from this, that the half rings, forming the
hæmal canal in the hindermost trunk vertebræ of the Salmon, are not (with
the exception of the last) completely homologous [Pg 799] with those of the
tail, but are formed by a connecting piece between the basal stumps (hæmal
processes), which originates as a paired median process of these
stumps.
The incomplete homology between the anterior hæmal arches and the true caudal hæmal arches which follow them is exactly what we suggest may be the case in Elasmobranchii, and if it be admitted in the one case, we see no reason why it should not also be admitted in the other.
Fig. 3.
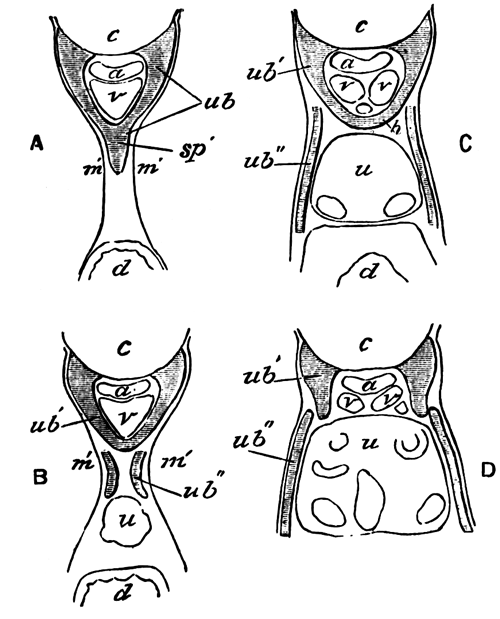
Semi-diagrammatic transverse sections through the first caudal vertebra (A), the last trunk vertebra (B), and the two trunk vertebræ in front (C and D), of a Salmon embryo of 2-3 centims. (From Götte.)
ub., hæmal arch; ub´., hæmal process; ub´´., rib; c., notochord; a., aorta; v., vein; h., connecting pieces between hæmal processes; u., kidney; d., intestine; sp´., hæmal spine; m´., muscles.
If this admission is made, the only ground for not regarding the ribs of Elasmobranchii as homologous with those of Ganoids is their different position, and we have already attempted to prove that this is not a fundamental point.
The results of our researches appear to us, then, to leave two alternatives as to the ribs of Fishes. One of these, which may be called Götte's view, may be thus stated:—The hæmal arches [Pg 800] are homologous throughout the Pisces: in Teleostei, Ganoidei, and Dipnoi[527], the ribs, placed on the inner face of the body-wall, are serially homologous with the ventral parts of the hæmal arches of the tail; in Elasmobranchii, on the other hand, the ribs are neither serially homologous with the hæmal arches of the tail nor homologous with the ribs of Teleostei and Ganoidei, but are outgrowths of the hæmal processes into the space between the dorso-lateral and ventro-lateral muscles, which may perhaps have their homologues in Teleostei and Ganoids in certain accessory processes of the vertebræ.
The other view, which we are inclined to adopt, and the arguments for which have been stated in the preceding pages, is as follows:—The Teleostei, Ganoidei, Dipnoi, and Elasmobranchii are provided with homologous hæmal arches, which are formed by the coalescence below the caudal vein of simple prolongations of the primitive hæmal processes of the embryo. The canal enclosed by the hæmal arches can be demonstrated embryologically to be the aborted body-cavity.
In the region of the trunk the hæmal processes and their prolongations behave somewhat differently in the different types.
In Ganoids and Dipnoi, in which the most primitive arrangement is probably retained, the ribs are attached to the hæmal processes, and are placed immediately without the peritoneal membrane at the insertions of the intermuscular septa. These ribs are in many instances (Lepidosteus, Acipenser), and very probably in all, developed continuously with the hæmal processes, and become subsequently segmented from them. They are serially homologous with the ventral parts of the hæmal arches of the tail, which, like them, are in many instances (Ceratodus, Lepidosteus, Polypterus, and to some extent in Amia) segmented off from the basal parts of the hæmal arches.
In Teleostei the ribs have the same position and relations as those in Ganoids and Dipnoi, but their serial homology with the ventral parts of the hæmal processes of the tail, is often (e.g., the Salmon) obscured by some of the anterior hæmal arches in the posterior part of the trunk being completed, not by the ribs, but [Pg 801] by independent outgrowths of the basal parts of the hæmal processes.
In Elasmobranchii a still further divergence from the primitive arrangement is present. The ribs appear to have passed outwards along the intermuscular septa into the muscles, and are placed between the dorso-lateral and ventro-lateral muscles (a change of position of the ribs of the same nature, but affecting only their ends, is observable in Lepidosteus). This change of position, combined probably with the secondary formation of a certain number of anterior hæmal arches similar to those in the Salmon, renders their serial homology with the ventral parts of the hæmal processes of the tail far less clear than in other types, and further proof is required before such homology can be considered as definitely established.
This is not the place to enter into the obscure question as to how far the ribs of the Amphibia and Amniota are homologous with those of Fishes. It is to be remarked, however, that the ribs of the Urodela (1) occupy the same position in relation to the muscles as the Elasmobranch ribs, (2) that they are connected with the neural arches, and (3) that they coexist in the tail with the hæmal arches, and seem, therefore, to be as different as possible from the ribs of the Dipnoi.
Part IV.—The skeleton of the ventral lobe of the tail fin, and its bearing on the nature of the tail fin of the various types of Pisces.
In the embryos or larvæ of all the Elasmobranchii, Ganoidei, and Teleostei which have up to this time been studied, the unpaired fins arise as median longitudinal folds of the integument on the dorsal and ventral sides of the body, which meet at the apex of the tail. The tail at first is symmetrical, having a form which has been called diphycercal or protocercal. At a later stage, usually, though not always, parts of these fins atrophy, while other parts undergo a special development and constitute the permanent unpaired fins.
Since the majority of existing as well as extinct Fishes are provided with discontinuous fins, those forms, such as the Eel (Anguilla), in which the fins are continuous, have probably reverted [Pg 802] to an embryonic condition: an evolutional process which is of more frequent occurrence than has usually been admitted.
In the caudal region there is almost always developed in the larvæ of the above groups a special ventral lobe of the embryonic fin a short distance from the end of the tail. In Elasmobranchii and Chondrostean Ganoids the portion of the embryonic tail behind this lobe persists through life, and a special type of caudal fin, which is usually called heterocercal, is thus produced. This type of caudal fin appears to have been the most usual in the earlier geological periods.
Simultaneously with the formation of the ventral lobe of the heterocercal caudal fin, the notochord with the vertebral tissues surrounding it, becomes bent somewhat dorsalwards, and thus the primitive caudal fin forms a dorsally directed lobe of the heterocercal tail. We shall call this part the dorsal lobe of the tail-fin, and the secondarily formed lobe the ventral lobe.
Lepidosteus and Amia (Wilder, No. 15) amongst the bony Ganoids, and, as has recently been shewn by A. Agassiz[528], most Teleostei acquire at an early stage of their development heterocercal caudal fins, like those of Elasmobranchii and the Chondrostean Ganoids; but in the course of their further growth the dorsal lobe partly atrophies, and partly disappears as such, owing to the great prominence acquired by the ventral lobe. A portion of the dorsally flexed notochord and of the cartilage or bone replacing or investing it remains, however, as an indication of the original dorsal lobe, though it does not project backwards beyond the level of the end of the ventral lobe, which in these types forms the terminal caudal fin.
The true significance of the dorsally flexed portion of the vertebral axis was first clearly stated by Huxley[529], but as A. Agassiz has fairly pointed out in the paper already quoted, this fact does not in any way militate against the view put forward by L. Agassiz that there is a complete parallelism between the embryonic development of the tail in these Fishes and the palæontological development of this organ. We think [Pg 803] that it is moreover convenient to retain the term homocercal for those types of caudal fin in which the dorsal lobe has atrophied so far as not to project beyond the ventral lobe.
We have stated these now well-known facts to enable the reader to follow us in dealing with the comparison between the skeleton supporting the fin-rays of the ventral lobe of the caudal fin, and that supporting the fin-rays of the remaining unpaired fins.
It has been shewn that in Lepidosteus the unpaired fins fall into two categories, according to the nature of the skeletal parts supporting them. The fin-rays of the true ventral lobe of the caudal fin are supported by the spinous processes of certain of the hæmal arches. The remaining unpaired fins, including the anal fin, are supported by the so-called interspinous bones, which are developed independently of the vertebral column and its arches.
The question which first presents itself is, how far does this distinction hold good for other Fishes? This question, though interesting, does not appear to have been greatly discussed by anatomists. Not unfrequently the skeletal supports of the ventral lobe of the caudal fin are assumed to be the same as those of the other fins.
Davidoff[530], for instance, in speaking of
the unpaired fins of Elasmobranch embryos, says (p. 514): The
cartilaginous rays of the dorsal fins agreed not only in number with the
spinous processes (as indeed is also found in the caudal fin of the
full-grown Dog-fish),
&c.
Thacker[531], again, in his memoir on the
Median and Paired Fins, states at p. 284: We shall here consider the
skeleton of the dorsal and anal fins alone. That of the caudal fin has
undergone peculiar modifications by the union of fin-rays with hæmal
spines.
Mivart[532] goes into the question more
fully. He points out (p. 471) that there is an essential difference
between the dorsal and ventral parts of the caudal fin in Elasmobranchii,
in that in [Pg
804] the former the radials are more numerous than the vertebræ and
unconformable to them, while in the latter they are equal in number to the
vertebræ and continuous with them. This,
he goes on to say,
seems to point to a difference in nature between the dorsal and ventral
portions of the caudal fin, in at least most Elasmobranchii.
He further
points out that Polyodon resembles Elasmobranchii. As to Teleostei,
he does not express himself decidedly except in the case of Muræna,
to which we shall return.
Mivart expresses himself as very doubtful as to the nature of the
supports of the caudal fin, and thinks that the caudal fin of different
kinds of Fishes may have arisen in different ways in different cases.
An examination of the ventral part of the caudal fin in various Ganoids, Teleostei, and Elasmobranchii appears to us to shew that there can be but little doubt that, in the majority of the members of these groups at any rate, and we believe in all, the same distinction between the ventral lobe of the caudal fin and the remaining unpaired fins is found as in Lepidosteus.
In the case of most Elasmobranchii, a simple inspection of the caudal fin suffices to prove this, and the anatomical features involved in this fact have usually been recognized; though, in the absence of embryological evidence, the legitimate conclusion has not always been drawn from them.
The difference between the ventral lobe of the caudal fin and the other fins in the mode in which the fin-rays are supported is as obvious in Chondrostean Ganoids as it is in Elasmobranchii; it would appear also to hold good for Amia. Polypterus we have had no opportunity of examining, but if, as there is no reason to doubt, the figure of its skeleton given by Agassiz (Poissons Fossiles) is correct, there can be no question that the ventral lobe of the caudal fin is supported by the hæmal arches, and not by interspinous bones. In Calamoicthys, the tail of which we have had an opportunity of dissecting through the kindness of Professor Parker, the fin-rays of the ventral lobe of the true caudal fin are undoubtedly supported by true hæmal arches.
There is no unanimity of opinion as to the nature of the elements supporting the fin-rays of the caudal fin of Teleostei.
[Pg
805] Huxley[533] in his paper on the development
of the caudal fin of the Stickleback, holds that these elements are of the
nature of interhæmal bones. He says (p. 39): "The last of these rings
lay just where the notochord began to bend up. It was slightly longer than
the bony ring which preceded it, and instead of having its posterior margin
parallel with the anterior, it sloped from above downwards and backwards.
Two short osseous plates, attached to the anterior part of the inferior
surface of the penultimate ring, or rudimentary vertebral centrum, passed
downwards and a little backwards, and abutted against a slender elongated
mass of cartilage. Similar cartilaginous bodies occupy the same relation to
corresponding plates of bone in the anterior vertebræ in the region of the
anal fin; and it is here seen, that while the bony plates coalesce and form
the inferior arches of the caudal vertebræ, the cartilaginous elements at
their extremities become the interhæmal bones. The cartilage connected with
the inferior arch of the penultimate centrum is therefore an
interhæmal
cartilage. The anterior part of the inferior surface of
the terminal ossification likewise has its osseous inferior arch, but the
direction of this is nearly vertical, and though it is connected below with
an element which corresponds in position with the interhæmal cartilage,
this cartilage is five or six times as large, and constitutes a broad
vertical plate, longer than it is deep, and having its longest axis
inclined downwards and backwards....
Immediately behind and above this anterior hypural
apophysis (as it may be termed) is another very much smaller vertical
cartilaginous plate, which may be called the posterior hypural
apophysis.
We have seen that Mivart expresses himself doubtful on the subject. Gegenbaur[534] appears to regard them as hæmal arches.
The latter view appears to us without doubt the correct one. An examination of the tail of normal Teleostei shews that the fin-rays of that part of the caudal fin which is derived from the ventral lobe of the larva are supported by elements serially homologous with the hæmal arches, but in no way homologous [Pg 806] with the interspinous bones of the anal fin. The elements in question formed of cartilage in the larva, become ossified in the adult, and are known as the hypural bones. They may appear in the form of a series of separate hæmal arches, corresponding in number with the primitive somites of this region, which usually, however, atrophy in the adult, or more often are from the first imperfectly segmented, and have in the adult the form of two or three or even of a single broad bony plate. The transitional forms between this state of things and that, for instance, in Lepidosteus are so numerous, that there can be no doubt that even the most peculiar forms of the hypural bones of Teleostei are simply modified hæmal arches.
This view of the hypural bones is, moreover, supported by embryological evidence, since Aug. Müller[535] (p. 205) describes their development in a manner which, if his statements are to be trusted, leaves no doubt on this point.
There are a considerable number of Fishes which are not provided with an
obvious caudal fin as distinct from the remaining unpaired fins,
i.e. Chimæra, Eels, and various Eel-like forms amongst Teleostei,
and the Dipnoi. Gegenbaur appears to hold that these Fishes ought to be
classed together in relation to the structure of the caudal portion of
their vertebral column, as he says on p. 431 of his Comparative
Anatomy (English Translation): In the Chimæræ, Dipnoi, and many
Teleostei, the caudal portion of the vertebral column ends by gradually
diminishing in size, but in most Fishes, &c.
For our purpose it will, however, be advisable to treat them separately.
The tail of Chimæra appears to us to be simply a peculiar modification of the typical Elasmobranch heterocercal tail, in which the true ventral lobe of the caudal fin may be recognized in the fin-fold immediately in front of the filamentous portion of the tail. In the allied genus Callorhynchus this feature is more distinct. The filamentous portion of the tail of Chimæra constitutes, according to the nomenclature adopted above, the true dorsal lobe, and may be partially paralleled in the filamentous dorsal lobe of the tail of the larval Lepidosteus (Plate 34, fig. 16).
[Pg 807] The tail of the eel-like Teleostei is again undoubtedly a modification of the normal form of tail characteristic of the Teleostei, in which, however, the caudal fin has become very much reduced and merged into the prolongations of the anal and dorsal fins.
This can be very clearly seen in Siluroid forms with an Eel-like tail, such as Cnidoglanis. Although the dorsal and ventral fins appear to be continuous round the end of the tail, and there is superficially no distinct caudal fin, yet an examination of the skeleton of Cnidoglanis shews that the end of the vertebral column is modified in the usual Teleostean fashion, and that the hæmal arches of the modified portion of the vertebral column support a small number of fin-rays; the adjoining ventral fin-rays being supported by independent osseous fin-supports (interspinous bones).
In the case of the Eel (Anguilla anguilla) Huxley (loc. cit.) long ago pointed out that the terminal portion of the vertebral column was modified in an analogous fashion to that of other Teleostei, and we have found that the modified hæmal arches of this part support a few fin-rays, though a still smaller number than in Cnidoglanis. The fin-rays so supported clearly constitute an aborted ventral lobe of the caudal fin.
Under these circumstances we think that the following statement by Mivart (Zool. Trans. Vol. X., p. 471) is somewhat misleading:—
As to the condition of this part (i.e. the
ventral lobe of the tail-fin) in Teleosteans generally, I will not venture
as yet to say anything generally, except that it is plain that in such
forms as Muræna, the dorsal and ventral parts of the caudal fin are similar
in nature and homotypal with ordinary dorsal and anal fins[536].
The italicized portion of this sentence is only true in respect to that part of the fringe of fin surrounding the end of the body, which is not only homotypal with, but actually part of, the dorsal and anal fins.
Having settled, then, that the tails of Chimæra and of Eel-like Teleostei are simply special modifications of the typical form of tail of the group of Fishes to which they respectively [Pg 808] belong, we come to the consideration of the Dipnoi, in which the tail-fin presents problems of more interest and greater difficulty than those we have so far had to deal with.
The undoubtedly very ancient and primitive character of the Dipnoi has led to the view, implicitly if not definitely stated in most text-books, that their tail-fin retains the character of the piscine tail prior to the formation of the ventral caudal lobe, a stage which is repeated embryologically in the pre-heterocercal condition of the tail in ordinary Fishes.
Through the want of embryological data, and in the absence of really careful histological examination of the tail of any of the Dipnoi, we are not willing to speak with very great confidence as to its nature; we are nevertheless of the opinion that the facts we can bring forward on this head are sufficient to shew that the tail of the existing Dipnoi is largely aborted, so that it is more or less comparable with that of the Eel.
We have had opportunities of examining the structure of the tail of Ceratodus and Protopterus in dissected specimens in the Cambridge Museum. The vertebral axis runs to the ends of the tail without shewing any signs of becoming dorsally flexed. At some distance from the end of the tail the fin-rays are supported by what are apparently segmented spinous prolongations of the neural and hæmal arches. The dorsal elements are placed above the longitudinal dorsal cord, and occupy therefore the same position as the independent elements of the neural arches of Lepidosteus. They are therefore to be regarded as homologous with the dorsal fin-supports or interspinous bones of other types. The corresponding ventral elements are therefore also to be regarded as interspinous bones.
In view of the fact that the fin-supports, whenever their development has been observed, are found to be formed independently of the neural and hæmal arches, we may fairly assume that this is also true for what we have identified as the interspinous elements in the Dipnoi.
The interspinous elements become gradually shorter as the end of the tail is approached, and it is very difficult from a simple examination of dissected specimens to make out how far any of the posterior fin-rays are supported by the hæmal arches only. To this question we shall return, but we may remark [Pg 809] that, although there is a prolongation backwards of the vertebral axis beyond the last interspinous elements, composed it would seem of the coalesced neural and hæmal arches but without the notochord, yet by far the majority of the fin-rays which constitute the apparent caudal fin are supported by interspinous elements.
The grounds on which we hold that the tail of the Dipnoi is to be regarded as a degenerate rather than primitive type of tail are the following:—
(1) If it be granted that a diphycercal or protocercal form of tail must have preceded a heterocercal form, it is also clear that the ventral fin-rays of such a tail must have been supported, as in Polypterus and Calamoicthys, by hæmal arches, and not by interspinous elements; otherwise, a special ventral lobe, giving a heterocercal character to the tail, and provided with fin-rays supported only by hæmal arches, could never have become evolved from the protocercal tail-fin. Since the ventral fin-rays of the tail of the Dipnoi are supported by interspinous elements and not by hæmal arches, this tail-fin cannot claim to have the character of that primitive type of diphycercal or protocercal tail from which the heterocercal tail must be supposed to have been evolved.
(2) Since the nearest allies of the Dipnoi are to be found in Polypterus and the Crossopterygidæ of Huxley, and since in these forms (as evinced by the structure of the tail-fin of Polypterus, and the transitional type between a heterocercal and diphycercal form of fin observable in fossil Crossopterygidæ) the ventral fin-rays of the caudal fin were clearly supported by hæmal arches and not by interspinous elements, it is rendered highly probable that the absence of fin-rays so supported in the Dipnoi is a result of degeneration of the posterior part of the tail.
[We use this argument without offering any opinion as to whether the diphycercal character of the tail of many Crossopterygidæ is primary or secondary.]
(3) The argument just used is supported by the degenerate and variable state of the end of the vertebral axis in the Dipnoi—a condition most easily explained by assuming that the terminal part of the tail has become aborted.
[Pg 810] (4) We believe that in Ceratodus we have been able to trace a small number of the ventral fin-rays supported by hæmal arches only, but these rays are so short as not to extend so far back as some of the rays attached to the interspinous elements in front. These rays may probably be interpreted, like the more or less corresponding rays in the tail of the Eel, as the last remnant of a true caudal fin.
The above considerations appear to us to shew with very considerable probability that the true caudal fin of the Dipnoi has become all but aborted like that of various Teleostei; and that the apparent caudal fin is formed by the anal and dorsal fins meeting round the end of the stump of the tail.
From the adult forms of Dipnoi we are, however, of opinion that no conclusion can be drawn as to whether their ancestors were provided with a diphycercal or a heterocercal form of caudal fin.
The general conclusions with reference to the tail-fin at which we have arrived are the following:—
(1) The ventral lobe of the tail-fin of Pisces differs from the other unpaired fins in the fact that its fin-rays are directly supported by spinous processes of certain of the hæmal arches instead of independently developed interspinous bones.
(2) The presence or absence of fin-rays in the tail-fin supported by hæmal arches may be used in deciding whether apparently diphycercal tail-fins are aborted or primitive.
[521] These specimens were given to us by Professor W. K. Parker, who received them from Professor Burt G. Wilder.
[522]
Gegenbaur (No. 6) takes a different view on
this subject, as is clear from the following passage in this memoir (pp. 369-370):—Each vertebra of
Lepidosteus thus consists of a section of the notochord, and of the
cartilaginous tissue surrounding its sheath, which gives origin to the
upper arches for the whole length of the vertebral column, and in the
caudal region to that of the lower arches also. The latter do not
however complete the enclosure of a lower canal, but this is effected by
special independent elements, which are to be interpreted as
homologues of the ribs.
(The italics are ours.) While we fully accept
the homology between the ribs and the lower elements of the hæmal arches
of the tail, the view expressed in the italicised section, to the effect
that the lower parts of the caudal arches are not true hæmal arches but
are independently formed elements, is entirely opposed to our
observations, and has we believe only arisen from the fact that Gegenbaur
had not the young larvæ to work with by which alone this question could be
settled.
[523]
Beiträge zur vergl. Morphol. d. Skeletsystems d.
Wirbelthiere.
Archiv f. Mikr. Anat. Vol. XVI. 1879.
[524]
Beiträge z. vergl. Morph. d. Skeletsystems d. Wirbelthiere.
II. Die Wirbelsäule u. ihre Anhänge.
Archiv f. Mikr.
Anat., Vol. XV., 1878, and Vol. XVI., 1879.
[525]
Ueb. d. Entwick. d. Wirbelsäule d. Lepidosteus, mit.
vergl. Anat. Bemerkungen.
Jenaische Zeitschrift,
Bd. III., 1863.
[526] Comparative Embryology, Vol. II., pp. 462, 463 [the original edition].
[527] We find the serial homology of the ribs and ventral parts of the hæmal arches to be very clear in Ceratodus. Wiedersheim states that it is not clear in Protopterus, although he holds that the facts are in favour of this view.
[528]
On the Young Stages of some Osseous Fishes.—I. The Development of
the Tail,
Proc. of the
American Academy of Arts and Sciences, Vol. XIII., 1877.
[529]
Observations on the Development of some Parts of the Skeleton of
Fishes,
Quart.
Journ. of Micr. Science, Vol.
VII.,
1859.
[530]
Beiträge z. vergl. Anat. d. hinteren Gliedmassen d.
Fische,
Morph. Jahrbuch, Vol. V., 1879.
[531] Trans. of the Connecticut Acad., Vol. III., 1877.
[532]
St George Mivart, Fins of Elasmobranchii,
Zool. Trans., Vol. X.
[533]
Observations on the Development of some parts of the Skeleton of
Fishes,
Quart.
Journ. Micr. Science, Vol.
VII.,
1859.
[534] Elements of Comparative Anatomy. (Translation), p. 431.
[535]
Beobachtungen zur vergl. Anat. d. Wirbelsäule,
Müller's
Archiv, 1853.
[536] The italics are ours.
I.—Anatomy.
The excretory organs of Lepidosteus have been described by Müller (No. 13) and Hyrtl (No. 11). These anatomists have given a fairly adequate account of the generative ducts in the female, and Hyrtl has also described the male generative ducts and the kidney and its duct, but his description is contradicted by our observations in some of the most fundamental points.
In the female example of 100.5 centims. which we dissected, the kidney forms a paired gland, consisting of a narrow strip of glandular matter placed on each side of the vertebral column, on [Pg 811] the dorsal aspect of the body-cavity. It is covered on its ventral aspect by the oviduct and by its own duct, but is separated from both of these by a layer of the tough peritoneal membrane, through which the collecting tubes pass. It extends forwards from the anus for about three-fifths of the length of the body-cavity, and in our example had a total length of about 28 centims. (Plate 39, fig. 60, k). Anteriorly the two kidneys are separated by a short interval in the median line, but posteriorly they come into contact, and are so intimately united as almost to constitute a single gland.
A superficial examination might lead to the supposition that the kidney extended forwards for the whole length of the body-cavity up to the region of the branchial arches, and Hyrtl appears to have fallen into this error; but what appears to be its anterior continuation is really a form of lymphatic tissue, something like that of the spleen, filled with numerous cells. This matter (Plate 39, fig. 60, ly.) continues from the kidney forwards without any break, and has a colour so similar to that of the kidney as to be hardly distinguishable from it with the naked eye. The true anterior end of the kidney is placed about 3 centims. in front on the left side, and on the same level on the right side as the wide anterior end of the generative duct (Plate 39, fig. 60, od.). It is not obviously divided into segments, and is richly supplied with malpighian bodies.
It is clear from the above description that there is no trace of head-kidney or pronephros visible in the adult. To this subject we shall, however, again return.
As will appear from the embryological section, the ducts of the kidneys are probably simply the archinephric ducts, but to avoid the use of terms involving a theory, we propose in the anatomical part of our work to call them kidney ducts. They are thin-walled widish tubes coextensive with the kidneys. If cut open there may be seen on their inner aspect the numerous openings of the collecting tubes of the kidneys. They are placed ventrally to and on the outer border of the kidneys (Plate 39, fig. 60, s.g.). Posteriorly they gradually enlarge, and approaching each other in the median line, coalesce, forming an unpaired vesicle or bladder (bl.)—about 6 centims. long in our example—opening by a median pore on a more or less [Pg 812] prominent papilla (u.g.) behind the anus. The dilated portions of the two ducts are called by Hyrtl the horns of the bladder.
The sides of the bladder and its so-called horns are provided with lateral pockets into which the collecting tubes of the kidney open. These pockets, which we have found in two female examples, are much larger in the horns of the bladder than in the bladder itself. Similar pockets, but larger than those we have found, have been described by Hyrtl in the male, but are stated by him to be absent in the female. It is clear from our examples that this is by no means always the case.
Hyrtl states that the wide kidney ducts, of which his description differs in no material point from our own, suddenly narrow in front, and, perforating the peritoneal lining, are continued forwards to supply the anterior part of the kidney. We have already shewn that the anterior part of the kidney has no existence, and the kidney ducts supplying it are, according to our investigations, equally imaginary.
It was first shewn by Müller, whose observations on this point have been confirmed by Hyrtl, &c., that the ovaries of Lepidosteus are continuous with their ducts, forming in this respect an exception to other Ganoids.
In our example of Lepidosteus the ovaries (Plate 39, fig. 60, ov.) were about 18 centims. in length. They have the form of simple sacks, filled with ova, and attached about their middle to their generative duct, and continued both backwards and forwards from their attachment into a blind process.
With reference to these sacks Müller has pointed out—and the importance of this observation will become apparent when we deal with the development—that the ova are formed in the thickness of the inner wall of the sack. We hope to shew that the inner wall of the sack is alone equivalent to the genital ridge of, for instance, the ovary of Scyllium. The outer aspect of this wall—i.e., that turned towards the interior of the sack—is equivalent to the outer aspect of the Elasmobranch genital ridge, on which alone the ova are developed[537]. The sack into which the ova fall is, as we shall shew in the embryological section, a special section of the body-cavity shut off from the remainder, [Pg 813] and the dehiscence of the ova into this cavity is equivalent to their discharge into the body-cavity in other forms.
The oviduct (Plate 39, fig. 60, od.) is a thin-walled duct of about 21 centims. in length in the example we are describing, continuous in front with the ovarian sack, and gradually tapering behind, till it ends (od´.) by opening into the dilated terminal section of the kidney duct on the inner side, a short distance before the latter unites with its fellow. It is throughout closely attached to the ureter and placed on its inner, and to some extent on its ventral, aspect. The hindermost part of the oviduct which runs beside the enlarged portion of the kidney duct—that portion called by Hyrtl the horn of the urinary bladder—is so completely enveloped by the wall of the horn of the urinary bladder as to appear like a projection into the lumen of the latter structure, and the somewhat peculiar appearance which it presents in Hyrtl's figure is due to this fact. In our examples the oviduct was provided with a simple opening into the kidney duct, on a slight papilla; the peculiar dilatations and processes of the terminal parts of the oviduct, which have been described by Hyrtl, not being present.
The results we have arrived at with reference to the male organs are very different indeed from those of our predecessor, in that we find the testicular products to be carried off by a series of vasa efferentia, which traverse the mesorchium, and are continuous with the uriniferous tubuli; so that the semen passes through the uriniferous tubuli into the kidney duct and so to the exterior. We have moreover been unable to find in the male a duct homologous with the oviduct of the female.
This mode of transportation outwards of the semen has not hitherto been known to occur in Ganoids, though found in all Elasmobranchii, Amphibia, and Amniota. It is not, however, impossible that it exists in other Ganoids, but has hitherto been overlooked.
Our male example of Lepidosteus was about 60 centims. in length, and was no doubt mature. It was smaller than any of our female examples, but this according to Garman (vide, p. 361) is usual. The testes (Plate 39, fig. 58A., t.) occupied a similar position to the ovaries, and were about 21 centims. long. They were, as is frequently the case with piscine testes, [Pg 814] divided into a series of lobes (10-12), and were suspended by a delicate mesentery (mesorchium) from the dorsal wall of the abdomen on each side of the dorsal aorta. Hyrtl (No. 11) states that air or quicksilver injected between the limbs of the mesentery, passed into a vas deferens homologous with the oviduct which joins the ureter. We have been unable to find such a vas deferens; but we have found in the mesorchium a number of tubes of a yellow colour, the colour being due to a granular substance quite unlike coagulated blood, but which appeared to us from microscopic examination to be the remains of spermatozoa[538]. These tubes to the number of 40-50 constitute, we believe, the vasa efferentia. Along the line of suspension of the testis on its inner border these tubes unite to form an elaborate network of tubes placed on the inner face of the testis—an arrangement very similar to that often found in Elasmobranchii (vide F. M. Balfour, Monograph on the Development of Elasmobranch Fishes, plate 20, figs. 4 and 8).
We have figured this network on the posterior lobe of the testis (fig. 58B), and have represented a section through it (fig. 59A, n.v.e.), and through one of the vasa efferentia (v.e.) in the mesorchium. Such a section conclusively demonstrates the real nature of these passages: they are filled with sperm like that in the body of the testis, and are, as may be seen from the section figured, continuous with the seminal tubes of the testis itself.
At the attached base of the mesorchium the vasa efferentia unite into a
longitudinal canal, placed on the inner side of the kidney duct (Plate 39,
fig. 58A, l.c., also shewn in section in
Plate 39, fig. 59B, l.c.). From this
canal tubules pass off which are continuous with the tubuli uriniferi, as
may be seen from fig. 59B, but the exact course of these tubuli through the
kidney could not be made out in the preparations we were able to make of
the badly conserved kidney. Hyrtl describes the arrangement of the vascular
trunks in the mesorchium in the following way (No. 11, p. 6): The mesorchium contains vascular
trunks, viz., veins, which through their
numerous anastomoses [Pg 815] form a plexus at the hilus of the testis,
whose efferent trunks, 13 in number, again unite into a plexus on the
vertebral column, which is continuous with the cardinal veins.
The
arrangement (though not the number) of Hyrtl's vessels is very similar to
that of our vasa efferentia, and we cannot help thinking that a confusion
of the two may have taken place; which, in badly conserved specimens, not
injected with semen, would be very easy.
We have, as already stated, been unable to find in our dissections any trace of a duct homologous with the oviduct of the female, and our sections through the kidney and its ducts equally fail to bring to light such a duct. The kidney ducts are about 19 centims. in length, measured from the genital aperture to their front end. These ducts are generally similar to those in the female; they unite about 2 centims. from the genital pore to form an unpaired vesicle. Their posterior parts are considerably enlarged, forming what Hyrtl calls the horns of the urinary bladder. In these enlarged portions, and in the wall of the unpaired urinary bladder, numerous transverse partitions are present, as correctly described by Hyrtl, which are similar to those in the female, but more numerous. They give rise to a series of pits, at the blind ends of which are placed the openings of the kidney tubules. The kidney duct without doubt serves as vas deferens, and we have found in it masses of yellowish colour similar to the substance in the vasa efferentia identified by us as remains of spermatozoa.
II.—Development.
In the general account of the development we have already called attention to the earliest stages of the excretory system.
We may remind the reader that the first part of the system to be formed is the segmental or archinephric duct (Plate 36, figs. 28 and 29, sg.). This duct arises, as in Teleostei and Amphibia, by the constriction of a hollow ridge of the somatic mesoblast into a canal, which is placed in contiguity with the epiblast, along the line of junction between the mesoblastic somites and the lateral plates of mesoblast. Anteriorly the duct [Pg 816] does not become shut off from the body-cavity, and also bends inwards towards the middle line. The inflected part of the duct is the first rudiment of the pronephros, and very soon becomes considerably dilated relatively to the posterior part of the duct.
The posterior part of each segmental duct acquires an opening into the cloacal section of the alimentary tract. Apart from this change, the whole of the ducts, except their pronephric sections, remain for a long time unaltered, and the next changes we have to speak of concern the definite establishment of the pronephros.
The dilated incurved portion of each segmental duct soon becomes convoluted, and by the time the embryo is about 10 millims. in length, but before the period of hatching, an important change is effected in the relations of their peritoneal openings[539].
Instead of leading into the body-cavity, they open into an isolated chamber on each side (Plate 38, fig. 51, pr.c.), which we will call the pronephric chamber. The pronephric chamber is not, however, so far as we can judge, completely isolated from the body-cavity. We have not, it is true, detected with certainty at this stage a communication between the two; but in later stages, in larvæ of from 11 to 26 millims., we have found a richly ciliated passage leading from the body-cavity into the pronephros on each side (Plate 38, fig. 52, p.f.p.). We have not succeeded in determining with absolute certainty the exact relations between this passage and the tube of the pronephros, but we are inclined to believe that it opens directly into the pronephric chamber just spoken of.
As we hope to shew, this chamber soon becomes largely filled by a vascular glomerulus. On the accomplishment of these changes, the pronephros is essentially provided with all the parts typically present in a segment of the mesonephros (woodcut, fig. 4). There is a peritoneal tube (f)[540], opening into a vesicle (v); from near the neck of the peritoneal tube there [Pg 817] comes off a convoluted tube (pr.n.), forming the main mass of the pronephros, and ending in the segmental duct (sd.).
Fig. 4.
Diagrammatic views of the pronephros of Lepidosteus.
A, pronephros supposed to be isolated and seen from the side; B, section through the vesicle of the pronephros and the ciliated peritoneal funnel leading into it; pr.n., coiled tube of pronephros; sd., segmental or archinephric duct; f., peritoneal funnel; v., vesicle of pronephros; bv., blood vessel of glomerulus; gl., glomerulus.
The different parts do not, however, appear to have the same morphological significance as those in the mesonephros.
Judging from the analogy of Teleostei, the embryonic structure of whose pronephros is strikingly similar to that of Lepidosteus, the two pronephric chambers into which the segmental ducts open are constricted off sections of the body-cavity.
[Pg 818] With the formation of the convoluted duct opening into the isolated section of the body-cavity we may speak of a definite pronephros as having become established. The pronephros is placed, as can be made out in later stages, on the level of the opening of the air-bladder into the throat.
The pronephros increases in size, so far as could be determined, by the further convolution of the duct of which it is mainly formed; and the next change of importance which we have noticed is the formation of a vascular projection into the pronephric chamber, forming the glomerulus already spoken of (vide woodcut, fig. 4, gl.), which is similar to that of the pronephros of Teleostei. We first detected these glomeruli in an embryo of about 15 millims., some days after hatching (Plate 38, fig. 52, gl.), but it is quite possible that they may be formed considerably earlier.
In the same embryo in which the glomeruli were found we also detected for the first time a mesonephros consisting of a series of isolated segmental or nephridial tubes, placed posteriorly to the pronephros along the dorsal wall of the abdomen.
These were so far advanced at this stage that we are not in a position to give any account of their mode of origin. They are, however, formed independently of the segmental ducts, and in the establishment of the junction between the two structures, there is no outgrowth from the segmental duct to meet the segmental tubes. We could not at this stage find peritoneal funnels of the segmental tubes, though we have met with them at a later stage (Plate 38, fig. 53, p.f.), and our failure to find them at this stage is not to be regarded as conclusive against their existence.
A very considerable space exists between the pronephros and the foremost segmental tube of the mesonephros. The anterior mesonephric tubes are, moreover, formed earlier than the posterior.
In the course of further development, the mesonephric tubules increase in size, so that there ceases to be an interval between them, the mesonephros thus becoming a continuous gland. In an embryo of 26 millims. there was no indication of the formation of segmental tubes to fill up the space between the pronephros and mesonephros.
[Pg 819] The two segmental ducts have united behind into an unpaired structure in an embryo of 11 millims. This structure is no doubt the future unpaired urinogenital chamber (Plate 39, figs. 58A, and 60, bl.). Somewhat later, the hypoblastic cloaca becomes split into two sections, the hinder one receiving the coalesced segmental ducts, and the anterior remaining continuous with the alimentary tract. The opening of the hinder one forms the urinogenital opening, and that of the anterior the anus.
In an older larva of about 5.5 centims. the pronephros did not exhibit any marked signs of atrophy, though the duct between it and the mesonephros was somewhat reduced and surrounded by the trabecular tissue spoken of in connection with the adult. In the region between the pronephros and the front end of the fully developed part of the mesonephros very rudimentary tubules had become established.
The latest stage of the excretory system which we have studied is in a young Fish of about 11 centims. in length. The special interest of this stage depends upon the fact that the ovary is already developed, and not only so, but the formation of the oviducts has commenced, and their condition at this stage throws considerable light on the obscure problem of their nature in the Ganoids.
Unfortunately, the head of the young Fish had been removed before it was put into our hands, so that it was impossible for us to determine whether the pronephros was still present; but as we shall subsequently shew, the section of the segmental duct, originally present between the pronephros and the front end of the permanent kidney or mesonephros, has in any case disappeared.
In addition to an examination of the excretory organs in situ, which shewed little except the presence of the generative ridges, we made a complete series of sections through the excretory organs for their whole length (Plate 39, figs. 54-57).
Posteriorly these sections shewed nothing worthy of note, the excretory organs and their ducts differing in no important particular from these organs as we have described them in the adult, except in the fact that the segmental ducts are not joined by the oviducts.
Some little way in front of the point where the two segmental [Pg 820] ducts coalesce to form the urinary bladder, the genital ridge comes into view. For its whole extent, except near its anterior part (of which more hereafter) this ridge projects freely into the body-cavity, and in this respect the young Fish differs entirely from the adult. As shewn in Plate 39, figs. 56 and 57 (g.r.), it is attached to the abdominal wall on the ventral side of, and near the inner border of each kidney. The genital ridge itself has a structure very similar to that which is characteristic of young Elasmobranchii, and it may be presumed of young Fishes generally. The free edge of the ridge is swollen, and this part constitutes the true generative region of the ridge, while its dorsal portion forms the supporting mesentery. The ridge itself is formed of a central stroma and a germinal epithelium covering it. The epithelium is thin on the whole of the inner aspect of the ridge, but, just as in Elasmobranchii, it becomes greatly thickened for a band-like strip on the outer aspect. Here, the epithelium is several layers deep, and contains numerous primitive germinal cells (p.o.).
Though the generative organs were not sufficiently advanced for us to decide the point with certainty, the structure of the organ is in favour of the view that this specimen was a female, and, as will be shewn directly, there can on other grounds be no doubt that this is so. The large size of the primitive germinal cells (primitive ova) reminded us of these bodies in Elasmobranchii.
In the region between the insertion of the genital ridge (or ovary, as we may more conveniently call it) and the segmental duct we detected the openings of a series of peritoneal funnels of the excretory tubes (Plate 39, fig. 57, p.f.), which clearly therefore persist till the young Fish has reached a very considerable size.
As we have already said, the ovary projects freely into the body-cavity for the greater part of its length. Anteriorly, however, we found that a lamina extended from the free ventral edge of the ovary to the dorsal wall of the body-cavity, to which it was attached on the level of the outer side of the segmental duct. A somewhat triangular channel was thus constituted, the inner wall of which was formed by the ovary, the outer by the lamina just spoken of, and the roof by the strip of the peritoneum [Pg 821] of the abdominal wall covering that part of the ventral surface of the kidney in which the openings of the peritoneal funnels of the excretory tubes are placed. The structure of this canal will be at once understood by the section of it shewn in Plate 39, fig. 55.
There can be no doubt that this canal is the commencing ovarian sack. On tracing it backwards we found that the lamina forming its outer wall arises as a fold growing upwards from the free edge of the genital ridge meeting a downward growth of the peritoneal membrane from the dorsal wall of the abdomen; and in Plate 39, fig. 56, these two laminæ may be seen before they have met. Anteriorly the canal becomes gradually smaller and smaller in correlation with the reduced size of the ovarian ridge, and ends blindly nearly on a level with the front end of the excretory organs.
It should be noted that, owing to the mode of formation of the ovarian sack, the outer side of the ovary with the band of thickened germinal epithelium is turned towards the lumen of the sack; and thus the fact of the ova being formed on the inner wall of the genital sack in the adult is explained, and the comparison which we instituted in our description of the adult between the inner wall of the genital sack and the free genital ridge of Elasmobranchii receives its justification.
It is further to be noticed that, from the mode of formation of the ovarian sack, the openings of the peritoneal funnels of the excretory organs ought to open into its lumen; and if these openings persist in the adult, they will no doubt be found in this situation.
Before entering on further theoretical considerations with reference to the oviduct, it will be convenient to complete our description of the excretory organs at this stage.
When we dissected the excretory organs out, and removed them from the body of the young Fish, we were under the impression that they extended for the whole length of the body-cavity. Great was our astonishment to find that slightly in front of the end of the ovary both excretory organs and segmental ducts grew rapidly smaller and finally vanished, and that what we had taken to be the front part of the kidney was nothing else but a linear streak of tissue formed of cells with [Pg 822] peculiar granular contents supported in a trabecular work (Plate 39, fig. 54). This discovery first led us to investigate histologically what we, in common with previous observers, had supposed to be the anterior end of the kidneys in the adult, and to shew that they were nothing else but trabecular tissue with cells like that of lymphatic glands. The interruption of the segmental duct at the commencement of this tissue demonstrates that if any rudiment of the pronephros still persists, it is quite functionless, in that it is not provided with a duct.
III.—Theoretical considerations.
There are three points in our observations on the urinogenital system which appear to call for special remark. The first of these concerns the structure and fate of the pronephros, the second the nature of the oviduct, and the third the presence of vasa efferentia in the male.
Although the history we have been able to give of the pronephros is not complete, we have nevertheless shewn that in most points it is essentially similar to the pronephros of Teleostei. In an early stage we find the pronephros provided with a peritoneal funnel opening into the body-cavity. At a later stage we find that there is connected with the pronephros on each side, a cavity—the pronephric cavity—into which a glomerulus projects. This cavity is in communication on the one hand with the lumen of the coiled tube which forms the main mass of the pronephros, and on the other hand with the body-cavity by means of a richly ciliated canal (woodcut, fig. 4, p. 817).
In Teleostei the pronephros has precisely the same characters, except that the cavity in which the glomerulus is placed is without a peritoneal canal.
The questions which naturally arise in connection with the pronephros are: (1) what is the origin of the above cavity with its glomerulus; and (2) what is the meaning of the ciliated canal connecting this cavity with the peritoneal cavity?
We have not from our researches been able to answer the first of these questions. In Teleostei, however, the origin of this [Pg 823] cavity has been studied by Rosenberg[541] and Götte[542]. According to the account of the latter, which we have not ourselves confirmed but which has usually been accepted, the front end of the segmental duct, instead of becoming folded off from the body-cavity, becomes included in a kind of diverticulum of the body-cavity, which only communicates with the remainder of the body-cavity by a narrow opening. On the inner wall of this diverticulum a projection is formed which becomes a glomerulus. At this stage in the development of the pronephros we have essentially the same parts as in the fully formed pronephros of Lepidosteus, the only difference being that the passage connecting the diverticulum containing the glomerulus with the remainder of the body-cavity is short in Teleostei, and in Lepidosteus forms a longish ciliated canal. In Teleostei the opening into the body-cavity becomes soon closed. If the above comparison is justified, and if the development of these parts in Lepidosteus takes place as it is described as doing in Teleostei, there can, we think, be no doubt that the ciliated canal of Lepidosteus, which connects the pronephric cavity with the body-cavity, is a persisting communication between this cavity and the body-cavity; and that Lepidosteus presents in this respect a more primitive type of pronephros than Teleostei.
It may be noted that in Lepidosteus the whole pronephros has exactly the character of a single segmental tube of the mesonephros. The pronephric cavity with its glomerulus is identical in structure with a malpighian body. The ciliated canal is similar in its relations to the peritoneal canal of such a segmental tube, and the coiled portion of the pronephros resembles the secreting part of the ordinary segmental tube. This comparison is no doubt an indication that the pronephros is physiologically very similar to the mesonephros, and so far justifies Sedgwick's[543] comparison between the two, but it does not appear to us to justify the morphological conclusions at [Pg 824] which he has arrived, or to necessitate any modification in the views on this subject expressed by one of us[544].
The genital ducts of Ganoids and Teleostei have for some time been a source of great difficulty to morphologists; and any contributions with reference to the ontogeny of these structures are of interest.
The essential point which we have made out is that the anterior part of the oviduct of Lepidosteus arises by a fold of the peritoneum attaching itself to the free edge of the genital ridge. We have not, unfortunately, had specimens old enough to decide how the posterior part of the oviduct is formed; and although in the absence of such stages it would be rash in the extreme to speak with confidence as to the nature of this part of the duct, it may be well to consider the possibilities of the case in relation to other Ganoids and Teleostei.
The simplest supposition would be that the posterior part of the genital duct had the same origin as the anterior, i.e., that it was formed for its whole length by the concrescence of a peritoneal fold with the genital ridge, and that the duct so formed opened into the segmental duct.
The other possible supposition is that a true Müllerian duct—i.e., a product of the splitting of the segmental duct—is subsequently developed, and that the open end of this duct coalesces with the duct which has already begun to be formed in our oldest larva.
In attempting to estimate the relative probability of these two views, one important element is the relation of the oviducts of Lepidosteus to those of other Ganoids.
In all other Ganoids (vide Hyrtl, No. II) there are stated to be genital ducts in both sexes which are provided at their anterior extremities with a funnel-shaped mouth open to the abdominal cavity. At first sight, therefore, it might be supposed that they had no morphological relationship with the oviducts of Lepidosteus, but, apart from the presence of a funnel-shaped mouth, the oviducts of Lepidosteus are very similar to those of Chondrostean Ganoids, being thin-walled tubes opening on a projecting papilla into the dilated kidney ducts (horns of the [Pg 825] urinary bladder, Hyrtl). These relations seem to prove beyond a doubt that the oviduct of Lepidosteus is for its major part homologous with the genital ducts of other Ganoids.
The relationship of the genital ducts to the kidney ducts in Amia and Polypterus is somewhat different from that in the Chondrostei and Lepidosteus. In Amia the ureters are so small that they may be described rather as joining the coalesced genital ducts than vice versâ, although the apparent coalesced portion of the genital ducts is shewn to be really part of the kidney ducts by receiving the secretion of a number of mesonephric tubuli. In Polypterus the two ureters are stated to unite, and open by a common orifice into a sinus formed by the junction of the two genital ducts, which has not been described as receiving directly the secretion of any part of the mesonephros.
It has been usual to assume that the genital ducts of Ganoids are true Müllerian ducts in the sense above defined, on the ground that they are provided with a peritoneal opening and that they are united behind with the kidney ducts. In the absence of ontological evidence this identification is necessarily provisional. On the assumption that it is correct we should have to accept the second of the two alternatives above suggested as to the development of the posterior parts of the oviduct in Lepidosteus.
There appear to us, however, to be sufficiently serious objections to this view to render it necessary for us to suspend our judgment with reference to this point. In the first place, if the view that the genital ducts are Müllerian ducts is correct, the true genital ducts of Lepidosteus must necessarily be developed at a later period than the secondary attachment between their open mouths and the genital folds, which would, to say the least of it, be a remarkable inversion of the natural order of development. Secondly, the condition of our oldest larva shews that the Müllerian duct, if developed later, is only split off from quite the posterior part of the segmental duct; yet in all types in which the development of the Müllerian duct has been followed, its anterior extremity, with the abdominal opening, is split off from either the foremost or nearly the foremost part of the segmental duct.
[Pg 826]Judging from the structure of the adult genital ducts of other Ganoids they must also be developed only from the posterior part of the segmental duct, and this peculiarity so struck one of us that in a previous paper[545] the suggestion was put forward that the true Ganoid genital ducts were perhaps not Müllerian ducts, but enlarged segmental tubes with persisting abdominal funnels belonging to the mesonephros.
If the possibility of the oviduct of Lepidosteus not being a Müllerian duct is admitted, a similar doubt must also exist as to the genital ducts of other Ganoids, and we must be prepared to shew that there is a reasonable ground for scepticism on this point. We would in this connexion point out that the second of the two arguments urged against the view that the genital duct of Lepidosteus is not a Müllerian duct applies with equal force to the case of all other Ganoids.
The short funnel-shaped genital duct of the Chondrostei is also very unlike undoubted Müllerian ducts, and could moreover easily be conceived as originating by a fold of the peritoneum, a slight extension of which would give rise to a genital duct like that of Lepidosteus.
The main difficulty of the view that the genital ducts of Ganoids are not Müllerian ducts lies in the fact that they open into the segmental duct. While it is easy to understand the genesis of a duct from a folding of the peritoneum, and also easy to understand how such a duct might lead to the exterior by coalescing, for instance, with an abdominal pore, it is not easy to see how such a duct could acquire a communication with the segmental duct.
We do not under these circumstances wish to speak dogmatically, either in favour of or against the view that the genital ducts of Ganoids are Müllerian ducts. Their ontogeny would be conclusive on this matter, and we trust that some of the anatomists who have the opportunity of studying the development of the Sturgeon will soon let us know the facts of the case. If there are persisting funnels of the mesonephric segmental tubes in adult Sturgeons, some of them ought to be situated within the genital ducts, if the latter are not Müllerian ducts; [Pg 827] and naturalists who have the opportunity ought also to look out for such openings.
The mode of origin of the anterior part of the genital duct of Lepidosteus appears to us to tell strongly in favour of the view, already regarded as probable by one of us[546], that the Teleostean genital ducts are derived from those of Ganoids; and if, as appears to us indubitable, the most primitive type of Ganoid genital ducts is found in the Chondrostei, it is interesting to notice that the remaining Ganoids present in various ways approximations to the arrangement typically found in Teleostei. Lepidosteus obviously approaches Teleostei in the fact of the ovarian ridge forming part of the wall of the oviduct, but differs from the Teleostei in the fact of the oviduct opening into the kidney ducts, instead of each pair of ducts having an independent opening in the cloaca, and in the fact that the male genital products are not carried to the exterior by a duct homologous with the oviduct. Amia is closer to the Teleostei in the arrangement of the posterior part of the genital ducts, in that the two genital ducts coalesce posteriorly; while Polypterus approaches still nearer to the Teleostei in the fact that the two genital ducts and the two kidney ducts unite with each other before they join; and in order to convert this arrangement into that characteristic of the Teleostei we have only to conceive the coalesced ducts of the kidneys acquiring an independent opening into the cloaca behind the genital opening.
The male genital ducts.—The discovery of the vasa efferentia in Lepidosteus, carrying off the semen from the testis, and transporting it to the mesonephros, and thence through the mesonephric tubes to the segmental duct, must be regarded as the most important of our results on the excretory system.
It proves in the first place that the transportation outwards of the genital products of both sexes by homologous ducts, which has been hitherto held to be universal in Ganoids, and which, in the absence of evidence to the contrary, must still be assumed to be true for all Ganoids except Lepidosteus, is a secondary arrangement. This conclusion follows from the fact that in Elasmobranchii, &c., which are not descendants of [Pg 828] the Ganoids, the same arrangement of seminal ducts is found as in Lepidosteus, and it must therefore have been inherited from an ancestor common to the two groups.
If, therefore, the current statements about the generative ducts of Ganoids are true, the males must have lost their vasa efferentia, and the function of vas deferens must have been taken by the homologue of the oviduct, presumably present in the male. The Teleostei must, moreover, have sprung from Ganoidei in which the vasa efferentia had become aborted.
Considerable phylogenetic difficulties as to the relationships of Ganoidei and Elasmobranchii are removed by the discovery that Ganoids were originally provided with a system of vasa efferentia like that of Elasmobranchii.
[537] Treatise on Comparative Embryology, Vol. I., p. 43 [the original edition].
[538] The females we examined, which were no doubt procured at the same time as the male, had their oviducts filled with ova: and it is therefore not surprising that the vasa efferentia should be naturally injected with sperm.
[539] The change is probably effected somewhat earlier than would appear from our description, but our specimens were not sufficiently well preserved to enable us to speak definitely as to the exact period.
[540] We feel fairly confident that there is only one pronephric opening on each side, though we have no single series of sections sufficiently complete to demonstrate this fact with absolute certainty.
[541] Rosenberg, Untersuch. ueb. d. Entwick. d. Teleostierniere, Dorpat, 1867.
[542] Götte, Entwick. d. Unke, p. 826.
[543]
Sedgwick, Early Development of the Wolffian Duct and anterior Wolffian
Tubules in the Chick; with some Remarks on the Vertebrate Excretory
System,
Quart.
Journ. of Micros. Science, Vol.
XXI.,
1881.
[544] F. M. Balfour, Comparative Embryology, Vol. II., pp. 600-603 [the original edition].
[545]
F. M. Balfour, On the Origin and History of the Urinogenital Organs of
Vertebrates,
Journ. of Anat. and Phys., Vol. X., 1876 [This
edition, No. VII].
[546] F. M. Balfour, Comparative Embryology, Vol. II., p. 605 [the original edition].
I.—Anatomy.
Agassiz (No. 2) gives a short description with a figure of the viscera of Lepidosteus as a whole. Van der Hœven has also given a figure of them in his memoir on the air-bladder of this form (No. 8), and Johannes Müller first detected the spiral valve and gave a short account of it in his memoir (No. 13). Stannius, again, makes several references to the viscera of Lepidosteus in his anatomy of the Vertebrata, and throws some doubt on Müller's determination of the spiral valve.
The following description refers to a female Lepidosteus of 100.5 centims. (Plate 40, fig. 66).
With reference to the mouth and pharynx, we have nothing special to remark. Immediately behind the pharynx there comes an elongated tube, which is not divisible into stomach and œsophagus, and may be called the stomach (st.). It is about 44.6 centims. long, and gradually narrows from the middle towards the hinder or pyloric extremity. It runs straight backwards for the greater part of its length, the last 3.8 centims., however, taking a sudden bend forwards. For about half its length the walls are thin, and the mucous membrane is smooth; [Pg 829] in the posterior half the walls are thick, and the mucous membrane is raised into numerous longitudinal ridges. The peculiar glandular structure of the epithelium of this part in the embryo is shewn in Plate 40, fig. 62 (st.). Its opening into the duodenum is provided with a very distinct pyloric valve (py.). This valve projects into a kind of chamber, freely communicating with the duodenum, and containing four large pits (c´), into each of which a group of pyloric cæca opens. These cæca form a fairly compact gland (c.) about 6.5 centims. long, which overlaps the stomach anteriorly, and the duodenum posteriorly.
Close to the pyloric valve, on its right side, is a small papilla, on the apex of which the bile duct opens (b.d´).
A small, apparently glandular, mass closely connected with the bile duct, in the position in which we have seen the pancreas in the larva (Plate 40, figs. 62 and 63, p.), is almost certainly a rudimentary pancreas, like that of many Teleostei; but its preservation was too bad for histological examination. We believe that the pancreas of Lepidosteus has hitherto been overlooked.
The small intestine passes straight backwards for about 8 centims., and then presents three compact coils. From the end of these a section, about 5 centims. long, the walls of which are much thicker, runs forwards. The intestine then again turns backwards, making one spiral coil. This spiral part passes directly, without any sharp line of demarcation, into a short and straight tube, which tapers slightly from before backwards, and ends at the anus. The mucous membrane of the intestine for about the first 3.5 centims. is smooth, and the muscular walls thin: the rest of the small intestine has thick walls, and the mucous membrane is reticulated.
A short spiral valve (sp.v.), with a very rudimentary epithelial fold, making nearly two turns, begins in about the posterior half of the spiral coil of the intestine, extending backwards for slightly less than half the straight terminal portion of the intestine, and ending 4 centims. in front of the anus. Its total length in one example was about 4.5 centims.
The termination of the spiral valve is marked by a slight constriction, and we may call the straight portion of the intestine behind it the rectum (rc.).
[Pg 830] The posterior part of the intestine, from the beginning of the spiral valve to the anus, is connected with the ventral wall of the abdomen by a mesentery.
The air-bladder (a.b.) is 45 centims. long, and opens into the alimentary canal by a slit-like aperture (a.b´.) on the median dorsal line, immediately behind the epipharyngeal teeth. Each lip of this aperture is largely formed by a muscular cushion, thickest at its posterior end, and extending about 6 millims. behind the aperture itself. A narrow passage is bounded by these muscular walls, which opens dorsally into the air-bladder.
The air-bladder is provided with two short anterior cornua, and tapers to a point behind: it shews no indication of any separation into two parts. A strong band of connective tissue runs along the inner aspect of its whole dorsal region, from which there are given off on each side—at intervals of about 12 millims. anteriorly, gradually increasing to 18 millims. posteriorly—bands of muscle, which pass outwards towards its side walls, and then spread out into the numerous reticulations with which the air-bladder is lined throughout. By the contraction of these muscles the cavity of the air-bladder can doubtless be very much diminished.
The main muscular bands circumscribe a series of more or less complete chambers, which were about twenty-seven in number on each side in our example. The chambers are confined to the sides, so that there is a continuous cavity running through the central part of the organ. The whole organ has the characteristic structure of a simple lung.
The liver (lr.) consists of a single elongated lobe, about 32 centims. long, tapering anteriorly and posteriorly, the anterior half being on the average twice as thick as the posterior half. The gall-bladder (g.b.) lies at its posterior end, and is of considerable size, tapering gradually so as to pass insensibly into the bile duct. The hepatic duct (hp.d.) opens into the gall-bladder at its anterior end.
The spleen (s.) is a large, compact, double gland, one lobe lying in the turn of the intestine immediately above the spiral valve, and the other on the opposite side of the intestine, so that the intestine is nearly embraced between the two lobes. [Pg 831]
II.—Development.
We have already described in detail the first formation of the alimentary tract so far as we have been able to work it out, and we need only say here that the anterior and posterior ends of the canal become first formed, and that these two parts gradually elongate, so as to approach each other; the growth of the posterior part is, however, the most rapid. The junction of the two parts takes place a very short distance behind the opening of the bile duct into the intestine.
For some time after the two parts of the alimentary tract have nearly met, the ventral wall of the canal at this point is not closed; so that there is left a passage between the alimentary canal and the yolk-sack, which forms a vitelline duct.
After the yolk-sack has ceased to be visible as an external appendage it still persists within the abdominal cavity. It has, however, by this stage ceased to communicate with the gut, so that the eventual absorption of the yolk is no doubt entirely effected by the vitelline vessels. At these later stages of development we have noticed that numerous yolk nuclei, like those met with in Teleostei and Elasmobranchii[547], are still to be found in the yolk.
It will be convenient to treat the history of sections of the alimentary tract in front of and behind the vitelline duct separately. The former gives rise to the pharyngeal region, the œsophagus, the stomach, and the duodenum.
The pharyngeal region, immediately after it has become established, gives rise to a series of paired pouches. These may be called the branchial pouches, and are placed between the successive branchial arches. The first or hyomandibular pouch, placed between the mandibular and hyoid arches, has rather the character of a double layer of hypoblast than of a true pouch, though in parts a slight space is developed between its two walls. It is shewn in section in Plate 37, fig. 43 (h.m.), from an embryo of about 10 millims., shortly before hatching. It [Pg 832] does not appear to undergo any further development, and, so far as we can make out, disappears shortly after the embryo is hatched, without acquiring an opening to the exterior.
It is important to notice that this cleft, which in the cartilaginous Ganoids and Polypterus remains permanently open as the spiracle, is rudimentary even in the embryo of Lepidosteus.
The second pouch is the hyobranchial pouch: its outer end meets the epiblast before the larva is hatched, and a perforation is effected at the junction of the two layers, converting the pouch into a visceral cleft.
Behind the hyobranchial pouch there are four branchial pouches, which become perforated and converted into branchial clefts shortly after hatching.
The region of the œsophagus following the pharynx is not separated from the stomach, unless a glandular posterior region (vide description of adult) be regarded as the stomach, a non-glandular anterior region forming the œsophagus. The lumen of this part appears to be all but obliterated in the stages immediately before hatching, giving rise for a short period to a solid œsophagus like that of Elasmobranchii and Teleostei[548].
From the anterior part of the region immediately behind the pharynx the air-bladder arises as a dorsal unpaired diverticulum. From the very first it has an elongated slit-like mouth (Plate 40, fig. 64, a.b´.), and is placed in the mesenteric attachment of the part of the throat from which it springs.
We have first noticed it in the stages immediately after hatching. At first very short and narrow, it grows in succeeding stages longer and wider, making its way backwards in the mesentery of the alimentary tract (Plate 40, fig. 65, a.b.). In the larva of a month and a half old (26 millims.) it has still a perfectly simple form, and is without traces of its adult lung-like structure; but in the larva of 11 centims. it has the typical adult structure.
The stomach is at first quite straight, but shortly after the larva is hatched its posterior end becomes bent ventralwards and forwards, so that the flexure of its posterior end (present in the adult) is very early established. The stomach is continuous behind [Pg 833] with the duodenum, the commencement of which is indicated by the opening of the bile duct.
The liver is the first-formed alimentary gland, and is already a compact body before the larva is hatched. We have nothing to say with reference to its development, except that it exhibits the same simple structure in the embryo that it does in the adult.
A more interesting glandular body is the pancreas. It has already been stated that in the adult we have recognized a small body which we believe to be the pancreas, but that we were unable to study its histological characters.
In the embryo there is a well-developed pancreas which arises in the same position and the same manner as in those Vertebrata in which the pancreas is an important gland in the adult.
We have first noticed the pancreas in a stage shortly after hatching (Plate 40, fig. 61, p.). It then has the form of a funnel-shaped diverticulum of the dorsal wall of the duodenum, immediately behind the level of the opening of the bile duct. From the apex of this funnel numerous small glandular tubuli soon sprout out.
The similarity in the development of the pancreas in Lepidosteus to that of the same gland in Elasmobranchii is very striking[549].
The pancreas at a later stage is placed immediately behind the end of the liver in a loop formed by the pyloric section of the stomach (Plate 40, fig. 62, p.). During larval life it constitutes a considerable gland, the anterior end of which partly envelopes the bile duct (Plate 40, fig. 63, p.).
Considering the undoubted affinities between Lepidosteus and the Teleostei, the facts just recorded with reference to the pancreas appear to us to demonstrate that the small size and occasional absence (?) of this gland in Teleostei is a result of the degeneration of this gland; and it seems probable that the pancreas will be found in the larvæ of most Teleostei. These conclusions render intelligible, moreover, the great development of the pancreas in the Elasmobranchii.
[Pg 834] We have first noticed the pyloric cæca arising as outgrowths of the duodenum in larvæ of about three weeks old, and they become rapidly longer and more prominent (Plate 40, fig. 62, c.).
The portion of the intestine behind the vitelline duct is, as in all the Vertebrata, at first straight. In Elasmobranchii the lumen of the part of the intestine in which a spiral valve is present in the adult, very early acquires a more or less semilunar form by the appearance of a fold which winds in a long spiral. In Lepidosteus there is a fold similar in every respect (Plate 38, fig. 53, sp.v.), forming an open spiral round the intestine. This fold is the first indication of the spiral valve, but it is relatively very much later in its appearance than in Elasmobranchii, not being formed till about three weeks after hatching. It is, moreover, in correlation with the small extent of the spiral valve of the adult, confined to a much smaller portion of the intestine than in Elasmobranchii, although owing to the relative straightness of the anterior part of the intestine it is proportionately longer in the embryo than in the adult.
The similarity of the embryonic spiral valve of Lepidosteus to that of Elasmobranchii shews that Stannius' hesitation in accepting Müller's discovery of the spiral valve in Lepidosteus is not justified.
J. Müller (Bau u. Entwick. d. Myxinoiden) holds that the so-called bursa entiana of Elasmobranchii (i.e., the chamber placed between the part of the intestine with the spiral valve and the end of the pylorus) is the homologue of the more elongated portion of the small intestine which occupies a similar position in the Sturgeon. This portion of the small intestine is no doubt homologous with the still more elongated and coiled portion of the small intestine in Lepidosteus placed between the chamber into which the pyloric cæca, &c., open and the region of the spiral valve. The fact that the vitelline duct in the embryo Lepidosteus is placed close to the pyloric end of the stomach, and that the greater portion of the small intestine is derived from part of the alimentary canal behind this, shews that Müller is mistaken in attempting to homologise the bursa entiana of Elasmobranchii, which is placed in front of the vitelline duct, with the coiled part of the small intestine of the above forms. The latter is either derived from an elongation of the very short [Pg 835] portion of the intestine between the vitelline duct and the primitive spiral valve, or more probably by the conversion of the anterior part of the intestine, originally provided with a spiral valve into a coiled small intestine not so provided.
We have already called attention to the peculiar mesentery present in the adult attaching the posterior straight part of the intestine to the ventral wall of the body. This mesentery, which together with the dorsal mesentery divides the hinder section of the body-cavity into two lateral compartments is, we believe, a persisting portion of the ventral mesentery which, as pointed out by one of us[550], is primitively present for the whole length of the body-cavity. The persistence of such a large section of it as that found in the adult Lepidosteus is, so far as we know, quite exceptional. This mesentery is shewn in section in the embryo in Plate 38, fig. 53 (v.mt.). The small vessel in it appears to be the remnant of the subintestinal vein.
[547] For a history of similar nuclei, vide Comp. Embryol., Vol. II., chapters III. and IV.
[548] Vide Comp. Embryol., Vol. II., pp. 50-63 [the original edition].
[549]
Vide F. M. Balfour, Monograph on Development of
Elasmobranch Fishes,
p. 226 [This edition, No. X., p. 454].
[550] Comparative Embryology, Vol. II. p. 514 [the original edition].
It is well known that Lepidosteus is provided with a gill on the hyoid arch, divided on each side into two parts. An excellent figure of this gill is given by Müller (No. 13, plate 5, fig. 6), who holds from a consideration of the vascular supply that the two parts of this gill represent respectively the hyoid gill and the mandibular gill (called by Müller pseudobranch). Müller's views on this subject have not usually been accepted, but it is the fashion to regard the whole of the gill as the hyoid gill divided into two parts. It appeared to us not improbable that embryology might throw some light on the history of this gill, and accordingly we kept a look out in our embryos for traces of gills on the hyoid and mandibular arches. The results we have arrived at are purely negative, but are not the less surprising for this fact. The hyomandibular cleft as shewn above, is never fully developed, and early undergoes a complete atrophy—a fact which is, on the whole, against Müller's view; but what astonished us most in connection with the gill in question is that we have been [Pg 836] unable to find any trace of it even in the oldest larva whose head we have had (26 millims.), and at a period when the gills on the hinder arches have reached their full development.
We imagined the gill in question to be the remnant of a gill fully formed in extinct Ganoid types, and therefore expected to find it better developed in the larva than in the adult. That the contrary is the fact appears to us fairly certain, although we cannot at present offer any explanation of it.
A. Agassiz concludes his memoir on the development of Lepidosteus
by pointing out that in spite of certain affinities in other directions
this form is not so far removed from the bony Fishes as has been
supposed.
Our own observations go far to confirm Agassiz' opinion.
Apart from the complete segmentation, the general development of Lepidosteus is strikingly Teleostean. In addition to the general Teleostean features of the embryo and larva, which can only be appreciated by those who have had an opportunity of practically working at the subject, we may point to the following developmental features[551] as indicative of Teleostean affinities:—
(1) The formation of the nervous system as a solid keel of the epiblast.
(2) The division of the epiblast into a nervous and epidermic stratum.
(3) The mode of development of the gut (vide pp. 752-754).
(4) The mode of development of the pronephros; though, as shewn on p. 822, the pronephros of Lepidosteus has primitive characters not retained by Teleostei.
(5) The early stages in the development of the vertebral column (vide p. 779).
In addition to these, so to speak, purely embryonic characters there are not a few important adult characters:—
(1) The continuity of the oviducts with the genital glands.
[Pg 837](2) The small size of the pancreas, and the presence of numerous so-called pancreatic cæca.
(3) The somewhat coiled small intestine.
(4) Certain characters of the brain, e.g., the large size of the cerebellum; the presence of the so-called lobi inferiores on the infundibulum; and of tori semicirculares in the mid-brain.
In spite of the undoubtedly important list of features to which we have just called attention, a list containing not less important characters, both embryological and adult, separating Lepidosteus from the Teleostei, can be drawn up:—
(1) The character of the truncus arteriosus.
(2) The fact of the genital ducts joining the ureters.
(3) The presence of vasa efferentia in the male carrying the semen from the testes to the kidney, and through the tubules of the latter into the kidney duct.
(4) The presence of a well-developed opercular gill.
(5) The presence of a spiral valve; though this character may possibly break down with the extension of our knowledge.
(6) The typical Ganoid characters of the thalamencephalon and the cerebral hemispheres (vide pp. 769 and 770).
(7) The chiasma of the optic nerves.
(8) The absence of a pecten, and presence of a vascular membrane between the vitreous humour and the retina.
(9) The opisthocœlous form of the vertebræ.
(10) The articulation of the ventral parts of the hæmal arches of the tail with processes of the vertebral column.
(11) The absence of a division of the muscles into dorso-lateral and ventro-lateral divisions.
(12) The complete segmentation of the ovum.
The list just given appears to us sufficient to demonstrate that Lepidosteus cannot be classed with the Teleostei; and we hold that Müller's view is correct, according to which Lepidosteus is a true Ganoid.
The existence of the Ganoids as a distinct group has, however, recently been challenged by so distinguished an Ichthyologist as Günther, and it may therefore be well to consider how far the group as defined by Müller is a natural one for living [Pg 838] forms[552], and how far recent researches enable us to improve upon Müller's definitions. In his classical memoir (No. 13) the characters of the Ganoids are thus shortly stated:—
"These Fishes are either provided with plate-like angular or rounded cement-covered scales, or they bear osseous plates, or are quite naked. The fins are often, but not always, beset with a double or single row of spinous plates or splints. The caudal fin occasionally embraces in its upper lobe the end of the vertebral column, which may be prolonged to the end of the upper lobe. Their double nasal openings resemble those of Teleostei. The gills are free, and lie in a branchial cavity under an operculum, like those of Teleostei. Many of them have an accessory organ of respiration, in the form of an opercular gill, which is distinct from the pseudobranch, and can be present together with the latter; many also have spiracles like Elasmobranchii. They have many valves in the stem of the aorta like the latter, also a muscular coat in the stem of the aorta. Their ova are transported from the abdominal cavity by oviducts. Their optic nerves do not cross each other. The intestine is often provided with a spiral valve, like Elasmobranchii. They have a swimming-bladder with a duct, like many Teleostei. Their pelvic fins are abdominal.
If we include in a definition only those characters
which are invariable, the Ganoids may be shortly defined as being those
Fish with numerous valves to the stem of the aorta, which is also provided
with a muscular coat; with free gills and an operculum, and with abdominal
pelvic fins.
To these distinctive characters, he adds in an appendix to his paper, the presence of the spiral valve, and the absence of a processus falciformis and a choroid gland.
To the distinctive set of characters given by Müller we may probably add the following:—
(1) Oviducts and urinary ducts always unite, and open by a common urinogenital aperture behind the anus.
(2) Skull hyostylic.
[Pg 839](3) Segmentation complete in the types so far investigated, though perhaps Amia may be found to resemble the Teleostei in this particular.
(4) A pronephros of the Teleostean type present in the larva.
(5) Thalamencephalon very large and well developed.
(6) The ventricle in the posterior part of the cerebrum is not divided behind into lateral halves, the roof of the undivided part being extremely thin.
(7) Abdominal pores always present.
The great number of characters just given are amply sufficient to differentiate the Ganoids as a group; but, curiously enough, the only characters amongst the whole series which have been given, which can be regarded as peculiar to the Ganoids, are (1) the characters of the brain, and (2) the fact of the oviducts and kidney ducts uniting together and opening by a common pore to the exterior.
This absence of characters peculiar to the Ganoids is an indication of how widely separated in organization are the different members of this great group.
At the same time, the only group with which existing Ganoids have close affinities is the Teleostei. The points they have in common with the Elasmobranchii are merely such as are due to the fact that both retain numerous primitive Vertebrate characters[553], and the gulf which really separates them is very wide.
There is again no indication of any close affinity between the Dipnoi and, at any rate, existing Ganoids.
Like the Ganoids, the Dipnoi are no doubt remnants of a very primitive stock; but in the conversion of the air-bladder into a true lung, the highly specialized character of their limbs[554], their peculiar autostylic skulls, the fact of their ventral nasal openings leading directly into the mouth, their multisegmented bars (interspinous bars), directly prolonged from the neural and hæmal arches and supporting the fin-rays of the unpaired dorsal and ventral fins, and their well-developed cerebral hemispheres, [Pg 840] very unlike those of Ganoids and approaching the Amphibian type, they form a very well-defined group, and one very distinctly separated from the Ganoids.
No doubt the Chondrostean Ganoids are nearly as far removed from the Teleostei as from the Dipnoi, but the links uniting these Ganoids with the Teleostei have been so fully preserved in the existing fauna of the globe, that the two groups almost run into each other. If, in fact, we were anxious to make any radical change in the ordinary classification of Fishes, it would be by uniting the Teleostei and Ganoids, or rather constituting the Teleostei into one of the sub-groups of the Ganoids, equivalent to the Chondrostei. We do not recommend such an arrangement, which in view of the great preponderance of the Teleostei amongst living Fishes would be highly inconvenient, but the step from Amia to the Teleostei is certainly not so great as that from the Chondrostei to Amia, and is undoubtedly less than that from the Selachii to the Holocephali.
[551] The features enumerated above are not in all cases confined to Lepidosteus and Teleostei, but are always eminently characteristic of the latter.
[552] We do not profess to be able to discuss this question for extinct forms of Fish, though of course it is a necessary consequence of the theory of descent that the various groups should merge into each other as we go back in geological time.
[553] As instances of this we may cite (1) the spiral valve; (2) the frequent presence of a spiracle; (3) the frequent presence of a communication between the pericardium and the body-cavity; (4) the heterocercal tail.
[554]
Vide F. M. Balfour, On the Development of the
Skeleton of the Paired Fins of Elasmobranchii,
Proc. Zool. Soc.,
1881 [This edition, No. XX.].
1. Agassiz, A. The
Development of Lepidosteus.
Part 1., Proc. Amer. Acad. Arts and
Sciences, Vol. XIV. 1879.
2. Agassiz, L. Recherches s. l. Poissons Fossiles. Neuchatel. 1833-45.
3. Boas, J. E. Ueber Herz u. Arterienbogen bei Ceradotus u.
Protopterus,
Morphol. Jahrbuch, Vol. VI. 1880.
4. Davidoff, M. von. Beiträge z. vergleich. Anat. d. hinteren Gliedmassen d.
Fische,
Morphol. Jahrbuch, Vol. VI. 1880.
5. Gegenbaur, C. Untersuch. z. vergleich. Anat. d. Wirbelthiere, Heft II., Schultergürtel d. Wirbelthiere. Brustflosse der Fische. Leipzig, 1865.
6. Gegenbaur, C. Zur Entwick. d. Wirbelsäule d. Lepidosteus, &c.
Jenaische
Zeitschrift, Vol. III. 1867.
7. Hertwig, O. Ueber d. Hautskelet d. Fische (Lepidosteus u.
Polypterus),
Morphol. Jahrbuch, Vol. V. 1879.
8. Hœven, Van der. Ueber d. zellige Schwimmblase d. Lepidosteus.
Müller's
Archiv, 1841.
[Pg 841] 9. Hyrtl, J. Ueber d. Schwimmblase von Lepidosteus osseus,
Sitz. d. Wiener Akad. Vol.
VIII.
1852.
10. Hyrtl, J. Ueber
d. Pori abdominales, d. Kiemen-Arterien, u. d. Glandula thyroidea d.
Ganoiden,
Sitz. d. Wiener Akad. Vol. VIII. 1852.
11. Hyrtl, J. Ueber d. Zusammenhang [TN19] d. Geschlechts u. Harnwerkzeuge bei d. Ganoiden, Wien, 1855.
12. Kölliker, A. Ueber d. Ende d. Wirbelsäule b. Ganoiden, Leipzig, 1860.
13. Müller, J. Ueber d. Bau u. d. Grenzen d. Ganoiden,
Berlin Akad. 1844.
14. Schneider, H. Ueber d. Augenmuskelnerven d. Ganoiden,
Jenaische Zeitschrift, Vol.
XV. 1881.
15. Wilder, Burt G. Notes on
the North American Ganoids, Amia, Lepidosteus,
Acipenser, and Polyodon.
Proc. Amer. Assoc. for the Advancement
of Science, 1875.
a. Anus. ab. Air-bladder. ab´. Aperture of air-bladder into throat. ac. Anterior commissure. af. Anal fin. al. Alimentary canal. ao. Aorta. ar. Artery. au. Auditory pit. b. Brain. bc. Body-cavity. bd. Bile duct. bd´. Aperture of bile duct into duodenum. bl. Coalesced portion of segmental ducts, forming urinogenital bladder. bra. Branchial arches. brc. Branchial clefts. c. Pyloric caæca. c´. Apertures of caæca into duodenum. cb. Cerebellum. cdv. Cardinal vein. ce. Cerebrum: in figs. 47A and B, anterior lobe of cerebrum. ce´. Posterior lobe of cerebrum. cf. Caudal fin. cn. Centrum. ch. Choroidal fissure. crv. Circular vein of vascular membrane of eye. csh. Cuticular sheath of notochord. cv. Caudal vein. d. Duodenum. dc. Dorsal cartilage of neural arch. df. Dermal fin-rays. dl. Dorsal lobe of caudal fin. dlf. Dorsal fin. e. Eye. ed. Epidermis. ep. Epiblast. fb. Fore-brain. fe. Pyriform bodies surrounding the zona radiata of the ovum, probably the remains of epithelial cells. gb. Gall-bladder. gd. Genital duct. gl. Glomerulus. gr. Genital ridge. h. Heart. ha. Hæmal arch. hb. Hind-brain. hc. Head-cavity. hpd. Hepatic duct. hm. Hyomandibular cleft. hop. Operculum. hy. Hypoblast; in fig. 10, hyoid arch. hyl. Hyaloid membrane. ic. Intercalated cartilaginous elements of the neural arches. in. Infundibulum. ir. Iris. is. Interspinous cartilage or bones. iv. subintestinal vein. ivr. Intervertebral ring of cartilage. k. Kidney. l. Lens. lc. Longitudinal canal, formed by union of the vasa efferentia. lin. Lobi inferiores. ll. Ligamentum longitudinale superius. lr. Liver. lt. Lateral line. ly. Lymphatic body in front of kidney. m. Mouth. mb. Mid-brain. mc. Medullary cord. mel. Membrana elastica externa. mes. Mesorchium. mn. Mandible. md. and mo. Medulla oblongata. ms. Mesoblast. na. Neural arch. na´. Dorsal element of neural arch. nc. Notochord. nve. Network formed by vasa efferentia on inner face of testis. od. Oviduct. od´. Aperture of oviduct into bladder. ol. Nasal pit or aperture. olf. Olfactory lobe. op. Optic vesicle. opch. Optic chiasma. opl. Optic lobes. opth. Optic thalami. orep. [Pg 842] Oral epithelium. ov. Ovary. p. Pancreas. pc. Pericardium. pcf. Pectoral fin. pch. Pigmented layer of choroid. pf. Peritoneal funnel of segmental tube of mesonephros. pfp. Peritoneal funnel leading into pronephric chamber. pg. Pectoral girdle. plf. Pelvic fin. pn. Pineal gland. po. Primitive germinal cells. pr. Mesoblastic somite. prc. Pronephric chamber. prn. Pronephros. prn´. Opening of pronephros into pronephric chamber. pt. Pituitary body. py. Pyloric valve. pz. Parietal zone of blastoderm. r. Rostrum. rb. Rib. rc. Rectum. s. Spleen. sc. Seminal vessels passing from the longitudinal canal into the kidney. sd. Suctorial disc. sg. Segmental or archinephric duct. sgt. Segmental tubules. sh. Granular outer portion of the sheath of the notochord in the vertebral regions. smx. Superior maxillary process. snc. subnotochordal rod. so. Somatic mesoblast. sp. Splanchnic mesoblast. spn. Spinal nerve. spv. Spiral valve. st. Stomach. st. Seminal tubes of the testis. sup. Suctorial papillæ. t. Testis. th. Thalamencephalon. thl. Lobes of the roof of the thalamencephalon. tr. Trabeculæ. ug. Urinogenital aperture. v. Ventricle. ve. Vasa efferentia. vh. Vitreous humour. vl. Ventral lobe of the caudal fin. vmt. Ventral mesentery. vn. Vein. vs. Blood-vessel. vsh. Vascular sheath between the hyaloid membrane and the vitreous humour. vth. Vesicle of the thalamencephalon. x. Groove in epiblast, probably formed in process of hardening. y. Yolk. z. Commissure in front of pineal gland. zr. Outer striated portion of investing membrane (zona radiata) of ovum. zr´. Inner non-striated portion of investing membrane of ovum. I. Olfactory nerve. II. Optic nerve. III. Oculomotor nerve. V. Trigeminal nerve. VIII. Facial and auditory nerves.
EXPLANATION OF PLATES 34-42.
Plate 34.
Figs. 1-4. Different stages in the segmentation of the ovum.
Fig. 1. Ovum with a single vertical furrow, from above.
Fig. 2. Ovum with two vertical furrows, from above.
Fig. 3. Side view of an ovum with a completely formed blastodermic disc.
Fig. 4. The same ovum as fig. 3, from below, shewing four vertical furrows nearly meeting at the vegetative pole.
Figs. 5-10. External views of embryos up to time of hatching.
Fig. 5. Embryo, 3.5 millims. long, third day after impregnation.
Fig. 6. Embryo on the fifth day after impregnation.
Fig. 7. Posterior part of same embryo as fig. 6, shewing tail swelling.
Fig. 8. Embryo on the sixth day after impregnation.
Fig. 9. Embryo on the seventh day after impregnation.
Fig. 10. Embryo on the eleventh day after impregnation (shortly before hatching).
Fig. 11. Head of embryo about the same age as fig. 10, ventral aspect.
Fig. 12. Side view of a larva about 11 millims. in length, shortly after hatching.
Fig. 13. Head of a larva about the same age as fig. 12, ventral aspect.
[Pg 843] Fig. 14. Side view of a larva about 15 millims. long, five days after hatching.
Fig. 15. Head of a larva 23 millims. in length.
Fig. 16. Tail of a larva 11 centims. in length.
Fig. 17. Transverse section through the egg-membranes of a just-laid ovum.
We are indebted to Professor W. K. Parker for figs. 12, 14 and 15.
Plate 35.
Figs. 18-22. Transverse sections of embryo on the third day after impregnation.
Fig. 18. Through head, shewing the medullary keel.
Fig. 19. Through anterior part of trunk.
Fig. 20. Through same region as fig. 19, shewing a groove (x) in the epiblast, probably artificially formed in the process of hardening.
Fig. 21. Through anterior part of tail region, shewing partial fusion of layers.
Fig. 22. Through posterior part of tail region, shewing more complete fusion of layers than fig. 21.
Figs. 23-25. Transverse sections of an embryo on the fifth day after impregnation.
Fig. 23. Through fore-brain and optic vesicles.
Fig. 24. Through hind-brain and auditory pits.
Fig. 25. Through anterior part of trunk.
Figs. 26-27. Transverse[TN20] sections of the head of an embryo on the sixth day after impregnation.
Fig. 26. Through fore-brain and optic vesicles.
Fig. 27. Through hind-brain and auditory pits.
Plate 36.
Figs. 28-29. Transverse sections of the trunk of an embryo on the sixth day after impregnation.
Fig. 28. Through anterior part of trunk (from a slightly older embryo than the other sections of this stage).
Fig. 29. Slightly posterior to fig. 28, shewing formation of segmental duct as a fold of the somatic mesoblast.
Fig. 30. Longitudinal horizontal section of embryo on the sixth day after impregnation, passing through the mesoblastic somites, notochord, and medullary canal.
Figs. 31-34. Transverse sections through an embryo on the seventh day after impregnation.
Fig. 31. Through anterior part of trunk.
Fig. 32. Through the trunk somewhat behind fig. 31.
Fig. 33. Through tail region.
Fig. 34. Further back than fig. 33, shewing constriction of tail from the yolk.
[Pg 844] Figs. 35-37. Transverse sections through an embryo on the eighth day after impregnation.
Fig. 35. Through fore-brain and optic vesicles.
Fig. 36. Through hind-brain, shewing closed auditory pits, &c.
Fig. 37. Through anterior part of trunk.
Fig. 38. Section through tail of an embryo on the ninth day after impregnation.
Plate 37.
Fig. 39. Section through the olfactory involution and part of fore-brain of a larva on the ninth day after impregnation, shewing olfactory nerve.
Fig. 40. Section through the anterior part of the head of the same larva, shewing pituitary involution.
Figs. 41-43. Transverse sections through an embryo on the eleventh day after impregnation.
Fig. 41. Through fore-part of head, shewing the pituitary body still connected with the oral epithelium.
Fig. 42. Slightly further back than fig. 41, shewing the pituitary body constricted off from the oral epithelium.
Fig. 43. Slightly posterior to fig. 42, to shew olfactory involution, eye, and hyomandibular cleft.
Fig. 44. Longitudinal section of the head of an embryo of 15 millims. in length, a few days after hatching, shewing the structure of the brain.
Fig. 45. Longitudinal section of the head of an embryo, about five weeks after hatching, 26 millims. in length, shewing the structure of the brain. In the front part of the brain the section passes slightly to one side of the median line.
Figs. 46A to 46G. Transverse sections through the brain of an embryo 25 millims. in length, about a month after hatching.
Fig. 46A. Through anterior lobes of cerebrum.
Fig. 46B. Through posterior lobes of cerebrum.
Fig. 46C. Through thalamencephalon.
Fig. 46D. Through optic thalami and optic chiasma.
Fig. 46E. Through optic lobes and infundibulum.
Fig. 46F. Through optic lobes and cerebellum.
Fig. 46G. Through optic lobes and cerebellum, slightly in front of fig. 46F.
Plate 38.
Figs. 47A, B, C. Figures of adult brain.
Fig. 47A. From the side.
Fig. 47B. From above.
Fig. 47C. From below.
Fig. 48. Longitudinal vertical section through the eye of an embryo, about a week after hatching, shewing the vascular membrane surrounding the vitreous humour.
[Pg 845] Fig. 49. Diagram shewing the arrangement of the vessels in the vascular membrane of the vitreous humour of adult eye.
Fig. 50. Capillaries of the same vascular membrane.
Fig. 51. Transverse section through anterior part of trunk of an embryo on the ninth day after impregnation, shewing the pronephros and pronephric chamber.
Fig. 52. Transverse section through the region of the stomach of an embryo 15 millims. in length, shortly after hatching, to shew the glomerulus and peritoneal funnel of pronephros.
Fig. 53. Transverse section through posterior part of the body of an embryo, about a month after hatching, shewing the structure of the mesonephros, the spiral valve, &c.
Plate 39.
Figs. 54, 55, 56, and 57 are a series of transverse sections through the genital ridge and mesonephros of one side from a larva of 11 centims.
Fig. 54. Section of the lymphatic organ which lies in front of the mesonephros.
Fig. 55. Section near the anterior end of the mesonephros, where the genital sack is completely formed.
Fig. 56. Section somewhat further back, shewing the mode of formation of the genital sack.
Fig. 57. Section posterior to the above, the formation of the genital sack not having commenced, and the genital ridge with primitive germinal cells projecting freely into the body-cavity.
Fig. 58A. View of the testis, mesorchium, and duct of the kidney of the left side of an adult male example of Lepidosteus, 60 centims. in length, shewing the vasa efferentia and the longitudinal canal at the base of the mesorchium. The kidney ducts have been cut open posteriorly to shew the structure of the interior.
Fig. 58B. Inner aspect of the posterior lobe of the testis from the same example, to shew the vasa efferentia forming a network on the face of the testis.
Figs. 59A and B. Two sections shewing the structure and relations of the efferent ducts of the testis in the same example.
Fig. 59A. Section through the inner aspect of a portion of the testis and mesorchium, to shew the network of the vasa efferentia (nve) becoming continuous with the seminal tubes (st). The granular matter nearly filling the vasa efferentia and the seminal tubes represent the spermatozoa.
Fig. 59B. Section through part of the kidney and its duct and the longitudinal canal (lc) at the base of the mesorchium. Canals (sc) are seen passing off from the latter, which enter the kidney and join the uriniferous tubuli. Some of the latter (as well as the seminal tubes) are seen to be filled with granular matter, which we believe to be the remains of spermatozoa.
[Pg 846] Fig. 60. Diagram of the urinogenital organs of the left side of an adult female example of Lepidosteus 100 centims. in length. This figure shews the oviduct (od) continuous with the investment of the ovary, opening at od´ into the dilated part of the kidney duct (segmental duct). It also shews the segmental duct and the junction of the latter with its fellow of the right side to form the so-called bladder, this part being represented as cut open. The kidney (k) and lymphatic organ (ly) in front of it are also shewn.
Plate 40.
Fig. 61. Transverse section through the developing pancreas (p) of a larva 11 millims. in length.
Fig. 62. Longitudinal section through portions of the stomach, liver, and duodenum of an embryo about a month after hatching, to shew the relations of the pancreas (p) to the surrounding parts.
Fig. 63. External view of portions of the liver, stomach, duodenum, &c., of a young Fish, 11 centims. in length, to shew the pancreas (p).
Fig. 64. Transverse section through the anterior part of the trunk of an embryo, about a month after hatching, shewing the connection of the air-bladder with the throat (ab´).
Fig. 65. Transverse section through the same embryo as fig. 64 further back, shewing the posterior part of the air-bladder (ab).
Fig. 66. Viscera of an adult female, 100 centims. in length, shewing the alimentary canal with its appended glands in natural position, and the air-bladder with its aperture into the throat (ab´). The proximal part of the duodenum and the terminal part of the intestine are represented as cut open, the former to shew the pyloric valve and the apertures of the pyloric cæca and bile duct, and the latter to shew the spiral valve.
This figure was drawn for us by Professor A. C. Haddon.
Plate 41.
Fig. 67. Transverse section through the tail of an advanced larva, shewing the neural and hæmal processes, the independently developed interneural and interhæmal elements (is), and the commencing dermal fin-rays (df).
Fig. 68. Side view of the tail of a larva, 21 minims. in length, dissected so as to shew the structure of the skeleton.
Fig. 69. Longitudinal horizontal section through the vertebral column of a larva, 5.5 centims. in length, on the level of the hæmal arches, shewing the intervertebral rings of cartilage continuous with the arches, the vertebral constriction of the notochord, &c.
Figs. 70 and 71. Transverse sections through the vertebral column of a larva of 5.5 centims. The red represents bone, and the blue cartilage.
Fig. 70. Through the vertebral region, shewing the neural and hæmal arches, the notochordal sheath, &c.
Fig. 71. Through the intervertebral region, shewing the intervertebral cartilage.
[Pg 847] Figs. 72 and 73. Transverse sections through the trunk of a larva of 5.5 centims. to shew the structure of the ribs and hæmal arches.
Fig. 72. Through the anterior part of the trunk.
Fig. 73. Through the posterior part of the trunk.
Plate 42.
Figs. 74-76. Transverse sections through the trunk of the same larva as figs. 72 and 73.
Fig. 74. Through the posterior part of the trunk (rather further back than fig. 73).
Fig. 75. Through the anterior part of the tail.
Fig. 76. Rather further back than fig. 75.
Fig. 77. Longitudinal horizontal section through the vertebral column of a larva of 11 centims., passing through the level of the hæmal arches, and shewing the intervertebral constriction of the notochord, the ossification of the cartilage, &c.
Fig. 78. Transverse section through a vertebral region of the vertebral column of a larva 11 centims. in length.
Fig. 79. Transverse section through an intervertebral region of the same larva as fig. 78.
Fig. 80. Side view of two trunk vertebræ of an adult Lepidosteus.
Fig. 81. Front view of a trunk vertebra of adult.
In figures 80 and 81 the red does not represent bone as in the other figures, but simply the ligamentum longitudinale superius.
While working at the anatomy of Lepidosteus I was led to doubt the accuracy of the accepted accounts of the anterior part of the kidneys in this[556] and in allied species of Fishes. In order to test my doubts I first examined the structure of the kidneys in the Sturgeon (Acipenser), of which I fortunately had a well-preserved specimen.
The bodies usually described as the kidneys consist of two elongated bands, attached to the dorsal wall of the abdomen, and extending for the greater part of the length of the abdominal cavity. In front each of these bands first becomes considerably narrowed, and then expands and terminates in a great dilatation, which is usually called the head-kidney. Along the outer border of the hinder part of each kidney is placed a wide ureter, which ends suddenly in the narrow part of the body, some little way behind the head-kidney. To the naked eye there is no distinction in structure between the part of the so-called kidney in front of the ureter and that in the region of the ureter. Any section through the kidney in the region of the ureter suffices to shew that in this part the kidney is really formed of uriniferous tubuli with numerous Malpighian bodies. Just in front, however, of the point where the ureter ends the true kidney substance rapidly thins out, and its place is taken by a peculiar tissue formed of a trabecular work filled with cells, [Pg 849] which I shall in future call lymphatic tissue. Thus the whole of that part of the apparent kidney in front of the ureter, including the whole of the so-called head-kidney, is simply a great mass of lymphatic tissue, and does not contain a single uriniferous tubule or Malpighian body.
The difference in structure between the anterior and posterior parts of the so-called kidney, although not alluded to in most modern works on the kidneys, appears to have been known to Stannius, at least I so interpret a note of his in the second edition of his Comparative Anatomy, p. 263, where he describes the kidney of the Sturgeon as being composed of two separate parts, viz. a spongy vascular substance (no doubt the so-called head-kidney) and a true secretory substance.
After arriving at the above results with reference to the Sturgeon I proceeded to the examination of the structure of the so-called head-kidney in Teleostei.
I have as yet only examined four forms, viz. the Pike (Esox lucius), the Smelt (Osmerus eperlanus), the Eel (Anguilla anguilla), and the Angler (Lophius piscatorius).
The external features of the apparent kidney of the Pike have been
accurately described by Hyrtl[557]. He
says: The kidneys extend from the second trunk vertebra to the end of
the abdominal cavity. Their anterior extremities, which have the form of
transversely placed coffee beans, are united together, and lie on the
anterior end of the swimming bladder. The continuation of the kidney
backwards forms two small bands, separated from each other by the whole
breadth of the vertebral column. They gradually, however, increase in
breadth, so that about the middle of the vertebral column they unite
together and form a single symmetrical, keel-shaped body,
&c.
The Pike I examined was a large specimen of about 58 centimètres in length, and with an apparent kidney of about 25½ centimètres. The relations of lymphatic tissue and kidney tissue were much as in the Sturgeon. The whole of the anterior swelling, forming the so-called head-kidney, together with a considerable portion of the part immediately behind, forming not far short of half the whole length of the apparent kidney, [Pg 850] was entirely formed of lymphatic tissue. The posterior part of the kidney was composed of true kidney substance, but even at 16 centimètres from the front end of the kidney the lymphatic tissue formed a large portion of the whole.
A rudiment of the duct of the kidney extended forwards for a short way into the lymphatic substance beyond the front part of the functional kidney.
In the Smelt (Osmerus eperlanus) the kidney had the typical Teleostean form, consisting of two linear bands stretching for the whole length of the body-cavity, and expanding into a great swelling in front on the level of the ductus Cuvieri, forming the so-called head-kidney. The histological examination of these bodies shewed generally the same features as in the case of the Sturgeon and Pike. The posterior part was formed of the usual uriniferous tubuli and Malpighian bodies. The anterior swollen part of these bodies, and the part immediately following, were almost wholly formed of a highly vascular lymphatic tissue; but in a varying amount in different examples portions of uriniferous tubules were present, mainly, however, in the region behind the anterior swelling. In some cases I could find no tubules in the lymphatic tissue, and in all cases the number of them beyond the region of the well-developed part of the kidney was so slight, that there can be little doubt that they are functionless remnants of the anterior part of the larval kidney. Their continuation into the anterior swelling, when present, consisted of a single tube only.
In the Eel (Anguilla anguilla), which, however, I have not examined with the same care as the Smelt, the true excretory part of the kidney appears to be confined to the posterior portion, and to the portion immediately in front of the anus, the whole of the anterior part of each apparent kidney, which is not swollen in front, being composed of lymphatic tissue.
Lophius piscatorius is one of the forms which, according to Hyrtl[558], is provided with a head-kidney only, i.e. with that part of the kidney which corresponds with the anterior swelling of the kidney of other types. For this reason I was particularly anxious to investigate the structure of its kidneys.
[Pg 851] Each of these bodies forms a compact oval mass, with the ureter springing from its hinder extremity, situated in a forward position in the body-cavity. Sections through the kidneys shewed that they were throughout penetrated by uriniferous tubules, but owing to the bad state of preservation of my specimens I could not come to a decision as to the presence of Malpighian bodies. The uriniferous tubules were embedded in lymphatic tissue, similar to that which forms the anterior part of the apparent kidneys in other Teleostean types.
With reference to the structure of the Teleostean kidneys, the account given by Stannius is decidedly more correct than that of most subsequent writers. In the note already quoted he gives it as his opinion that there is a division of the kidney into the same two parts as in the Sturgeon, viz. into a spongy vascular part and a true secreting part; and on a subsequent page he points out the absence or poverty of the uriniferous tubules in the anterior part of the kidney in many of our native Fishes.
Prior to the discovery that the larvæ of Teleosteans and Ganoids were provided with two very distinct excretory organs, viz. a pronephros or head-kidney, and a mesonephros or Wolffian body, which are usually separated from each other by a more or less considerable interval, it was a matter of no very great importance to know whether the anterior part of the so-called kidney was a true excretory organ. In the present state of our knowledge the question is, however, one of considerable interest.
In the Cyclostomata and Amphibia the pronephros is a purely larval organ, which either disappears or ceases to be functionally active in the adult state.
Rosenberg, to whom the earliest satisfactory investigations on the development of the Teleostean pronephros are due, stated that he had traced in the Pike (Esox lucius) the larval organ into the adult part of the kidney, called by Hyrtl the pronephros; and subsequent investigators have usually assumed that the so-called head-kidney of adult Teleosteans and Ganoids is the persisting larval pronephros.
We have already seen that Rosenberg was entirely mistaken on this point, in that the so-called head-kidney of the adult is [Pg 852] not part of the true kidney. From my own studies on young Fishes I do not believe that the oldest larvæ investigated by Rosenberg were sufficiently advanced to settle the point in question; and, moreover, as Rosenberg had no reason for doubting that the so-called head-kidney of the adult was part of the excretory organ, he does not appear to have studied the histological structure of the organ which he identified with the embryonic pronephros in his oldest larva.
The facts to which I have called attention in this paper demonstrate that in the Sturgeon the larval pronephros undoubtedly undergoes atrophy before the adult stage is reached. The same is true for Lepidosteus, and may probably be stated for Ganoids generally.
My observations on Teleostei are clearly not sufficiently extensive to prove that the larval pronephros never persists in this group. They appear to me, however, to shew that in the normal types of Teleostei the organ usually held to be the pronephros is actually nothing of the kind.
A different interpretation might no doubt be placed upon my observations on Lophius piscatorius, but the position of the kidney in this species appears to me to be far from affording a conclusive proof that it is homologous with the anterior swelling of the kidney of more normal Teleostei.
When, moreover, we consider that Lophius, and the other forms mentioned by Hyrtl as being provided with a head-kidney only, are all of them peculiarly modified and specialized types of Teleostei, it appears to me far more natural to hold that their kidney is merely the ordinary Teleostean kidney, which, like many of their other organs, has become shifted in position, than to maintain that the ordinary excretory organ present in other Teleostei has been lost, and that a larval organ has been retained, which undergoes atrophy in less specialized Teleostei.
As the question at present stands, it appears to me that the probabilities are in favour of there being no functionally active remains of the pronephros in adult Teleostei, and that in any case the burden of proof rests with those who maintain that such remnants are to be found.
The general result of my investigations is thus to render it probable that the pronephros, though found in the larvæ or embryos [Pg 853] of almost all the Ichthyopsida, except the Elasmobranchii, is always a purely larval organ, which never constitutes an active part of the excretory system in the adult state.
This conclusion appears to me to add probability to the view of Gegenbaur that the pronephros is the primitive excretory gland of the Chordata; and that the mesonephros or Wolffian body, by which it is replaced in existing Ichthyopsida, is phylogenetically a more recent organ.
In the preceding pages I have had frequent occasion to allude to the lymphatic tissue which has been usually mistaken for part of the excretory organ. This tissue is formed of trabecular work, like that of lymphatic glands, in the meshes of which an immense number of cells are placed, which may fairly be compared with the similarly placed cells of lymphatic glands. In the Sturgeon a considerable number of cells are found with peculiar granular nuclei, which are not found in the Teleostei. In both groups, but especially in the Teleostei, the tissue is highly vascular, and is penetrated throughout by a regular plexus of very large capillaries, which appear to have distinct walls, and which pour their blood into the posterior cardinal vein as it passes through the organ. The relation of this tissue to the lymphatic system I have not made out.
The function of the tissue is far from clear. Its great abundance, highly vascular character, and presence before the atrophy of the pronephros, appear to me to shew that it cannot be merely the non-absorbed remnant of the latter organ. From its size and vascularity it probably has an important function; and from its structure this must either be the formation of lymph corpuscles or of blood corpuscles.
In structure it most resembles a lymphatic gland, though, till it has been shewn to have some relation to the lymphatic system, this can go for very little.
On the whole, I am provisionally inclined to regard it as a form of lymphatic gland, these bodies being not otherwise represented in fishes.
[555] From the Quarterly Journal of Microscopical Science, Vol. XXII., 1882.
[556] I am about to publish, in conjunction with Mr Parker, a full account of the anatomy and development of Lepidosteus [No. XXII. of this edition], and shall therefore in this paper make no further allusion to it.
[557]
Das Uropoëtische System der Knochenfische,
Sitz. d. Wien. Akad., 1830.
[558]
Das Uropoëtische System der Knochenfische,
Sitz. d. Wien. Akad., 1830.
(With Plates 43, 44, 45.)
The formation of the germinal layers in the chick has been so often and so fully dealt with in recent years, that we consider some explanation to be required of the reasons which have induced us to add to the long list of memoirs on this subject. Our reasons are twofold. In the first place the principal results we have to record have already been briefly put forward in a Treatise on Comparative Embryology by one of us; and it seemed desirable that the data on which the conclusions there stated rest should be recorded with greater detail than was possible in such a treatise. In the second place, our observations differ from those of most other investigators, in that they were primarily made with the object of testing a theory as to the nature of the primitive streak. As such they form a contribution to comparative embryology; since our object has been to investigate how far the phenomena of the formation of the germinal layers in the chick admit of being compared with those of lower and less modified vertebrate types.
We do not propose to weary the reader by giving a new version of the often told history of the views of various writers on the germinal layers in the chick, but our references to other investigators will be in the main confined to a comparison of our results with those of two embryologists who have published their memoirs since our observations were made. One of them is L. Gerlach, who published a short memoir[560] in April last, and [Pg 855] the other is C. Koller, who has published his memoir[561] still more recently. Both of them cover part of the ground of our investigations, and their results are in many, though not in all points, in harmony with our own. Both of them, moreover, lay stress on certain features in the development which have escaped our attention. We desired to work over these points again, but various circumstances have prevented our doing so, and we have accordingly thought it best to publish our observations as they stand, in spite of their incompleteness, merely indicating where the most important gaps occur.
Our observations commence at a stage a few hours after hatching, but before the appearance of the primitive streak.
The area pellucida is at this stage nearly spherical. In it there is a large oval opaque patch, which is continued to the hinder border of the area. This opaque patch has received the name of the embryonic shield—a somewhat inappropriate name, since the structure in question has no very definite connection with the formation of the embryo.
Koller describes, at this stage, in addition to the so-called embryonic shield, a sickle-shaped opaque appearance at the hinder border of the area pellucida.
We have not made any fresh investigations for the purpose of testing Koller's statements on this subject.
Embryologists are in the main agreed as to the structure of the blastoderm at this stage. There is (Pl. 43, Ser. A, 1 and 2) the epiblast above, forming a continuous layer, extending over the whole of the area opaca and area pellucida. In the former its cells are arranged as a single row, and are cubical or slightly flattened. In the latter the cells are more columnar, and form, in the centre especially, more or less clearly, a double row; many of them, however, extend through the whole thickness of the layer.
We have obtained evidence at this stage which tends to shew that at its outer border the epiblast grows not merely by the division of its own cells, but also by the addition of cells derived from the yolk below. The epiblast has been observed to extend itself over the yolk by a similar process in many invertebrate forms.
[Pg 856] Below the epiblast there is placed, in the peripheral part of the area opaca, simply white yolk; while in a ring immediately outside and concentric with the area pellucida, there is a closely-packed layer of cells, known as the germinal wall. The constituent cells of this wall are in part relatively small, of a spherical shape, with a distinct nucleus, and a granular and not very abundant protoplasm; and in part large and spherical, filled up with highly refracting yolk particles of variable size, which usually render the nucleus (which is probably present) invisible (A, 1 and 2). This mass of cell rests, on its outer side, on a layer of white yolk.
The sickle-shaped structure, visible in surface veins, is stated by Koller to be due to a special thickening of the germinal wall. We have not found this to be a very distinctly marked structure in our sections.
In the region of the area pellucida there is placed below the epiblast a more or less irregular layer of cells. This layer is continuous, peripherally, with the germinal wall; and is composed of cells, which are distinguished both by their flattened or oval shape and more granular protoplasm from the epiblast-cells above, to which, moreover, they are by no means closely attached. Amongst these cells a few larger cells are usually present, similar to those we have already described as forming an important constituent of the germinal wall.
We have figured two sections of a blastoderm of this age (Ser. A, 1 and 2) mainly to shew the arrangement of these cells. A large portion of them, considerably more flattened than the remainder, form a continuous membrane over the whole of the area pellucida, except usually for a small area in front, where the membrane is more or less interrupted. This layer is the hypoblast (hy.). The remaining cells are interposed between this layer and the epiblast. In front of the embryonic shield there are either comparatively few or none of these cells present (Ser. A, 1), but in the region of the embryonic shield they are very numerous (ser. A, 2), and are, without doubt, the main cause of the opacity of this part of the area pellucida. These cells may be regarded as not yet completely differentiated segmentation spheres.
In many blastoderms, not easily distinguishable in surface [Pg 857] views from those which have the characters just described, the hypoblastic sheet is often much less completely differentiated, and we have met with other blastoderms, again, in which the hypoblastic sheet was completely established, except at the hinder part of the embryonic shield; where, in place of it and of the cells between it and the epiblast, there was only to be found a thickish layer of rounded cells, continuous behind with the germinal wall.
In the next stage, of which we have examined surface views and sections, there is already a well-formed primitive streak.
The area pellucida is still nearly spherical, the embryonic shield has either disappeared or become much less obvious, but there is present a dark linear streak, extending from the posterior border of the area pellucida towards the centre, its total length being about one third, or even less, of the diameter of the area. This streak is the primitive streak. It enlarges considerably behind, where it joins the germinal wall. By Koller and Gerlach it is described as joining the sickle-shaped structure already spoken of. We have in some instances found the posterior end of the primitive streak extending laterally in the form of two wings (Pl. 45, fig. L). These extensions are, no doubt, the sickle; but the figures given by Koller appear to us somewhat diagrammatic. One or two of the figures of early primitive streaks in the sparrow, given by Kupffer and Benecke[562], correspond more closely with what we have found, except that in these figures the primitive streak does not reach the end of the area pellucida, which it certainly usually does at this early stage in the chick.
Sections through the area pellucida (Pl. 43, Ser. B and C) give the following results as to the structure of its constituent parts.
The epiblast cells have undergone division to a considerable extent, and in the middle part, especially, are decidedly more columnar than at an earlier stage, and distinctly divided into two rows, the nuclei of which form two more or less distinct layers.
In the region in front of the primitive streak the cells of the lower part of the blastoderm have arranged themselves as a [Pg 858] definite layer, the cells of which are not so flat as is the case with the hypoblast cells of the posterior part of the blastoderm, and in the older specimens of this stage they are very decidedly more columnar than in the younger specimens.
The primitive streak is however the most interesting structure in the area pellucida at this stage.
The feature which most obviously strikes the observer in transverse sections through it is the fact, proved by Kölliker, that it is mainly due to a proliferation of the epiblast cells along an axial streak, which, roughly speaking, corresponds with the dark line visible in surface views. In the youngest specimens and at the front end of the primitive streak, the proliferated cells do not extend laterally beyond the region of their origin, but in the older specimens they have a considerable lateral extension.
The hypoblast can, in most instances, be traced as a distinct layer underneath the primitive streak, although it is usually less easy to follow it in that region than elsewhere, and in some cases it can hardly be distinctly separated from the superjacent cells.
The cells, undoubtedly formed by a proliferation of the epiblast, form a compact mass extending downwards towards the hypoblast; but between this mass and the hypoblast there are almost always present along the whole length of the primitive streak a number of cells, more or less loosely arranged, and decidedly more granular than the proliferated cells. Amongst these loosely arranged cells there are to be found a certain number of large spherical cells filled with yolk granules. Sometimes these cells are entirely confined to the region of the primitive streak, at other times they are continuous laterally with cells irregularly scattered between the hypoblast and epiblast (Ser. C, 2), which are clearly the remnants of the undifferentiated cells of the embryonic shield. The junction between these cells and the cells of the primitive streak derived from the epiblast is often obscure, the two sets of cells becoming partially intermingled. The facility with which the cells we have just spoken of can be recognized varies moreover greatly in different instances. In some cases they are very obvious (Ser. C), while in other cases they can only be distinguished by a careful examination of good sections.
[Pg 859] The cells of the primitive streak between the epiblast and the hypoblast are without doubt mesoblastic, and constitute the first portion of the mesoblast which is established. The section of these cells attached to the epiblast, in our opinion, clearly originates from the epiblast; while the looser cells adjoining the hypoblast must, it appears to us, be admitted to have their origin in the indifferent cells of the embryonic shield, placed between the epiblast and the hypoblast, and also very probably in a distinct proliferation from the hypoblast below the primitive streak.
Posteriorly the breadth of the streak of epiblast which buds off the cells of the primitive streak widens considerably, and in the case of the blastoderm with the earliest primitive streaks extends into the region of the area opaca. The widening of the primitive streak behind is shewn in Ser. B, 3; Ser. C, 2; and Ser. E, 4. Where very marked it gives rise to the sickle-shaped appearance upon which so much stress has been laid by Koller and Gerlach. In the case of one of the youngest of our blastoderms of this stage in which we found in surface views (Pl. 45, fig. L) a very well-marked sickle-shaped appearance at the hind end of the primitive streak, the appearance was caused, as is clearly brought out by our sections, by a thickening of the hypoblast of the germinal wall.
There is a short gap in our observations between the stage with a young primitive streak and the first described stage in which no such structure is present. This gap has been filled up both by Gerlach and Koller.
Gerlach states that during this period a small portion of the epiblast, within the region of the area opaca, but close to the posterior border of the area pellucida, becomes thickened by a proliferation of its cells. This portion gradually grows outwards laterally, forming in this way a sickle-shaped structure. From the middle of this sickle a process next grows forward into the area pellucida. This process is the primitive streak, and it is formed, like the sickle, of proliferating epiblast cells.
Koller[563] described the sickle and the growth forwards from it of the primitive streak in surface views somewhat before Gerlach; [Pg 860] and in his later memoir has entered with considerable detail into the part played by the various layers in the formation of this structure.
He believes, as already mentioned, that the sickle-shaped structure,
which appears according to him at an earlier stage than is admitted by
Gerlach, is in the first instance due to a thickening of the hypoblast. At
a later stage he finds that the epiblast in the centre of the sickle
becomes thickened, and that a groove makes its appearance in this
thickening which he calls the Sichel-rinne.
This groove is
identical with that first described by Kupffer and Benecke[564] in the sparrow and fowl. We have never, however, found
very clear indications of it in our sections.
In the next stage, Koller states that, in the region immediately in
front of the Sichel-rinne,
a prominence appears which he
calls the Sichelknopf, and from this a process grows forwards which
constitutes the primitive streak. This structure is in main derived from a
proliferation of epiblast cells, but Koller admits that some of the cells
just above the hypoblast in the region of the Sichelknopf are probably
derived from the hypoblast. Since these cells form part of the mesoblast it
is obvious that Koller's views on the origin of the mesoblast of the
primitive streak closely approach those which we have put forward.
The primitive streak starting, as we have seen, at the hinder border of the area pellucida, soon elongates till it eventually occupies at least two-thirds of the length of the area. As Koller (loc. cit.) has stated, this can only be supposed to happen in one of two ways, viz. either by a progression forward of the region of epiblast budding off mesoblast, or by an interstitial growth of the area of budding epiblast. Koller adopts the second of these alternatives, but we cannot follow him in doing so. The simplest method of testing the point is by measuring the distance between the front end of the primitive streak and the front border of the area pellucida at different stages of growth of the primitive streak. If this distance diminishes with the elongation of the primitive streak then clearly the second of the two alternatives is out of the question.
[Pg 861] We have made measurements to test this point, and find that the diminution of the space between the front end of the primitive streak and the anterior border of the area pellucida is very marked up to the period in which the medullary plate first becomes established. We can further point in support of our view to the fact that the extent of the growth lateralwards of the mesoblast from the sides of the primitive streak is always less in front than behind; which would seem to indicate that the front part of the streak is the part formed latest. Our view as to the elongation of the primitive streak appears to be that adopted by Gerlach.
Our next stage includes roughly the period commencing slightly before the first formation of a groove along the primitive streak, known as the primitive groove, and terminating immediately before the first trace of the notochord makes its appearance. After the close of the last stage the primitive streak gradually elongates, till it occupies fully two-thirds of the diameter of the area pellucida. The latter structure also soon changes its form from a circular to an oval, and finally becomes pyriform with the narrow end behind, while the primitive streak occupying two-thirds of its long axis becomes in most instances marked by a light linear band along the centre, which constitutes the primitive groove.
In surface views the primitive streak often appears to stop short of the hinder border of the area pellucida.
During the period in which the external changes, which we have thus briefly described, take place in the area pellucida, great modifications are effected in the characters of the germinal layers. The most important of these concern the region in front of the primitive streak; but they will be better understood if we commence our description with the changes in the primitive streak itself.
In the older embryos belonging to our last stage we pointed out that the mesoblast of the primitive streak was commencing to extend outwards from the median line in the form of two lateral sheets. This growth of the mesoblast is continued rapidly during the present stage, so that during the latter part of it any section through the primitive streak has approximately the characters of Ser. I, 5.
[Pg 862] The mesoblast is attached in the median line to the epiblast. Laterally it extends outwards to the edge of the area pellucida, and in older embryos may even form a thickening beyond the edge (fig. G). Beneath the denser part of the mesoblast, and attached to the epiblast, a portion composed of stellate cells may in the majority of instances be recognized, especially in the front part of the primitive streak. We believe these stellate cells to be in the main directly derived from the more granular cells of the previous stage. The hypoblast forms a sheet of flattened cells, which can be distinctly traced for the whole breadth of the area pellucida, though closely attached to the mesoblast above.
In sections we find that the primitive streak extends back to the border of the area pellucida, and even for some distance beyond. The attachment to the epiblast is wider behind; but the thickness of the mesoblast is not usually greater in the median line than it is laterally, and for this reason probably the posterior part of the streak fails to shew up in surface views. The thinning out of the median portion of the mesoblast of the primitive streak is shewn in a longitudinal section of a duck's blastoderm of this stage (fig. D). The same figure also shews that the hypoblastic sheet becomes somewhat thicker behind, and more independent of the parts above.
A careful study of the peripheral part of the area pellucida, in the region of the primitive streak, in older embryos of this stage, shews that the hypoblast is here thickened, and that its upper part, i.e. that adjoining the mesoblast, is often formed of stellate cells, many of which give the impression of being in the act of passing into the mesoblast above. At a later stage the mesoblast of the vascular area undoubtedly receives accessions of cells from the yolk below; so that we see no grounds for mistrusting the appearances just spoken of, or for doubting that they are to be interpreted in the sense suggested.
We have already stated that during the greater part of the present stage a groove, known as the primitive groove, is to be found along the dorsal median line of the primitive streak.
The extent to which this groove is developed appears to be subject to very great variation. On the average it is, perhaps, slightly deeper than it is represented in Ser. I, 5. In some cases [Pg 863] it is very much deeper. One of the latter is represented in fig. G. It has here the appearance of a narrow slit, and sections of it give the impression of the mesoblast originating from the lips of a fold; in fact, the whole structure appears like a linear blastopore, from the sides of which the mesoblast is growing out; and this as we conceive actually to be the true interpretation of the structure. Other cases occur in which the primitive groove is wholly deficient, or at the utmost represented by a shallow depression along the median axial line of a short posterior part of the primitive streak.
We may now pass to the consideration of the part of the area pellucida in front of the primitive streak.
We called attention to a change in the character of the hypoblast cells of this region as taking place at the end of the last stage. During the very early part of this stage the change in the character of these cells becomes very pronounced.
What we consider to be our earliest stage in this change we have only so far met with in the duck, and we have figured a longitudinal and median section to shew it (Pl. 43, fig. D). The hypoblast (hy) has become a thick layer of somewhat cubical cells several rows deep. These cells, especially in front, are characterized by their numerous yolk spherules, and give the impression that part of the area pellucida has been, so to speak, reclaimed from the area opaca. Posteriorly, at the front end of the primitive streak, the thick layer of hypoblast, instead of being continuous with the flattened hypoblast under the primitive streak, falls, in the axial line, into the mesoblast of the primitive streak (Pl. 43, fig. D).
In a slightly later stage, of which we have specimens both of the duck and chick, but have only figured selected sections of a chick series, still further changes have been effected in the constitution of the hypoblast (Pl. 44, Ser. H, 1 and 2).
Near the front border of the area pellucida (1) it has the general characters of the hypoblast of the duck's blastoderm just described. Slightly further back the cells of the hypoblast have become differentiated into stellate cells several rows deep, which can hardly be resolved in the axial line into hypoblast and mesoblast, though one can fancy that in places, especially laterally, they are partially differentiated into two layers. The axial [Pg 864] sheet of stellate cells is continuous laterally with cubical hypoblast cells.
As the primitive streak is approached an axial prolongation forwards of the rounded and closely-packed mesoblastic elements of the primitive streak is next met with; and at the front end of the primitive streak, where this prolongation unites with the epiblast, it also becomes continuous with the stellate cells just spoken of. In fact, close to the end of the primitive streak it becomes difficult to say which mesoblast cells are directly derived from the primitive layer of hypoblast in front of the primitive streak, and which from the forward growth of the mesoblast of the primitive streak. There is, in fact, as in the earlier stage, a fusion of the layers at this point.
Sections of a slightly older chick blastoderm are represented in Pl. 45, Ser. I, 1, 2, 3, 4 and 5.
Nearly the whole of the hypoblast in front of the primitive streak has now undergone a differentiation into stellate cells. In the second section the products of the differentiation of this layer form a distinct mesoblast and hypoblast laterally, while in the median line they can hardly be divided into two distinct layers.
In a section slightly further back the same is true, except that we have here, in the axial line above the stellate cells, rounded elements derived from a forward prolongation of the cells of the primitive streak. In the next section figured, passing through the front end of the primitive streak, the axial cells have become continuous with the axial mesoblast of the primitive streak, while below there is an independent sheet of flattened hypoblast cells.
The general result of our observations on the part of the blastoderm in front of the primitive streak during this stage is to shew that the primitive hypoblast of this region undergoes considerable changes, including a multiplication of its cells; and that these changes result in its becoming differentiated on each side of the middle line, with more or less distinctness, into (1) a hypoblastic sheet below, formed of a single row of flattened cells, and (2) a mesoblast plate above formed of stellate cells, while in the middle line there is a strip of stellate cells in which there is no distinct differentiation into two layers.
[Pg 865] Since the region in which these changes take place is that in which the medullary plate becomes subsequently formed, the lateral parts of the mesoblast plate are clearly the permanent lateral plates of the trunk, from which the mesoblastic somites, &c., become subsequently formed; so that the main part of the mesoblast of the trunk is not directly derived from the primitive streak.
Before leaving this stage we would call attention to the presence, in one of our blastoderms of this stage, of a deep pit at the junction of the primitive streak with the region in front of it (Pl. 44, Ser. F, 1 and 2). Such a pit is unusual, but we think it may be regarded as an exceptionally early commencement of that most variable structure in the chick, the neurenteric canal.
The next and last stage we have to deal with is that during which the first trace of the notochord and of the medullary plate make their appearance.
In surface views this stage is marked by the appearance of a faint dark line, extending forwards, from the front end of the primitive streak, to a fold, which has in the mean time made its appearance near the front end of the area pellucida, and constitutes the head fold.
Pl. 45, Ser. K, represents a series of sections through a blastoderm of this stage, which have been selected to illustrate the mode of formation of the notochord.
In a section immediately behind the head fold the median part of the epiblast is thicker than the lateral parts, forming the first indication of a medullary plate (Ser. K, 1). Below the median line of the epiblast is a small cord of cells, not divided into two layers, but continuous laterally, both with the hypoblast and mesoblast, which are still more distinctly separated than in the previous stage.
A section or so further back (Ser. K, 2) the axial cord, which we need scarcely say is the rudiment of the notochord, is thicker, and causes a slight projection in the epiblast above. It is, as before, continuous laterally, both with the mesoblast and with the hypoblast. The medullary plate is more distinct, and a shallow but unmistakable medullary groove has made its appearance.
[Pg 866] As we approach the front end of the primitive streak the notochord becomes (Ser. K, 3) very much more prominent, though retaining the same relation to the germinal layers as in front.
In the section immediately behind (Ser. K, 4) the convex upper surface of the notochord has become continuous with the epiblast for a very small region. The section, in fact, traverses the front end of the primitive streak.
In the next section the attachment between the epiblast and the cells below becomes considerably wider. It will be noticed that this part of the primitive streak is placed on the floor of the wide medullary groove, and there forms a prominence known as the anterior swelling of the primitive streak.
It will further be noticed that in the two sections passing through the primitive streak, the hypoblast, instead of simply becoming continuous with the axial thickening of the cells, as in front, forms a more or less imperfect layer underneath it. This layer becomes in the sections following still more definite, and forms part of the continuous layer of hypoblast present in the region of the primitive streak.
A comparison of this stage with the previous one shews very clearly that the notochord is formed out of the median plate of cells of the earlier stage, which was not divided into mesoblast and hypoblast, together with the short column of cells which grew forwards from the primitive streak.
The notochord, from its mode of origin, is necessarily continuous behind with the axial cells of the primitive streak.
The sections immediately behind the last we have represented shew a rudiment of the neurenteric canal of the same form as that first figured by Gasser, viz. a pit perforating the epiblast with a great mass of rounded cells projecting upwards through it.
* * * * *
The observations just recorded practically deal with two much disputed points in the ontogeny of birds, viz. the origin of the mesoblast and the origin of the notochord.
With reference to the first of these our results are briefly as follows:
The first part of the mesoblast to be formed is that which arises in connection with the primitive streak. This part is in [Pg 867] the main formed by a proliferation from an axial strip of the epiblast along the line of the primitive streak, but in part also from a simultaneous differentiation of hypoblast cells also along the axial line of the primitive streak. The two parts of the mesoblast so formed become subsequently indistinguishable. The second part of the mesoblast to be formed is that which gives rise to the lateral plates of mesoblast of the head and trunk of the embryo. This part appears as two plates—one on each side of the middle line—which arise by direct differentiation from the hypoblast in front of the primitive streak. They are continuous behind with the lateral wings of mesoblast which grow out from the primitive streak, and on their inner side are also at first continuous with the cells which form the notochord.
In addition to the parts of mesoblast, formed as just described, the mesoblast of the vascular area is in a large measure developed by a direct formation of cells round the nuclei of the germinal wall.
The mesoblast formed in connection with the primitive streak gives rise in part to the mesoblast of the allantois, and ventral part of the tail of the embryo (?), and in part to the vascular structures found in the area pellucida.
With reference to the formation of the mesoblast of the primitive streak, our conclusions are practically in harmony with those of Koller; except that Koller is inclined to minimise the share taken by the hypoblast in the formation of the mesoblast of the primitive streak.
Gerlach, with reference to the formation of this part of the mesoblast, adopts the now generally accepted view of Kölliker, according to which the whole of the mesoblast of the primitive streak is derived from the epiblast.
As to the derivation of the lateral plates of mesoblast of the trunk from the hypoblast of the anterior part of the primitive streak, our general result is in complete harmony with Gerlach's results, although in our accounts of the details of the process we differ in some not unimportant particulars.
As to the origin of the notochord, our main result is that this structure is formed as an actual thickening of the primitive hypoblast of the anterior part of the area pellucida. We find [Pg 868] that it unites posteriorly with a forward growth of the axial tissue of the primitive streak, while it is laterally continuous, at first, both with the mesoblast of the lateral plates and with the hypoblast. At a later period its connection with the mesoblast is severed, while the hypoblast becomes differentiated as a continuous layer below it.
As to the hypoblastic origin of the notochord, we are again in complete accord with Gerlach; but we differ from him in admitting that the notochord is continuous posteriorly with the axial tissue of the primitive streak, and also at first continuous with the lateral plates of mesoblast.
The account we have given of the formation of the mesoblast may appear to the reader somewhat fantastic, and on that account not very credible. We believe, however, that if the view which has been elsewhere urged by one of us, that the primitive streak is the homologue of the blastopore of the lower vertebrates is accepted, the features we have described receive an adequate explanation.
The growth outwards of part of the mesoblast from the axial line of the primitive streak is a repetition of the well-known growth from the lips of the blastopore. It might have been anticipated that all the layers would fuse along the line of the primitive streak, and that the hypoblast as well as part of the mesoblast would grow out from it. There is, however, clearly a precocious formation of the hypoblast; but the formation of the mesoblast of the primitive streak, partly from the epiblast and partly from the hypoblast, is satisfactorily explained by regarding the whole structure as the blastopore. The two parts of the mesoblast subsequently become indistinguishable, and their difference in origin is, on the above view, to be regarded as simply due to a difference of position, and not as having a deeper significance.
The differentiation of the lateral plates of mesoblast of the trunk directly from the hypoblast is again a fundamental feature of vertebrate embryology, occurring in all types from Amphioxus upwards, the meaning of which has been fully dealt with in the Treatise on Comparative Embryology by one of us. Lastly, the formation of the notochord from the hypoblast is the typical vertebrate mode of formation of this organ, while [Pg 869] the fusion of the layers at the front end of the primitive streak is the universal fusion of the layers at the dorsal lip of the blastopore, which is so well known in the lower vertebrate types.
EXPLANATION OF PLATES 43-45.
N. B. The series of sections are in all cases numbered from before backwards.
List of Reference Letters.
a.p. Area pellucida. ep. Epiblast. ch. Notochord. gr. Germinal wall. hy. Hypoblast. m. Mesoblast. o.p. Area opaca. pr.g. Primitive groove. pvs. Primitive streak. yk. Yolk of germinal wall.
Plate 43.
Series A, 1 and 2. Sections through the blastoderm before the appearance of primitive streak.
1. Section through anterior part of area pellucida in front of embryonic shield. The hypoblast here forms an imperfect layer. The figure represents about half the section. 2. Section through same blastoderm, in the region of the embryonic shield. Between the epiblast and hypoblast are a number of undifferentiated cells. The figure represents considerably more than half the section.
Series B, 1, 2 and 3. Sections through a blastoderm with a very young primitive streak.
1. Section through the anterior part of the area pellucida in front of the primitive streak. 2. Section through about the middle of the primitive streak. 3. Section through the posterior part of the primitive streak.
Series C, 1 and 2. Sections through a blastoderm with a young primitive streak.
1. Section through the front end of the primitive streak. 2. Section through the primitive streak, somewhat behind 1. Both figures shew very clearly the difference in character between the cells of the epiblastic mesoblast of the primitive streak, and the more granular cells of the mesoblast derived from the hypoblast.
Fig. D. Longitudinal section through the axial line of the primitive streak, and the part of the blastoderm in front of it, of an embryo duck with a well-developed primitive streak.
Plate 44.
Series E, 1, 2, 3 and 4. Sections through blastoderm with a primitive streak, towards the end of the first stage.
1. Section through the anterior part of the area pellucida. 2. Section a little way behind 1 shewing a forward growth of mesoblast from the primitive streak. 3. Section through primitive streak. 4. Section through posterior part of primitive streak, shewing the great widening of primitive streak behind.
[Pg 870] Series F, 1 and 2. Sections through a blastoderm with primitive groove.
1. Section shewing a deep pit in front of primitive streak, probably an early indication of the neurenteric canal. 2. Section immediately following 1.
Fig. G. Section through blastoderm with well-developed primitive streak, shewing an exceptionally deep slit-like primitive groove.
Series H, 1 and 2. Sections through a blastoderm with a fully-developed primitive streak.
1. Section through the anterior part of area pellucida, shewing the cubical granular hypoblast cells in this region. 2. Section slightly behind 1, shewing the primitive hypoblast cells differentiated into stellate cells, which can hardly be resolved in the middle line into hypoblast and mesoblast.
Plate 45.
Series I, 1, 2, 3, 4 and 5. Sections through blastoderm somewhat older than Series H.
1. Section through area pellucida well in front of primitive streak. 2. Section through area pellucida just in front of primitive streak. 3. Section through the front end of primitive streak. 4. Section slightly behind 3. 5. Section slightly behind 4.
Series K, 1, 2, 3, 4 and 5. Sections through a blastoderm in which the first traces of notochord and medullary groove have made their appearance. Rather more than half the section is represented in each figure, but the right half is represented in 1 and 3, and the left in 2 and 4.
1. Section through notochord immediately behind the head fold. 2. Section shewing medullary groove a little behind 1. 3. Section just in front of the primitive streak. 4 and 5. Sections through the front end of the primitive streak.
Fig. L. Surface view of blastoderm with a very young primitive streak.
[559] From the Quarterly Journal of Microscopical Science, Vol. XXII. N. S. 1882.
[560]
Ueb. d. entodermale Entstehungsweise d. Chorda dorsalis,
Biol. Centralblatt, Vol. 1. Nos. 1 and 2.
[561]
Untersuch. üb. d. Blätterbildung im Hühnerkeim,
Archiv f. mikr. Anat. Vol. XX. 1881.
[562]
Photogramme d. Ontogenie d. Vogel.
Nova Acta. K. Leop.
Carol, Deutschen Akad. d. Naturfor. Bd. X. 41, 1879.
[563]
Beitr. z. Kenntuiss d. Hühmerkeims im Beginne d.
Bebrütung,
Sitz. d. k. Akad. Wiss. IV. Abth. 1879.
[564] Die erste Entwick. an Eier d. Reptilien. Königsberg. 1878.
(With Plates 46-53.)
The late Professor Balfour was engaged just before his death in investigating the structure and embryology of Peripatus capensis, with the view of publishing a complete monograph of the genus. He left numerous drawings intended to serve as illustrations to the monograph, together with a series of notes and descriptions of a large part of the anatomy of Peripatus capensis. Of this manuscript some portions were ready for publication, others were more or less imperfect; while of the figures many were without references, and others were provided with only a few words of explanation.
It was obviously necessary that Professor Balfour's work—embodying as it did much important discovery—should be published without delay; and the task of preparing his material for the press was confided to us. We have printed all his notes and descriptions without alteration[566]. Explanations which appeared to be necessary, and additions to the text in cases in which he had prepared figures without writing descriptions, together with full descriptions of all the plates, have been added by us, and are distinguished by enclosure in square brackets[567].
We have to thank Miss Balfour, Professor Balfour's sister, for the important service which she has rendered by preparing [Pg 872] a large part of the beautiful drawings with which the monograph is illustrated. Many of these had been executed by her under Professor Balfour's personal supervision; and the knowledge of his work which she then acquired has been of the greatest assistance to us in preparing the MSS. and drawings for publication.
Since his death she has spared no pains in studying the structure of Peripatus, so as to enable us to bring out the first part of the monograph in as complete a state as possible. It is due to her skill that the first really serviceable and accurate representation of the legs of any species of Peripatus available for scientific purposes are issued with the present memoir[568].
We have purposely refrained from introducing comments on the general bearing of the new and important results set forth in this memoir, and have confined ourselves to what was strictly necessary for the presentation of Mr Balfour's discoveries in a form in which they could be fully comprehended.
Mr Balfour had at his disposal numerous specimens of Peripatus novæ
zealandiæ, collected for him by Professor Jeffrey Parker, of
Christchurch, New Zealand; also specimens from the Cape of Good Hope
collected by Mr Lloyd Morgan, and brought to England by Mr Roland Trimen in
1881; and others given to him by Mr Wood Mason, together with all the
material collected by Mr Moseley during the Challenger
voyage.
A preliminary account of the discoveries as to the embryology of Peripatus has already been communicated to the Royal Society[569]. It is intended that the present memoir shall be followed by others, comprising a complete account of all the species of the genus Peripatus.
H. M. Moseley.
A. Sedgwick.
[565] From the Quarterly Journal of Microscopical Science, April, 1883.
[566] Excepting in an unimportant matter of change of nomenclature used with regard to the buccal cavity.
[567] The account of the external characters, generative organs, and develop ment, has been written by the editors.
[568] The drawings on Pl. 47, figs. 9 and 10 on Pl. 48, and the drawings of the embryos (except fig. 37), have been made by Miss Balfour since Professor Balfour's death.
[569] Proc. Royal Soc. 1883.
Peripatus capensis (fig. 1).
[The body is elongated, and slightly flattened dorso-ventrally. The dorsal surface is arched, and darkly pigmented; while the ventral surface is nearly flat, and of a lighter colour.
The mouth is placed at the anterior end of the body, on the ventral surface.
The anus is posterior and terminal.
The generative opening is single and median, and placed in both sexes on the ventral surface, immediately in front of the anus.
There are a pair of ringed antennæ projecting from the anterior end of the head, and a pair of simple eyes, placed on the dorsal surface at the roots of the antennæ.
The appendages of the body behind the antennæ are disposed in twenty pairs.
1. The single pair of jaws placed within the buccal cavity in front of the true mouth opening, and consisting each of a papilla, armed at its termination with two cutting blades.
2. The oral papillæ placed on each side of the mouth. At their apices the ducts of the slime glands open.
3. The seventeen pairs of ambulatory appendages, each provided with a pair of chitinous claws at its extremity.
4. The anal papillæ placed on each side of the generative opening.
Colour.—The following statements on this head are derived from observations of spirit specimens. The colour varies in different individuals. It always consists of a groundwork of green and bluish grey, with a greater or less admixture of brown. The chief variations in the appearance of the animal, so far as colour is concerned, depend on the shade of the green. In some it is dark, as in the specimen figured (fig. 1); in others it is of a lighter shade.
There is present in most specimens a fairly broad light band on each side of the body, immediately dorsal to the attachment [Pg 874] of the legs. This band is more prominent in the lighter coloured varieties than in the dark, and is especially conspicuous in large individuals. It is due to a diminution in the green pigment, and an increase in the brown.
There is a dark line running down the middle of the dorsal surface, in the middle of which is a fine whitish line.
The ventral surface is almost entirely free from the green pigment, but possesses a certain amount of light brown. This brown pigment is more conspicuous and of a darker shade on the spinous pads of the foot.
In parts of the body where the pigment is scarce, it is seen to be confined to the papillæ. This is especially evident round the mouth, where the sparse green pigment is entirely confined to the papillæ.
In some specimens a number of white papillæ, or perhaps light brown, are scattered over the dorsal surface; and sometimes there is a scattering of green papillæ all over the ventral surface. These two peculiarities are more especially noticeable in small specimens.
Ridges and Papillæ of the Skin.—The skin is thrown into a number of transverse ridges, along which the primary wart-like papillæ are placed.
The papillæ, which are found everywhere, are specially developed on the dorsal surface, less so on the ventral. The papillæ round the lips differ from the remaining papillæ of the ventral surface in containing a green pigment. Each papilla bears at its extremity a well-marked spine.
The ridges of the skin are not continued across the dorsal middle line, being interrupted by the whitish line already mentioned. Those which lie in the same transverse line as the legs are not continued on to the latter, but stop at the junction of the latter with the body. All the others pass round to the ventral surface and are continued across the middle line; they do not, however, become continuous with the ridges of the other side, but passing between them gradually thin off and vanish.
The ridges on the legs are directed transversely to their long axes, i.e. are at right angles to the ridges of the rest of the body.
[Pg 875] The antennæ are ringed and taper slightly till near their termination, where they present a slight enlargement in spirit specimens, which in its turn tapers to its termination.
The rings consist essentially of a number of coalesced primary papillæ, and are, therefore, beset by a number of spines like those of the primary papillæ (described below). They are more deeply pigmented than the rest of the antenna.
The free end of the antenna is covered by a cap of tissue like that of the rings. It is followed by four or more rings placed close together on the terminal enlargement. There appears to be about thirty rings on the antennæ of all adults of this species. But they are difficult to count, and a number of small rings occur between them, which are not included in the thirty.
The antennæ are prolongations of the dorso-lateral parts of the anterior end of the body.
The eyes are paired and are situated at the roots of the antennæ on the dorso-lateral parts of the head. Each is placed on the side of a protuberance which is continued as the antenna, and presents the appearance of a small circular crystalline ball inserted on the skin in this region.
The rings of papillæ on that part of the head from which the antennæ arise lose their transverse arrangement. They are arranged concentrically to the antennal rings, and have a straight course forwards between the antennæ.
The oral papillæ are placed at the side of the head. They are attached ventro-laterally on each side of the lips. The duct of the slime gland opens through their free end. They possess two main rings of projecting tissue, which are especially pigmented on the dorsal side; and their extremities are covered by papillæ irregularly arranged.
The buccal cavity, jaws, and lips are described below.
The Ambulatory Appendages.—The claw-bearing legs are usually seventeen in number; but in two cases of small females we have observed that the anal papillæ bear claws, and present all the essential features of the ambulatory appendages. In one small female specimen there were twenty pairs of claw-bearing appendages, the last being like the claw-bearing anal papillæ last mentioned, and the generative opening being placed between them.
[Pg 876] The ambulatory appendages, with the exception of the fourth and fifth pairs in both sexes, and the last pair (seventeenth) in the male, all resemble each other fairly closely. A typical appendage (figs. 2 and 3) will first be described, and the small variations found in the appendages just mentioned will then be pointed out. Each consists of two main divisions, a larger proximal portion, the leg, and a narrow distal claw-bearing portion, the foot.
The leg has the form of a truncated cone, the broad end of which is attached to the ventro-lateral body-wall, of which it appears to be, and is, a prolongation. It is marked by a number of rings of primary papillæ, placed transversely to the long axis of the leg, the dorsal of which contain a green and the ventral a brown pigment. These rings of papillæ, at the attachment of the leg, gradually change their direction and merge into the body rings. At the narrow end of the cone there are three ventrally placed pads, in which the brown pigment is dark, and which are covered by a number of spines precisely resembling the spines of the primary papillæ. These spinous pads are continued dorsally, each into a ring of papillæ.
The papillæ of the ventral row next the proximal of these spinous pads are intermediate in character between the primary papillæ and the spinous pads. Each of these papillæ is larger than a normal papilla, and bears several spines (fig. 2). This character of the papilla of this row is even more marked in some of the anterior legs than in the one figured; it seems probable that the pads have been formed by the coalescence of several rows of papillæ on the ventral surface of the legs. On the outer and inner sides of these pads the spines are absent, and secondary papillæ only are present.
In the centre of the basal part of the ventral surface of the foot there are present a group of larger papillæ, which are of a slightly paler colour than the others. They are arranged so as to form a groove, directed transversely to the long axis of the body, and separated at its internal extremity by a median papilla from a deep pit which is placed at the point of junction of the body and leg. The whole structure has the appearance, when viewed with the naked eye, of a transverse slit placed at the base of the leg. The segmental organs open by the deep pit placed [Pg 877] at the internal end of this structure. The exact arrangement of the papillæ round the outer part of the slit does not appear to be constant.
The foot is attached to the distal end of the leg. It is slightly narrower at its attached extremity than at its free end, which bears the two claws. The integument of the foot is covered with secondary papillæ, but spines and primary papillæ are absent, except at the points now to be described.
On each side of the middle ventral line of the proximal end of the foot is placed an elliptical elevation of the integument covered with spines. Attached to the proximal and lateral end of this is a primary papilla. At the distal end of the ventral side of the foot on each side of the middle line is a group of inconspicuous pale elevations, bearing spines.
On the front side of the distal end of the foot, close to the socket in which the claws are placed, are two primary papillæ, one dorsal and the other ventral.
On the posterior side of the foot the dorsal of these only is present. The claws are sickle-shaped, and placed on papillæ on the terminal portion of the foot. The part of the foot on which they are placed is especially retractile, and is generally found more or less telescoped into the proximal part (as in the figure).
The fourth and fifth pairs of legs exactly resemble the others, except in the fact that the proximal pad is broken up into three, a small central and two larger lateral. The enlarged segmental organs of these legs open on the small central division.
The last (17) leg of the male (Pl. 47, fig. 4) is characterized by possessing a well-marked white papilla on the ventral surface. This papilla, which presents a slit-like opening at its apex, is placed on the second row of papillæ counting from the innermost pad, and slightly posterior to the axial line of the leg.
The anal papillæ, or as they should be called, generative papillæ, are placed one on each side of the generative aperture. They are most marked in small and least so in large specimens. That they are rudimentary ambulatory appendages is shewn by the fact that they are sometimes provided with claws, and resemble closely the anterior appendages.]
The alimentary canal of Peripatus capensis forms, in the extended condition of the animal, a nearly straight tube, slightly longer than the body, the general characters of which are shewn in figs. 6 and 7.
For the purposes of description, it may conveniently be divided into five regions, viz. (1) the buccal cavity with the tongue, jaws, and salivary glands, (2) pharynx, (3) the œsophagus, (4) the stomach, (5) the rectum.
The Buccal Cavity.—The buccal cavity has the form of a fairly deep pit, of a longitudinal oval form, placed on the ventral surface of the head, and surrounded by a tumid lip.
[The buccal cavity has been shewn by Moseley to be formed in the embryo by the fusion of a series of processes surrounding the true mouth-opening, and enclosing in their fusion the jaws.]
The lip is covered by a soft skin, in which are numerous organs of touch, similar to those in other parts of the skin having their projecting portions enclosed in delicate spines formed by the cuticle. The skin of the lips differs, however, from the remainder of the skin, in the absence of tubercles, and in the great reduction of the thickness of the dermis. It is raised into a series of papilliform ridges, whose general form is shewn in fig. 5; of these there is one unpaired and median behind, and a pair, differing somewhat in character from the remainder, in front, and there are, in addition, seven on each side.
The structures within the buccal cavity are shewn as they appear in surface views in figs. 5 and 7, but their real nature is best seen in sections, and is illustrated by Pl. 49, figs. 11 and 12, representing the oral cavity in transverse section, and by Pl. 49, figs. 17 and 18, representing it in horizontal longitudinal sections. In the median line of the buccal cavity in front is placed a thick muscular protuberance, which may perhaps conveniently be called the tongue, though attached to the dorsal instead of the ventral wall of the mouth. It has the form of an elongated [Pg 879] ridge, which ends rather abruptly behind, becoming continuous with the dorsal wall of the pharynx. Its projecting edge is armed by a series of small teeth, which are thickenings of the chitinous covering, prolonged from the surface of the body over the buccal cavity. Where the ridge becomes flatter behind, the row of teeth divides into two, with a shallow groove between them (Pl. 48, fig. 7).
The surface of the tongue is covered by the oral epithelium, in parts of which are organs of special sense, similar to those in the skin; but its interior is wholly formed of powerful muscles. The muscles form two groups, intermingled amongst each other. There are a series of fibres inserted in the free edge of the tongue, which diverge, more or less obliquely, towards the skin at the front of the head anteriorly, and towards the pharynx behind. The latter set of fibres are directly continuous with the radial fibres of the pharynx. The muscular fibres just described are clearly adapted to give a sawing motion to the tongue, whose movements may thus, to a certain extent, be compared to those of the odontophore[TN21] of a mollusc.
In addition to the above set of muscles, there are also transverse muscles, forming laminæ between the fibres just described. They pass from side to side across the tongue, and their action is clearly to narrow it, and so cause it to project outwards from the buccal cavity.
On each side of the tongue are placed the jaws, which are, no doubt, a pair of appendages, modified in the characteristic arthropodan manner, to subserve mastication. Their structure has never been satisfactorily described, and is very complicated. They are essentially short papillæ, moved by an elaborate and powerful system of muscles, and armed at their free extremities by a pair of cutting blades or claws. The latter structures are, in all essential points, similar to the claws borne by the feet, and, like these, are formed as thickenings of the cuticle. They have therefore essentially the characters of the claws and jaws of the Arthropoda, and are wholly dissimilar to the setæ of Chætopoda. The claws are sickle-shaped and, as shewn in Pl. 47, fig. 5, have their convex edge directed nearly straight forwards, and their concave or cutting edge pointed backwards. Their form differs somewhat in the different species, and, as will [Pg 880] be shewn in the systematic part of this memoir[570], forms a good specific character. In Peripatus capensis (Pl. 48, fig. 10) the cutting surface of the outer blade is smooth and without teeth, while that of the inner blade (fig. 9), which is the larger of the two, is provided with five or six small teeth, in addition to the main point. A more important difference between the two blades than that in the character of the cutting edge just spoken of, is to be found in their relation to the muscles which move them. The anterior parts of both blades are placed on two epithelial ridges, which are moved by muscles common to both blades (Pl. 49, fig. 11). Posteriorly, however, the behaviour of the two blades is very different. The epithelial ridge bearing the outer blade is continued back for a short distance behind the blade, but the cuticle covering it becomes very thin, and it forms a simple epithelial ridge placed parallel to the inner blade. The cuticle covering the epithelial ridge of the inner blade is, on the contrary, prolonged behind the blade itself as a thick rod, which, penetrating backwards along a deep pocket of the buccal epithelium, behind the main part of the buccal cavity for the whole length of the pharynx, forms a very powerful lever, on which a great part of the muscles connected with the jaws find their insertion. The relations of the epithelial pocket bearing this lever are somewhat peculiar.
The part of the epithelial ridge bearing the proximal part of this lever is bounded on both its outer and inner aspect by a deep groove. The wall of the outer groove is formed by the epithelial ridge of the outer blade, and that of the inner by a special epithelial ridge at the side of the tongue. Close to the hinder border of the buccal cavity (as shewn in Pl. 49, fig. 12, on the right hand side), the outer walls of these two grooves meet over the lever, so as completely to enclose it in an epithelial tube, and almost immediately behind this point the epithelial tube is detached from the oral epithelium, and appears in section as a tube with a chitinous rod in its interior, lying freely in the body-cavity (shewn in Pl. 49, figs. 13-16, le). This apparent tube is the section of the deep pit already spoken of. It may [Pg 881] be traced back even beyond the end of the pharynx, and serves along its whole length for the attachment of muscles.
The greater part of the buccal cavity is filled with the tongue and jaws just described. It opens dorsally and behind by the mouth into the pharynx, there being no sharp line of demarcation between the buccal cavity and the pharynx. Behind the opening into the pharynx there is a continuation of the buccal cavity shewn in transverse section in fig. 13, and in longitudinal and horizontal section in fig. 17, into which there opens the common junction of the two salivary glands. This diverticulum is wide at first and opens by a somewhat constricted mouth into the pharynx above (Pl. 49, fig. 13, also shewn in longitudinal and horizontal section in fig. 17). Behind it narrows, passing insensibly into what may most conveniently be regarded as a common duct for the two salivary glands (Pl. 49, fig. 17).
The Salivary Glands.—These two bodies were originally described by Grube, by whom their nature was not made out, and subsequently by Moseley, who regarded them as fat bodies. They are placed in the lateral compartments of the body-cavity immediately dorsal to the ventral nerve cords, and extend for a very variable distance, sometimes not more than half the length of the body, and in other instances extending for nearly its whole length. Their average length is perhaps about two-thirds that of the body. Their middle portion is thickest, and they thin off very much behind and to a slight extent in front. Immediately behind the mouth and in front of the first pair of legs, they bend inwards and downwards, and fall (fig. 7) one on each side into the hind end of the narrow section of the oral diverticulum just spoken of as the common duct for the two salivary glands. The glandular part of these organs is that extending back from the point where they bend inwards. This part (fig. 16) is formed of very elongated cells supported by a delicate membrana propria. The section of this part is somewhat triangular, and the cells are so long as to leave a comparatively small lumen. The nuclei of the cells are placed close to the supporting membrane, and the remainder of the cells are filled with very closely packed secretory globules, which have a high index of refraction. It was the presence of these globules which probably led Moseley to regard the salivary glands as fat [Pg 882] bodies. The part of each gland which bends inwards must be regarded as the duct.
The cells lining the ducts are considerably less columnar than those of the gland proper. Their nuclei (fig. 14) are situated at the free extremities instead of at the base of the cells, and they are without secretory globules. The cells lining the ducts of the salivary glands pass, without any sharp line of demarcation, into those of the oral epithelium, which are flatter and have their nuclei placed in the middle.
The Pharynx.—The Pharynx is a highly muscular tube (fig. 7) with a triangular lumen (figs. 14, 15), which extends from the mouth to about half way between the first and second pair of legs. It is lined by a flattish epithelium bounded by a cuticle continuous with that of the mouth. On the dorsal side is a ridge projecting into the lumen of the pharynx. This ridge may be traced forwards (Pl. 49, figs. 11-14) into the tongue, and the two grooves at the side of this ridge, forming the two upper angles of the triangular lumen, may be followed into those at the sides of the tongue. The muscles of the pharynx are very highly developed, consisting of an intrinsic and an extrinsic set. The former consists, as is best seen in longitudinal sections, of (Pl. 51, fig. 23) radial fibres, arranged in somewhat wedge-shaped laminæ, between which are rings of circular fibres. The latter are thicker externally than internally, and so also appear wedge-shaped in longitudinal sections. Very characteristic of the pharynx are the two sympathetic nerves placed close to the two dorsal angles of the triangular lumen (fig. 14, sy).
The pharynx of Peripatus is interesting in that it is unlike, so far as I know, the pharynx of any true Arthropod, in all of which the region corresponding with the pharynx of Peripatus is provided with relatively very thin walls.
The pharynx of Peripatus has, on the other hand, a very close and obvious resemblance to that of many of the Chætopoda, a resemblance which is greatly increased by the characteristic course of the sympathetic nerves.
The form of the lumen, as already pointed out by Grube, resembles that of the Nematoda.
The Œsophagus.—Behind the pharynx there follows a narrow œsophagus (fig. 7, œ) shewn in section in fig. 16. It has somewhat [Pg 883] folded and fairly thick walls, and lies freely in the central division of the body-cavity without any mesenteric support. Its walls are formed of five layers, viz. from without inwards.
(1) A peritoneal investment.
(2) A layer of longitudinal fibres.
(3) A layer of circular fibres, amongst which are numerous nuclei.
(4) A connective-tissue layer supporting (5) a layer of fairly columnar hyaline epithelium, bounded on its inner aspect by a cuticle continued from that of the pharynx. In front it passes insensibly into the pharynx, and beyond the region where the dorsal walls of the pharynx have clearly commenced, the ventral walls still retain the characters of the œsophageal walls. The œsophagus is vertically oval in front, but more nearly circular behind. Characteristic of the œsophagus is the junction of the two sympathetic nerves on its dorsal wall (fig. 16). These nerves cannot be traced far beyond their point of junction.
The Stomach.—The next section of the alimentary tract is the stomach or mesenteron (fig. 6). It is by far the largest part of the alimentary tract, commencing at about the second pair of legs and extending nearly to the hind end of the body. It tapers both in front and behind, and is narrowest in the middle, and is marked off sharply both from the œsophagus in front and the rectum behind, and is distinguished from both of these by its somewhat pinker hue. In the retracted condition of the animal it is, as pointed out by Moseley, folded in a single short dorsal loop, at about the junction of its first with its second third, and also, according to my observations, at its junction with the rectum; but in the extended condition it is nearly straight, though usually the posterior fold at the junction of the rectum is not completely removed. Its walls are always marked by plications which, as both Moseley and Grube have stated, do not in any way correspond with the segmentation of the body. In its interior I have frequently found the chitinous remains of the skins of insects, so that we are not justified in considering that the diet is purely vegetable. It lies free, and is, like the remainder of the alimentary tract, without a mesentery. The structure of the walls of the stomach has not hitherto been very satisfactorily described.
[Pg 884] The connective tissue and muscular coats are extremely thin. There is present everywhere a peritoneal covering, and in front a fairly well-marked though very thin layer of muscles formed of an external circular and an internal longitudinal layer. In the middle and posterior parts, however, I was unable to recognize these two layers in section; although in surface view Grube found an inner layer of circular fibres and an outer layer formed of bands of longitudinal fibres, which he regards as muscular.
The layer supporting the epithelium is reduced to a basement membrane. The epithelial part of the wall of the stomach is by far the thickest (fig. 20), and is mainly composed of enormously elongated, fibre-like cells, which in the middle part of the stomach, where they are longest, are nearly half a millimètre in length, and only about .006 mm. in breadth. Their nuclei, as seen in fig. 20, are very elongated, and are placed about a quarter of the length from the base.
The cells are mainly filled with an immense number of highly refracting spherules, probably secretory globules, but held by Grube, from the fact of their dissolving in ether, to be fat. The epithelial cells are raised into numerous blunt processes projecting into the lumen of the stomach.
In addition to the cells just described there are present in the anterior part of the stomach a fair sprinkling of mucous cells. There are also everywhere present around the bases of the columnar cells short cells with spherical nuclei, which are somewhat irregularly scattered in the middle and posterior parts of the stomach, but form in the front part a definite layer. I have not been able to isolate these cells, and can give no account of their function.
The rectum extends from the end of the stomach to the anus. The region of junction between the stomach and the rectum is somewhat folded. The usual arrangement of the parts is shewn in fig. 6, where the hind end of the stomach is seen to be bent upon itself in a U-shaped fashion, and the rectum extending forwards under this bent portion and joining the front end of the dorsal limb of the U. The structure of the walls of the rectum is entirely different to that of the stomach, and the transition between the two is perfectly sudden. [Pg 885] Within the peritoneal investment comes a well-developed muscular layer with a somewhat unusual arrangement of its layers, there being an external circular layer and an internal layer formed of isolated longitudinal bands. The epithelium is fairly columnar, formed of granular cells with large nuclei, and is lined by a prolongation of the external cuticle. It is raised into numerous longitudinal folds, which are visible from the surface, and give a very characteristic appearance to this part of the alimentary tract. The muscular layers do not penetrate into the epithelial folds, which are supported by a connective tissue layer.
The central nervous system consists of a pair of supra-œsophageal ganglia united in the middle line, and of a pair of widely divaricated ventral cords, continuous in front with the supra-œsophageal ganglia.
It will be convenient in the first instance to deal with the general anatomy of the nervous system and then with the histology.
Ventral Cords.—The ventral cords at first sight appear to be without ganglionic thickenings, but on more careful examination they are found to be enlarged at each pair of legs (Pl. 48, fig. 8). These enlargements may be regarded as imperfect ganglia. There are, therefore, seventeen such pairs of ganglia corresponding to the seventeen pairs of legs. There is in addition a ganglionic enlargement at the commencement of the œsophageal commissures, where the nerves to the oral papillæ are given off (Pl. 51, fig. 22, or.g.), and the region of junction between the œsophageal commissures with the supra-œsophageal ganglia, where another pair of nerves are given off to the jaws (Pl. 51, fig. 22, jn), may be regarded as the anterior ganglion of the ventral cords. There are, therefore, according to the above reckoning, nineteen pairs of ganglia connected with the ventral cords.
The ventral cords are placed each in the lateral compartments of the body-cavity, immediately within the longitudinal layer of muscles.
[Pg 886] They are connected with each other, rather like the pedal nerves of Chiton and the lower Prosobranchiata, by a number of commissures. These commissures exhibit a fairly regular arrangement from the region included between the first and the last pair of true feet. There are nine or ten of them between each pair of feet (Pl. 52, fig. 26). They pass along the ventral wall of the body, perforating the ventral mass of longitudinal muscles. On their way they give off nerves which innervate the skin.
In Peripatus novæ zealandiæ, and probably also in P. capensis, two of these nerves, coming off from each pair of ganglia, are distinguished from the remainder by the fact that they are provided with numerous nerve-cells, instead of being composed of nerve-fibres only, like the remaining commissures (Pl. 52, fig. 26 gco). In correlation with the nerves given off from them to the skin the commissures are smaller in the middle than at the two ends.
Posteriorly the two nerve-cords nearly meet immediately in front of the generative aperture, and between this aperture and the last pair of feet there are about six commissures passing between them (Pl. 48, fig. 8). Behind the generative aperture the two cords bend upwards, and, as is shewn in fig. 8, fall into each other dorsally to the rectum. The section of the two cords placed dorsally to the rectum is solely formed of nerve-fibres; the nerve-cells, present elsewhere, being here absent.
In front of the ganglion of the first foot the commissures have a more dorsal situation than in the remainder of the body. The median longitudinal ventral muscle here gradually thins out and comes to an end, while the commissures pass immediately below the wall of the pharynx (Pl. 49, figs. 14, 15). The ventral cords themselves at first approach very close to each other in this region, separating again, however, to envelope between them the pharynx (Pl. 51, fig. 22).
There are eleven commissures in front of the first pair of legs (Pl. 51, fig. 22). The three foremost of these are very close together, the middle one arising in a more ventral position than the other two, and joining in the median ventral line a peculiar mass of cells placed in contact with the oral epithelium (fig. 14). It is probably an organ of special sense.
[Pg 887] The ventral cords give off a series of nerves from their outer borders, which present throughout the trunk a fairly regular arrangement. From each ganglion two large nerves (figs. 8, 22, 26) are given off, which, diverging somewhat from each other, pass into the feet, and, giving off branches on their way, may be traced for a considerable distance within the feet along their anterior and posterior borders.
In front of each of the pair of pedal nerves a fairly large nerve may be seen passing outwards towards the side of the body (fig. 22). In addition to this nerve there are a number of smaller nerves passing off from the main trunk, which do not appear to be quite constant in number, but which are usually about seven or eight. Similar nerves to those behind are given off from the region in front of the first pair of legs, while at the point where the two ventral cords pass into the œsophageal commissures two large nerves (fig. 22), similar to the pairs of pedal nerves, take their origin. These nerves may be traced forwards into the oral papillæ, and are therefore to be regarded as the nerves of these appendages. On the ventral side of the cords, where they approach most closely, between the oral papillæ and the first pair of legs, a number of small nerves are given off to the skin, whose distribution appears to be to the same region of the skin as that of the branches from the commissures behind the first pair of legs.
From the œsophageal commissures, close to their junction with the supra-œsophageal ganglia, a nerve arises on each side which passes to the jaws, and a little in front of this, apparently from the supra-œsophageal ganglion itself, a second nerve to the jaws also takes its origin (Pl. 51, fig. 22, jn). These two nerves I take to be homologous with a pair of pedal nerves.
Between the nerves to the jaws and those to the oral papillæ a number of small nerves take their origin. Three of these on each side pass in a dorsal direction and one or two in a ventral one.
The Supra-œsophageal Ganglia.—The supra-œsophageal ganglia (figs. 8 and 22) are large, somewhat oval masses, broader in front than behind, completely fused in the middle, but free at their extremities. Each of them is prolonged anteriorly into an antennary nerve, and is continuous behind with one of the [Pg 888] œsophageal commissures. On the ventral surface of each, rather behind the level of the eye, is placed a very peculiar protuberance (fig. 22, d), of which I shall say more in dealing with the histology of the nervous system.
A number of nerves arise from the supra-œsophageal ganglia, mainly from their dorsal surface.
In front are the immense antennary nerves extending along the whole length of each antenna, and giving off numerous lateral twigs to the sense organs. Near the origin of the antennary nerves, and rather on the dorsal surface, there spring a few small twigs, which pass to the skin, and are presumably sensory. The largest of them is shewn in Pl. 50, fig. 19A. About one-third of the way back the two large optic nerves take their origin, also arising laterally, but rather from the dorsal surface (Pl. 50, fig. 19D and E). Each of them joins a large ganglionic mass placed immediately behind the retina. Nearly on a level with the optic nerves and slightly nearer the middle dorsal line a pair of small nerves (fig. 19D) spring from the brain and pass upwards, while nearly in the same line with the optic nerves and a little behind them a larger pair of nerves take their origin.
Behind all these nerves there arises from the line of suture between the two supra-œsophageal ganglia a large median nerve which appears to supply the integument of the dorsal part of the head (Pl. 48, fig. 8; Pl. 49, figs. 11-14, dn).
Sympathetic System.—In addition to the nerves just described there are two very important nerves which arise near the median ventral line, close to the hind end of the supra-œsophageal ganglia. The origin of these two nerves is shewn in the surface view (fig. 22, sy, and in section in fig. 11). They at first tend somewhat forwards and pass into the muscles near the epithelium lining the groove on each side of the tongue. Here they suddenly bend backwards again and follow the grooves into the pharynx.
The two grooves are continuous with the two dorsal angles of the pharynx; and embedded in the muscles of the pharynx, in juxtaposition with the epithelium, these two nerves may easily be traced in sections. They pass backwards the whole length of the pharynx till the latter joins the œsophagus. [Pg 889] Here they at once approach and shortly meet in the median dorsal line (fig. 16). They can only be traced for a very short distance beyond their meeting point. These nerves are, without doubt, the homologues of the sympathetic system of Chætopods, occupying as they do the exact position which Semper has shewn to be characteristic of the sympathetic nerves in that group, and arising from an almost identical part of the brain[571].
Histology of the Nervous System.
Ventral Cords.—The histology of the ventral cords and œsophageal commissures is very simple and uniform. They consist of a cord almost wholly formed of nerve-fibres, placed dorsally, and a ventral layer of ganglion cells (figs. 16 and 20).
The fibrous portion of the cord has the usual structure, being formed mainly of longitudinal fibres, each probably being a bundle of fibres of various sizes, enveloped in a sponge-work of connective tissue. The larger bundles of fibres are placed near the inner borders of the cords. In this part of the cord there are placed a very small number of ganglion cells.
The layer of ganglion cells is somewhat crescent-shaped in section, and, as shewn in figs. 16 and 20, envelopes the whole ventral aspect of the fibrous parts of the cord, and even creeps up slightly on to the dorsal side. It is thicker on the inner than on the outer side, and increases considerably in bulk at each ganglionic enlargement. The cells of which it is composed are for the most part of a nearly uniform size, but at the border of the fibrous matter a fair sprinkling of larger cells is found.
The tracheal vessels supplying the nervous system are placed amongst the larger cells, at the boundary between the ganglionic and fibrous regions of the cords.
With reference to the peripheral nerve-stems there is not much to be said. They have for the most part a similar structure to the fibrous parts of the main cord, but are provided with a somewhat larger number of cells.
[Pg 890]Sheath of the Ventral Cords.—The ventral cords are enveloped by a double sheath, the two layers of which are often in contact, while in other cases they may be somewhat widely separated from each other. The inner layer is extremely thin and always very closely envelopes the nerve-cords. The outer layer is thick and fibrous, and contains a fair sprinkling of nuclei.
Supra-œsophageal Ganglia.—In the present state of our knowledge a very detailed description of the histology of the supra-œsophageal ganglia would be quite superfluous, and I shall confine myself to a description of the more obvious features in the arrangement of the ganglionic and fibrous portions (Pl. 50, fig. 19A-G).
The ganglion cells are in the first place confined, for the most part, to the surface. Along the under side of each ganglion there is a very thick layer of cells, continuous behind, with the layer of ganglion cells which is placed on the under surface of the œsophageal commissures. These cells have, moreover, an arrangement very similar to that in the ventral cords, so that a section through the supra-œsophageal ganglia has an obvious resemblance to what would be the appearance of a section through the united ventral cords. On the outer borders of the ganglia the cells extend upwards, but they end on about the level of the optic nerve (fig. 19D). Immediately dorsal to this point the fibrous matter of the brain is exposed freely on the surface (fig. 19A, B, &c., a). I shall call the region of fibrous matter so exposed the dorso-lateral horn of white matter.
Where the two ganglia separate in front the ganglion cells spread up the inner side, and arch over so as to cover part of the dorsal side. Thus, in the anterior part, where the two ganglia are separate, there is a complete covering of ganglionic substance, except for a narrow strip, where the dorso-lateral lobe of white matter is exposed on the surface (fig. 19A). From the point where the two ganglia meet in front the nerve-cells extend backwards as a median strip on the dorsal surface (fig. 19D and E). This strip, becoming gradually smaller behind, reaches nearly, though not quite, the posterior limit of the junction of the ganglia. Behind it there is, however, a region where [Pg 891] the whole dorsal surface of the ganglia is without any covering of nerve-cells.
This tongue of ganglion cells sends in, slightly behind the level of the eyes, a transverse vertical prolongation inwards into the white matter of the brain, which is shewn in the series of transverse sections in fig. 19E, and also in the vertical longitudinal section (Pl. 51, fig. 21), and in horizontal section in Pl. 51, fig. 22.
On the ventral aspect of each lobe of the brain there is present a very
peculiar, bluntly conical protuberance of ganglion cells (Pl. 51, fig. 22), which was first detected by Grube (No. 10), and described by him as a white thick
body of a regular tetrahedral form, and exhibiting an oval dark spot in the
middle of two of the faces.
He further states that it is united by a
delicate nerve to the supra-œsophageal ganglion, and regards it as an
organ of hearing.
In Peripatus capensis the organ in question can hardly be described as tetrahedral. It is rather of a flattened oval form, and consists, as shewn in sections (Pl. 50, fig. 19C and D, d), mainly of ganglion cells. In its interior is a cavity with a distinct bounding membrane: the cells of which it is composed vary somewhat in size, being smallest near the point of attachment. At its free end is placed a highly refractive, somewhat oval body, probably forming what Grube describes as a dark spot, half embedded in its substance, and kept in place by the sheath of nervous matter surrounding it. This body appears to have fallen out in my sections. The whole structure is attached to the under surface of the brain by a very short stalk formed of a bundle of cells and nervous fibres.
It is difficult to offer any interpretation of the nature of this body. It is removed considerably from the surface of the animal, and is not, therefore, so far as I can see, adapted to serve as an organ of hearing.
The distribution of the white or fibrous matter of the ganglia is not very easy to describe.
There is a central lobe of white matter (fig. 19E), which is continuous from ganglion to ganglion, where the two are united. It is smaller behind than in front. On its ventral side it exhibits fairly well-marked transverse commissural fibres, connecting [Pg 892] the two halves of the ganglion. Laterally and somewhat ventrally it is prolonged into a horn (fig. 19D, E, b), which I propose calling the ventro-lateral horn. In front it is placed in a distinct protuberance of the brain, which is placed ventrally to and nearly in the same vertical plane as the optic nerve. This protuberance is best shewn in the view of the brain from below given in Pl. 51, fig. 22. This part of the horn is characterized by the presence of large vertically-directed bundles of nerve-fibres, shewn in transverse section in fig. 19 D. Posteriorly the diameter of this horn is larger than in front (fig. 19E, F, G), but does not give rise to a protuberance on the surface of the brain owing to the smaller development of the median lobe behind.
The median lobe of the brain is also prolonged into a dorso-lateral lobe (fig. 19, a), which, as already mentioned, is freely exposed on the surface. On its ventral border there springs the optic nerve, and several pairs of sensory nerves already described (fig. 19D, E), while from its dorsal border a pair of sensory nerves also spring, nearly in the same vertical plane as the optic nerves.
Posteriorly where the dorsal surface of the brain is not covered in with ganglion cells the dorso-lateral horn and median lobe of the brain become indistinguishable.
In the front part of the brain the median lobe of white matter extends dorsalwards to the dorsal strip of ganglion cells, but behind the region of the transverse prolongation of these cells, into the white matter already described (p. 890), there is a more or less distinctly defined lobe of white matter on the dorsal surface, which I propose calling the postero-dorsal lobe of white matter. It is shewn in the transverse sections (fig. 19F and G, c). It gradually thins away and disappears behind. It is mainly characterized by the presence on the ventral border of definite transverse commissural fibres.
The skin is formed of three layers.
1. The cuticle.
2. The epidermis or hypodermis.
3. The dermis.
The cuticle is a layer of about 0.002 mm. in thickness. Its surface is not, however, smooth, but is everywhere, with the exception of the perioral region, raised into minute secondary papillæ, the base of which varies somewhat in diameter, but is usually not far from 0.02 mm. On the ventral surface of the body these papillæ are for the most part somewhat blunt, but on the dorsal surface they are more or less sharply pointed. In most instances they bear at their free extremity a somewhat prominent spine. The whole surface of each of the secondary papillæ just described is in its turn covered by numerous minute spinous tubercles. In the perioral region, where the cuticle is smooth, it is obviously formed of two layers which easily separate from each other, and there is I believe a similar division elsewhere, though it is not so easy to see. It is to be presumed that the cuticle is regularly shed.
The epidermis, placed immediately within the cuticle, is composed of a single row of cells, which vary, however, a good deal in size in different regions of the body. The cells excrete the cuticle, and, as shewn in fig. 32, they stand in a very remarkable relation to the secondary papillæ of the cuticle just described. Each epidermis cell is in fact placed within one of these secondary papillæ, so that the cuticle of each secondary papilla is the product of a single epidermis cell. This relation is easily seen in section, while it may also be beautifully shewn by taking a part of the skin which is not too much pigmented, and, after staining it, examining from the surface.
In fig. 32 a region of the epidermis is figured, in which the cells are exceptionally columnar. The cuticle has, moreover, in the process of cutting the section, been somewhat raised and carried away from the subjacent cells. The cells of the epidermis are provided with large oval nuclei, which contain a well-developed [Pg 894] reticulum, giving with low powers a very granular appearance to the nuclei. The protoplasm of the cells is also somewhat granular, and the granules are frequently so disposed as to produce a very well-marked appearance of striation on the inner end of the cells. The pigment which gives the characteristic colour to the skin is deposited in the protoplasm of the outer ends of the cells in the form of small granules. An attempt is made to shew this in fig. 32.
At the apex of most, if not all, the primary wart-like papillæ there are present oval aggregations, or masses of epidermis cells, each such mass being enclosed in a thickish capsule (fig. 31). The cells of these masses appear to form the wall of a cavity which leads into the hollow interior of a long spine. These spines when carefully examined with high objectives present a rather peculiar structure. The base of the spine is enveloped by the normal cuticle, but the spine itself, which terminates in a very fine point, appears, as shewn in fig. 31, to be continuous with the inner layer of the cuticle. In the perioral region the outer layer of the cuticle, as well as the inner, appear to be continued to the end of the spines. Within the base of the spine there is visible a finely striated substance which may often be traced into the cavity enclosed by the cells, and appears to be continuous with the cells. Attached to the inner ends of most of the capsules of these organs a delicate fibrillated cord may be observed, and although I have not in any instance succeeded in tracing this cord into one of the nerve-stems, yet in the antennæ, where the nerve-stems are of an enormous size, I have satisfied myself that the minute nerves leaving the main nerve-stems and passing out towards the skin are histologically not to be distinguished from these fibrillated cords. I have therefore but little hesitation in regarding these cords as nerves.
In certain regions of the body the oval aggregations of cells are extremely numerous; more especially is this the case in the antennæ, lips, and oral papillæ. On the ventral surface of the peripheral rings of the thicker sections of the feet they are also very thick set (fig. 20, P). They here form a kind of pad, and have a more elongated form than in other regions. In the antennæ they are thickly set side by side on the rings of skin [Pg 895] which give such an Arthropod appearance to these organs in Peripatus.
The arrangement of the cells in the bodies just described led me at first to look upon them as glands, but a further investigation induced me to regard them as a form of tactile organ. The arguments for this view are both of a positive and a negative kind.
The positive arguments are the following:
(1) The organs are supplied with large nerves, which is distinctly in favour of their being sense organs rather than glands.
(2) The peculiar striæ at the base of the spines appear to me like the imperfectly preserved remains of sense hairs.
(3) The distribution of these organs favours the view that they are tactile organs. They are most numerous on the antennæ, where such organs would naturally be present, especially in a case like that of Peripatus, where the nerve passing to the antennæ is simply gigantic. On the other hand, the antennæ would not be a natural place to look for an enormous development of dermal glands.
The lips, oral papillæ, and under surface of the legs, where these bodies are also very numerous, are situations where tactile organs would be of great use.
Under the head of negative arguments must be classed those which tell against these organs being glandular. The most important of these is the fact that they have no obvious orifice. Their cavities open no doubt into the spines, but the spines terminate in such extremely fine points that the existence of an orifice at their apex is hardly credible.
Another argument, from the distribution of these organs over the body is practically the converse of that already used. The distribution being as unfavourable to the view that they are glands, as it is favourable to that of their being sense organs.
The apertures of the tracheal system are placed in the depressions between the papillæ or ridges of the skin. Each of them leads into a tube, which I shall call the tracheal pit (fig. 30), the walls of which are formed of epithelial cells bounded [Pg 896] towards the lumen of the pit by a very delicate cuticular membrane continuous with the cuticle covering the surface of the body. The pits vary somewhat in depth; the pit figured was about 0.09 mm. It perforates the dermis and terminates in the subjacent muscular layer. The investigation of the inner end of the pit gave me some little trouble.
Transverse sections (fig. 30) through the trunk containing a tracheal opening shew that the walls of the pit expanded internally in a mushroom-like fashion, the narrow part being, however, often excentric in relation to the centre of the expanded part.
Although it was clear that the tracheæ started from the expanded region of the walls of the pit, I could not find that the lumen of the pit dilated into a large vesicle in this part, and further investigation proved that the tracheæ actually started from the slightly swollen inner extremity of the narrow part of the pit, the expanded walls of the pit forming an umbrella-like covering for the diverging bundles of tracheæ.
I have, in fig. 30, attempted to make clear this relation between the expanded walls of the tracheal pits and the tracheæ. In longitudinal sections of the trunk the tracheal pits do not exhibit the lateral expansion which I have just described, which proves that the divergence of the bundles of tracheæ only takes place laterally and not in an antero-posterior direction. Cells similar in general character to those of the walls of the tracheal pits are placed between the branches of tracheæ, and somewhat similar cells, though generally with more elongated nuclei, accompany the bundles of tracheæ as far as they can be followed in my sections. The structure of these parts in the adult would, in fact, lead one to suppose that the tracheæ had originated at the expense of the cells of pits of the epidermis, and that the cells accompanying the bundles of tracheæ were the remains of cords of cells which sprouted out from the blind ends of the epidermis pits and gave rise in the first instance to the tracheæ.
The tracheæ themselves are extremely minute, unbranched (so far as I could follow them) tubes. Each opening by a separate aperture into the base of the tracheal pit, and measuring about 0.002 mm. in diameter. They exhibit a faint transverse striation, which I take to be the indication of a spiral fibre. [Pg 897] [Moseley (Phil. Trans., 1874, Pl. 73, fig. 1) states that the tracheæ branch, but only exceptionally.]
Situation of the tracheal apertures.—Moseley states (No. 13) that the tracheæ arise from the skin all
over the surface of the body, but are especially developed in certain
regions. He finds a row of minute oval openings on the ventral surface of
the body,
the openings being situate with tolerable regularity in the
centres of the interspaces between the pairs of members, but additional
ones occurring at irregular intervals. Other similar openings occur in
depressions on the inner side of the conical foot protuberance.
It is
difficult in preserved specimens to make out the exact distributions of the
tracheal apertures, but I have been able to make out certain points about
them.
There is a double row of apertures on each side of the median dorsal line, forming two sub-dorsal rows of apertures. The apertures are considerably more numerous than the legs. There is also a double row of openings, again more numerous than the legs, on each side of the median ventral line between the insertions of the legs. Moseley speaks of a median row in this position. I think this must be a mistake.
Posteriorly the two inner rows approach very close to each other in the median ventral line, but I have never seen them in my section opening quite in the middle line. Both the dorsal and ventral rows are very irregular.
I have not found openings on the ventral or dorsal side of the feet but there are openings at the anterior and posterior aspects of the feet. There are, moreover, a considerable number of openings around the base of the feet.
The dorsal rows of tracheal apertures are continued into the head and give rise in this situation to enormous bundles of tracheæ.
In front of the mouth there is a very large median ventral tracheal pit, which gives off tracheæ to the ventral part of the nervous system, and still more in front a large number of such pits close together. The tracheæ to the central nervous system in many instances enter the nervous system bound up in the same sheath as the nerves.
The general muscular system consists of—(1) the general wall of the body; (2) the muscles connected with the mouth, pharynx, and jaws; (3) the muscles of the feet; (4) the muscles of the alimentary tract.
The muscular wall of the body is formed of—(1) an external layer of circular fibres; (2) an internal layer of longitudinal muscles; (3) a layer of transverse fibres.
The layer which I have spoken of as formed of circular fibres is formed of two strata of fibres which girth the body somewhat obliquely (Pl. 51, fig. 25). In the outer stratum the rings are arranged so that their ventral parts are behind, while the ventral parts of the rings of the inner stratum are most forward. Both in the median dorsal and ventral lines the layer of circular fibres become somewhat thinner, and where the legs are attached the regularity of both strata is somewhat interfered with, and they become continuous with a set of fibres inserted in the wall of the foot.
The longitudinal muscles are arranged as five bands (vide fig. 16), viz. two dorsal, two lateral, and three ventral. The three ventral may be spoken of as the latero-ventral and medio-ventral bands.
The transverse fibres consist of (1) a continuous sheet on each side inserted dorsally in the cutis, along a line opposite the space between the dorsal bands of longitudinal fibres, and ventrally between the ventro-median and ventro-lateral bands. Each sheet at its insertion slightly breaks up into separate bands. They divide the body-cavity into three regions—a median, containing the alimentary tract, slime glands, &c., and two lateral, which are less well developed, and contain the nervous system, salivary glands, segmental organs, &c.
(2) Inserted a little dorsal to the transverse band just described is a second band which immediately crosses the first, and then passes on the outer side of the nervous cord and salivary gland, where such is present, and is inserted ventrally in the space between the ventro-lateral and lateral longitudinal band.
[Pg 899] Where the feet are given off the second transverse band becomes continuous with the main retractor muscular fibres in the foot, which are inserted both on to the dorsal side and ventral side.
Muscular system of the feet.—This consists of the retractors of the feet connected with the outer transverse muscle and the circular layer of muscles. In addition to these muscles there are intrinsic transverse muscles which cross the cavity of the feet in various directions (Pl. 51, fig. 20). There is no special circular layer of fibres.
Histology of the muscle.—The main muscles of the body are unstriated and divided into fibres, each invested by a delicate membrane. Between the membrane and muscle are scattered nuclei, which are never found inside the muscle fibres. The muscles attached to the jaws form an exception in that they are distinctly transversely striated.
The body-cavity, as already indicated, is formed of three compartments—one central and two lateral. The former is by far the largest, and contains the alimentary tract, the generative organs, and the mucous glands. It is lined by a delicate endothelial layer, and is not divided into compartments nor traversed by muscular fibres.
The lateral divisions are much smaller than the central, and are shut off from it by the inner transverse band of muscles. They are almost entirely filled with the nerve-cord and salivary gland in front and with the nerve-cord alone behind, and their lumen is broken up by muscular bands. They further contain the segmental organs which open into them. They are prolonged into the feet, as is the embryonic body-cavity of most Arthropoda.
The vascular system is usually stated to consist of a dorsal heart. I find between the dorsal bands of longitudinal fibres a vessel in a space shut off from the body-cavity by a continuation of the endothelial lining of the latter (fig. 16). The vessel has definite walls and an endothelial lining, but I could not make out whether the walls were muscular. The ventral [Pg 900] part of it is surrounded by a peculiar cellular tissue, probably, as suggested by Moseley, equivalent to the fat bodies of insects. It is continued from close to the hind end of the body to the head, and is at its maximum behind. In addition to this vessel there is present a very delicate ventral vessel, by no means easy to see, situated between the cutis and the outer layer of circular muscles.
A series of glandular organs are found in Peripatus which have their external openings situated on the ventral surface of a certain number of the legs, and which, to the best of my belief, end internally by opening into the lateral compartments of the body-cavity. These organs are probably of an excretory nature, and I consider them homologous with the nephridia or segmental organs of the Chætopoda.
In Peripatus capensis they are present in all the legs. In all of them (except the first three) the following parts may be recognized:
(1) A vesicular portion opening to the exterior by a narrow passage.
(2) A coiled portion, which is again subdivided into several sections.
(3) A terminal section ending by a somewhat enlarged opening into the lateral compartment of the body-cavity.
The last twelve pairs of these organs are all constructed in a very similar manner, while the two pairs situated in the fourth and fifth pairs of legs are considerably larger than those behind, and are in some respects very differently constituted.
It will be convenient to commence with one of the hinder nephridia. Such a nephridium from the ninth pair of legs is represented in fig. 28. The external opening is placed at the outer end of a transverse groove placed at the base of one of the feet, while the main portion of the organ lies in the body-cavity in the base of the leg, and extends into the trunk to about the level of the outer edge of the nerve-cord of its side. The external opening (os) leads into a narrow tube (sd), which gradually dilates into a large sack (s).
[Pg 901] The narrow part is lined by small epithelial cells, which are directly continuous with and perfectly similar to those of the epidermis (fig. 20). It is provided with a superficial coating of longitudinal muscular fibres, which thins out where it passes over the sack, along which it only extends for a short distance.
The sack itself, which forms a kind of bladder or collecting vesicle for the organ, is provided with an extremely thin wall, lined with very large flattened cells. These cells are formed of granular protoplasm, and each of them is provided with a large nucleus, which causes a considerable projection into the lumen of the sack (figs. 20, 29, s). The epithelial wall of the sack is supported by a membrana propria, over which a delicate layer of the peritoneal epithelium is reflected.
The coiled tube forming the second section of the nephridium varies in length, and by the character of the epithelium lining it may be divided into four regions. It commences with a region lined by a fairly columnar epithelium with smallish nuclei (fig. 28, sc1). The boundaries of the cells of this epithelium are usually very indistinct, and the protoplasm contains numerous minute granules, which are usually arranged in such a manner as to give to optical or real sections of the wall of this part of the tube a transversely striated appearance. These granules are very probably minute balls of excretory matter.
The nuclei of the cells are placed near their free extremities, contrary to what might have been anticipated, and the inner ends of the cells project for very different lengths into the interior, so causing the inner boundary of the epithelium of this part of the tube to have a very ragged appearance. This portion of the coiled tube is continuous at its outer end with the thin-walled vesicle. At its inner end it is continuous with region No. 2 of the coiled tube (fig. 28, sc2), which is lined by small closely-packed columnar cells. This portion is followed by region No. 3, which has a very characteristic structure (fig. 28 sc3). The cells lining this part are very large and flat, and contain large disc-shaped nuclei, which are usually provided with large nucleoli, and often exhibit a beautiful reticulum. They may frequently be observed in a state of division. The protoplasm of this region is provided with similar granules to that in the first region, and the boundaries of the cells are usually [Pg 902] very indistinct. The fourth region is very short (fig. 28, sc4), and is formed of small columnar cells. It gradually narrows till it opens suddenly into the terminal section (sot), which ends by opening into the body-cavity, and constitutes the most distinct portion of the whole organ. Its walls are formed of columnar cells almost filled by oval nuclei, which absorb colouring matters with very great avidity, and thus renders this part extremely conspicuous. The nuclei are arranged in several rows.
The study of the internal opening of this part gave me some trouble. No specimens ever shew it as rounded off in the characteristic fashion of tubes ending in a cul-de-sac. It is usually somewhat ragged and apparently open. In the best preserved specimens it expands into a short funnel-shaped mouth, the free edge of which is turned back. Sections confirm the results of dissections. Those passing longitudinally through the opening prove its edges are turned back, forming a kind of rudimentary funnel. This is represented in fig. 29, from the last leg of a female. I have observed remains of what I consider to be cilia in this section of the organ. The fourth region of the organ is always placed close to the thin-walled collecting vesicle (figs. 28 and 29). In the whole of the coiled tube just described the epithelium is supported by a membrana propria, which in its turn is invested by a delicate layer of peritoneal epithelium.
The fourth and fifth pairs are very considerably larger than those behind, and are in other respects peculiar. The great mass of each organ is placed behind the leg, on which the external opening is placed, immediately outside one of the lateral nerve-cords. Its position is shewn in fig. 8.
The external opening, instead of being placed near the base of the leg, is placed on the ventral side of the third ring (counting from the outer end) of the thicker portion of the leg. It leads (fig. 27) into a portion which clearly corresponds with the collecting vesicle of the hinder nephridia. This part is not, however, dilated into a vesicle in the same sort of way, and the cells which form the lining epithelium have not the same characteristic structure, but are much smaller. Close to the point where the vesicle joins the coiled section of the nephridium the [Pg 903] former has a peculiar nick or bend in it. At this nick it is firmly attached to the ventral side of the foot by muscles and tracheæ, and when cut away from its attachment the muscles and tracheæ cannot easily be detached from it. The main part of the coils are formed by region No. 1, and the epithelial cells lining this part present very characteristically the striated appearance which has already been spoken of. The large-celled region of the coiled tube (fig. 27) is also of considerable dimensions, and the terminal portion is wedged in between this and the commencing part of the coiled tube. The terminal portion with its internal opening is in its histological characters exactly similar to the homologous region in the hinder nephridia.
The three pairs of nephridia in the three foremost pairs of legs are very rudimentary, consisting, so far as I have been able to make out, solely of the collecting vesicle and the duct leading from them to the exterior. The external opening is placed on the ventral side of the base of the feet, in the same situation as that of the posterior nephridia, but the histological characters of the vesicle are similar to those of the fourth and fifth pairs.
[The sexes are distinct, and the average size of the females appears to be greater than that of the males.
The only outward characteristic by which the males can be distinguished from the females is the presence in the former of a small white papilla on the ventral side of the 17th pair of legs (Pl. 47, fig. 4). At the extremity of this papilla the modified crural gland of the last leg opens by a slit-like aperture.
The generative orifice in both sexes is placed on the ventral surface of the body, close to the anus, and between the two anal papillæ, which are much more marked in small specimens than in large ones, and in two cases (of females) were observed to bear rudimentary claws.
1. The Male Organs. Pl. 53, fig. 43.
The male organs consist of a pair of testes (te), a pair of prostrates (pr) and vasa deferentia (vd) and accessory glandular tubules (f).
[Pg 904] All the above parts lie in the central compartment of the body-cavity. In addition, the accessory glandular bodies or crural glands of the last (17th) pair of legs [TN22] are enlarged and prolonged into an elongated tube placed in the lateral compartment of the body-cavity (ag).
The arrangement of these parts represented in the figure appears essentially that which Moseley has already described for this species. The dilatations on the vasa deferentia, which he calls vesiculæ seminales, is not so marked; nor can the peculiar spiral twisting of this part of the vas deferens which he figures (No. 13) be made out in this specimen. The testes are placed at different levels in the median compartment of the body-cavity, and both lie on the same side of the intestine (right side).
The arrangement of the terminal portions of the vas deferens is precisely that described by Moseley. The right vas deferens passes under both nerve-cords to join the left, and from the enlarged tube (p), which, passing beneath the nerve-cord of its side, runs to the external orifice. The enlarged terminal portion possesses thick muscular walls, and possibly constitutes a spermatophore maker, as has been shewn to be the case in P. N. Zealandiæ, by Moseley.
In some specimens a different arrangement obtains, in that the left vas deferens passes under both nerve-cords to join the right.
In addition to the above structures, which are all described by Moseley, there are a pair of small glandular tubes (f), which open with the unpaired terminal portion of the vas deferens at the generative orifice.
2. Female Organs. Pl. 52, fig. 33.
The female organs consist of a median unpaired ovary and a pair of oviducts, which are dilated for a great part of their course to perform a uterine function, and which open behind into a common vestibule communicating directly with the exterior.
Ovary.—In the specimen figured the following is the arrangement:
The ovary lies rather to the dorsal side in the central compartment of the body-cavity, and is attached to one of the [Pg 905] longitudinal septa separating this from the lateral compartment. It lies between the penultimate and antepenultimate pair of legs.
The oviducts cross before opening to the exterior. The right oviduct passes under the rectum, and the left over the rectum. They meet by opening into a common vestibule, which in its turn opens to the exterior immediately ventral to the anus. It has not been ascertained how far this arrangement, which differs from that observed by Moseley, is a normal one. The young undergo nearly the whole of their development within the uterus. They possess at birth the full number of appendages, and differ from the parent only in size and colour.]
1. They are present in all except the first.
2. They open externally to the nephridia (Pl. 51, fig. 20), except in the fourth and fifth pairs of legs, in which they are internal.
3. A muscular layer covers the whole gland, consisting, I believe, of an oblique circular layer.
4. The accessory gland in the male (fig. 43, ag) is probably a modification of one of these organs.
[The structure and relations of these glands may be best understood by reference to Pl. 51, fig. 20. Each consists of a dilated vesicular portion (fgl) placed in the lateral compartment of the body-cavity in the foot, and of a narrow duct leading to the exterior, and opening on the ventral surface amongst the papillæ of the second row (counting from the internal of the three foot pads—fig. 20, P).
The vesicular portion is lined by columnar cells, with very large oval nuclei, while the duct is lined by cells similar to the epidermic cells, with which they are continuous at the opening.
In the last (17th) leg of the males of this species, this gland (vide above, note 4) possesses a slit-like opening placed at the [Pg 906] apex of a well-developed white papilla (Pl. 47, fig. 4). It is enormously enlarged, and is prolonged forward as a long tubular gland, the structure of which resembles that of the vesicles of the crural glands in the other legs. This gland lies in the lateral compartment of the body-cavity, and extends forward to the level of the 9th leg (Pl. 48, fig. 8, and Pl. 53, fig. 43). It is described by Professor Balfour as the accessory gland of the male, and is seen in section lying immediately dorsal to the nerve-cord in fig. 20, ag.]
[570] Some material for this memoir was left by Prof. Balfour, which will be published separately.
[571]
Vide Spengel, Oligognathus Bonelliæ."
Naples Mittheilungen, Bd.III. pl. IV. fig. 52.
[The remarkable discoveries about the early development of Peripatus, which Balfour made in June last, shortly before starting for Switzerland, have already been the subject of a short communication to the Royal Society (Proc. Roy. Soc. No. 222, 1882). They relate (1) to the blastopore, (2) to the origin of the mesoblast.
Balfour left no manuscript account or notes of his discovery in connection with the drawings which he prepared in order to illustrate it, but he spoke about it to Professor Ray Lankester and also to us, and he further gave a short account of the matter in a private letter to Professor Kleinenberg.
In this letter, which by the courtesy of Professor Kleinenberg we have been permitted to see, he describes the blastopore as an elongated slit-like structure extending along nearly the whole ventral surface; and further states, as the result of his examination of the few and ill-preserved embryos in his possession, that the mesoblast appears to originate as paired outgrowths from the lips of the blastopore.
The drawings left by Balfour in connection with the discoveries are four in number: one of the entire embryo, shewing the slit-like blastopore and the mesoblastic somites, the other three depicting the transverse sections of the same embryo.
[Pg 907] The first drawing (fig. 37), viz. that of the whole embryo, shews an embryo of an oval shape, possessing six somites, whilst along the middle of its ventral surface there are two slit-like openings, lying parallel to the long axis of the body, and placed one behind the other. The mesoblastic somites are arranged bilaterally in pairs, six on either side of these slits. The following note in his handwriting is attached to this drawing:
Young larva of Peripatus capensis.—I could not make out
for certain which was the anterior end. Length 1.34 millimetres.
Balfour's three remaining drawings (figs. 40-42) are, as already stated, representations of transverse sections of the embryo figured by him as a whole. They tend to shew, as he stated in the letter referred to above, that the mesoblast originates as paired outgrowths from the hypoblast, and that these outgrowths are formed near the junction of the hypoblast with the epiblast at the lips of the blastopore.
In fig. 42 the walls of the mesoblastic somites appear continuous with those of the mesenteron near the blastopore.
In fig. 40, which is from a section a little in front of fig. 42, the walls of the mesoblastic somites are independent of those of the mesenteron.
Fig. 41 is from a section made in front of the region of the blastopore.
In all the sections the epiblast lying over the somites is thickened, while elsewhere it is formed of only one layer of cells; and this thickening subsequently appears to give rise to the nervous system. Balfour in his earlier investigations on the present subject found in more advanced stages of the embryo the nerve-cords still scarcely separated from the epiblast[572].
We have since found, in Balfour's material, embryos of a slightly different age to that just described. Of these, three (figs. 34, 35, 36) are younger, while one (fig. 38) is older than Balfour's embryo.
Stage A.—The youngest (fig. 34) is of a slightly oval form, and its greatest length is .48 mm. It possesses a blastopore, [Pg 908] which is elongated in the direction of the long axis of the embryo, and is slightly narrower in its middle than at either end. From one end of the blastopore there is continued an opaque band. This we consider to be the posterior end of the blastopore of the embryo. The blastopore leads into the archenteron.
Stage B.—In the next stage (fig. 35) the embryo is elongate-oval in form. Its length is .7 mm. The blastopore is elongated and slightly narrowed in the middle. At the posterior end of the embryo there is a mass of opaque tissue. On each side of the blastopore are three mesoblastic somites. The length of the blastopore is .45 mm.
Stage C.—In the next stage (fig. 36) the features are much the same as in the preceding. The length of the whole embryo is .9 mm.
The following were the measurements of an embryo of this stage with five somites, but slightly younger than that from which fig. 36 was drawn.
| Length of embryo | .74 mm. |
| Length of blastopore | .46 mm. |
| Distance between hind end of blastopore and hind end of body | .22 mm. |
| Distance between front end of body and front end of blastopore | .06 mm. |
The somites have increased to five, and there are indications of a sixth being budded off from the posterior mass of opaque tissue. The median parts of the lips of the blastopore have come together preparatory to the complete fusion by which the blastopore becomes divided into two parts.
Stage D.—The next stage is Balfour's stage, and has been already described.
The length is 1.34.
It will be observed, on comparing it with the preceding embryos, that while the anterior pair of somites in figs. 35 and 36 lie at a considerable distance from what we have called the anterior end of the embryo (a), in the embryo now under consideration they are placed at the anterior end of the body, one on each side of the middle line. We cannot speak positively as to how they come there, whether by a pushing forward of [Pg 909] the anterior somites of the previous stage, or by the formation of new somites anteriorly to those of the previous stage.
In the next stage it is obvious that this anterior pair of somites has been converted into the præoral lobes.
The anterior of the two openings to which the blastopore gives rise is placed between the second pair of somites; we shall call it the embryonic mouth. The posterior opening formed from the blastopore is elongated, being dilated in front and continued back as a narrow slit (?) to very near the hind end of the embryo, where it presents a second slight dilatation. The anterior dilatation of the posterior open region of the blastopore we shall call the embryonic anus.
Lately, but too late to be figured with this memoir, we have been fortunate enough to find an embryo of apparently precisely the same stage as fig. 37. We are able, therefore, to give a few more details about the stage.
The measurements of this embryo were:
| Length of whole embryo | 1.32 mm. |
| Distance from front end of body to front end of mouth | .32 mm. |
| Distance from embryonic mouth to hind end of embryonic anus | .52 mm. |
| Distance from hind end of embryonic anus to hind end of body | .45 mm. |
| Length of embryonic anus | .20 mm. |
| Length of part of blastopore behind embryonic anus | .20 mm. |
| Greatest width of embryo | .64 mm. |
Stage E.—In the next stage (figs. 38 and 39) the flexure of the hind end of the body has considerably increased. The anterior opening of the blastopore, the embryonic mouth, has increased remarkably in size. It is circular, and is placed between the second pair of mesoblastic somites. The anterior dilatation of the posterior opening of the blastopore, the embryonic anus, has, like the anterior opening, become much enlarged. It is circular, and is placed on the concavity of the ventral flexure. From its hind end there is continued to the hind end of the body a groove (shewn in fig. 39 as a dotted line), which we take to be the remains of the posterior slit-like part of the posterior opening of the blastopore of the preceding stage. The posterior dilatation has disappeared. The [Pg 910] embryo has apparently about thirteen somites, which are still quite distinct from one another, and apparently do not communicate at this stage with the mesenteron.
The epiblast lying immediately over the somites is, as in the, earlier stages, thickened, and the thickenings of the two sides join each other in front of the embryonic mouth, where the anterior pair of mesoblastic somites (the præoral lobes) are almost in contact.
The median ventral epiblast, i.e. the epiblast in the area, bounded by the embryonic mouth and anus before and behind and by the developing nerve-cords laterally, is extremely thin, and consists of one layer of very flat cells. Over the dorsal surface of the body the epiblast cells are cubical, and arranged in one layer.
| Measurements of Embryo of Stage E. | |
|---|---|
| Length of embryo | 1.12 mm. |
| Greatest width | .64 mm. |
| Distance from front end of embryonic mouth to hind end of embryonic anus | .48 mm. |
| Greatest length of embryonic mouth | .16 mm. |
| Length between hind end of embryonic mouth and front end of embryonic anus | .29 mm. |
These measurements were made with a micrometer eyepiece, with the embryo lying on its back in the position of fig. 38, so that they simply indicate the length of the straight line connecting the respective points.
This is the last embryo of our series of young stages. The next and oldest embryo was 3.2 mm. in length. It had ringed antennæ, seventeen (?) pairs of legs, and was completely doubled upon itself, as in Moseley's figure.
The pits into the cerebral ganglia and a mouth and anus were present. There can be no doubt that the mouth and anus of this embryo become the mouth and anus of the adult.
The important question as to the connection between the adult mouth and anus, and the embryonic mouth and anus of the Stage E, must, considering the great gap between Stage E and the next oldest embryo, be left open. Meanwhile, we may point out that the embryonic mouth of Stage E has exactly the [Pg 911] same position as that of the adult; but that the anus is considerably in front of the hind end of the body in Stage E, while it is terminal in the adult.
If the embryonic mouth and anus do become the adult mouth and anus, there would appear to be an entire absence of stomodæum and proctodæum in Peripatus, unless the buccal cavity represents the stomodæum. The latter is formed, as has been shewn by Moseley, by a series of outgrowths round the simple mouth-opening of the embryo, which enclosing the jaws give rise to the tumid lips of the adult.
For our determination of the posterior and anterior ends of each of these embryos, Stage A to E, we depend upon the opaque tissue seen in each case at one end of the blastopore.
In Stage A it has the form of a band, extending backwards from the blastopore.
In Stages B-D, it has the form of an opaque mass of tissue occupying the whole hind end of the embryo, and extending a short distance on either side of the posterior end of the blastopore.
This opacity is due in each case to a proliferation of cells of the hypoblast, and, perhaps, of the epiblast (?).
There can be no doubt that the mesoblast so formed gives rise to the great majority of the mesoblastic somites.
This posterior opacity is marked in Stage C by a slight longitudinal groove extending backwards from the hind end of the blastopore. This is difficult to see in surface views, and has not been represented in the figure, but is easily seen in sections.
But in Stage D this groove has become very strongly marked in surface views, and looks like a part of the original blastopore of Stage C.
Sections shew that it does not lead into the archenteron, but only into the mass of mesoblast which forms the posterior opacity. It presents an extraordinary resemblance to the primitive streak of vertebrates, and the ventral groove of insect embryos.
We think that there can be but little doubt that it is a part of the original blastopore, which, on account of its late appearance (this being due to the late development of the posterior [Pg 912] part of the body to which it belongs), does not acquire the normal relations of a blastopore, but presents only those rudimentary features (deep groove connected with origin of mesoblast) which the whole blastopore of other tracheates presents.
We think it probable that the larval anus eventually shifts to the hind end of the body, and gives rise to the adult anus. We reserve the account of the internal structure of these embryos (Stages A-E) and of the later stages for a subsequent memoir.
We may briefly summarise the more important facts of the early development of Peripatus capensis, detailed in the preceding account.
1. The greater part of the mesoblast is developed from the walls of the archenteron.
2. The embryonic mouth and anus are derived from the respective ends of the original blastopore, the middle part of the blastopore closing up.
3. The embryonic mouth almost certainly becomes the adult mouth, i.e. the aperture leading from the buccal cavity into the pharynx, the two being in the same position. The embryonic anus is in front of the position of the adult anus, but in all probability shifts back, and persists as the adult anus.
4. The anterior pair of mesoblastic somites gives rise to the swellings of the præoral lobes, and to the mesoblast of the head[573].
There is no need for us to enlarge upon the importance of these facts. Their close bearing upon some of the most important problems of morphology will be apparent to all, and we may with advantage quote here some passages from Balfour's Comparative Embryology, which shew that he himself long ago had anticipated and in a sense predicted their discovery.
Although the mesoblastic groove of insects is not a gastrula, it is
quite possible that it is the rudiment of a blastopore, the gastrula
corresponding to which has now vanished [Pg 913] from
development.
(Comparative Embryology, Vol. I. p. 378, the
original edition[574].)
Tracheata.—Insecta. It (the mesoblast)
grows inwards from the lips of the germinal groove, which probably
represents the remains of a blastopore.
(Comparative
Embryology, Vol. II. p. 291, the
original edition[575].)
It is, therefore, highly probable that the paired ingrowths of the
mesoblast from the lips of the blastopore may have been, in the first
instance, derived from a pair of archenteric diverticula.
(Comparative Embryology, Vol.
II.
p. 294, the original edition[576].)
The facts now recorded were discovered in June last, only a short time before Balfour started for Switzerland; we know but little of the new ideas which they called up in his mind. We can only point to passages in his published works which seem to indicate the direction which his speculations would have taken.
After speculating as to the probability of a genetic connection between the circumoral nervous system of the Cœlenterata, and the nervous system of Echinodermata, Platyhelminthes [TN23], Chætopoda, Mollusca, &c., he goes on to say:
A circumoral nerve-ring, if longitudinally
extended, might give rise to a pair of nerve-cords united in front and
behind—exactly such a nervous system, in fact, as is present in many
Nemertines (the Enopla and Pelagonemertes), in Peripatus and in
primitive molluscan types (Chiton, Fissurella, &c.). From the lateral parts of this ring it would be
easy to derive the ventral cord of the Chætopoda and Arthropoda. It is
especially deserving of notice, in connection with the nervous system of
the above mentioned Nemertines and Peripatus, that the commissure
connecting the two nerve-cords behind is placed on the dorsal side of the
intestines. As is at once obvious, by referring to the diagram (fig. 231
B), this is the position this commissure
ought, undoubtedly, to occupy if derived from part of a nerve-ring which
originally followed more or less closely the ciliated edge of the body of
the supposed radiate ancestor.
(Comparative Embryology,
Vol. II. pp. 311, 312,
the original edition[577].)
[Pg 914] The facts of development here recorded give a strong additional support to this latter view, and seem to render possible a considerable extension of it along the same lines.]
List of Memoirs on Peripatus.
1. M. Lansdown Guilding. An
Account of a New Genus of Mollusca,
Zoological Journal,
Vol. II. p. 443, 1826.
2. M. Andouin and Milne-Edwards. Classific. des Annélides et
description de celles qui habitent les côtes de France,
p. 411,
Ann. Scien. Nat. ser.
I. Vol. XXX. 1833.
3. M. Gervais. Études p. servir à l'histoire naturelle des Myriapodes,
Ann. Scien. Nat. ser. II. Vol. VII. 1837, p. 38.
4. Wiegmann. Wiegmann's Archiv, 1837.
5. H. Milne-Edwards. Note sur le Peripate juluforme,
Ann. Scien.
Nat. ser. II. Vol. XVIII. 1842.
6. Blanchard. Sur
l'organisation des Vers,
chap. IV. pp. 137-141, Ann. Scien. Nat.
ser. III. Vol. VIII. 1847.
7. Quatrefages. Anat. des Hermelles, note on,
p. 57, Ann. Scien. Nat. ser. III. Vol. X. 1848.
8. Quatrefages. Hist. Nat. des Annelés, 1865, Appendix, pp. 675-6.
9. De Blainville. Suppl. au Dict. des Sc. Nat. Vol. I.
10. Ed. Grube. Untersuchungen üb. d. Bau von Peripatus Edwardsii,
Archiv für Anat. und Physiol. 1853.
11. Saenger. Moskauer Naturforscher Sammlung,
Abth.
Zool. 1869.
12. H. N. Moseley. On the
Structure and Development of Peripatus capensis,
Proc. Roy. Soc. No. 153, 1874.
13. H. N. Moseley. On the
Structure and Development of Peripatus capensis,
Phil. Trans. Vol. CLXIV. 1874.
14. H. N. Moseley. Remarks on
Observations by Captain Hutton, Director of the Otago Museum, on
Peripatus novæ zealandiæ,
Ann. and Mag. of Nat. History, Jan. 1877.
15. Captain Hutton.
Observations on Peripatus novæ zealandiæ,
Ann. and Mag. of Nat.
History, Nov. 1876.
16. F. M. Balfour. On Certain
Points in the Anatomy of Peripatus capensis,
Quart. Journ. of Micr.
Science, Vol. XIX. 1879.
17. A. Ernst. Nature, March 10th, 1881.
EXPLANATION OF PLATES 46-53[578].
Complete List of Reference Letters.
A. Anus. a. Dorso-lateral horn of white matter in brain. a.g. Accessory gland of male (modified accessory leg gland). at. Antenna. at.n. Antennary nerve. b. Ventro-lateral horn of white matter of brain. b.c. Body-cavity. bl. Blastopore. C. Cutis. c. Postero-dorsal lobe of white matter of brain. c.g. Supra-œsophageal ganglia. cl. Claw. c.m. Circular layer of muscles. co. Commissures between the ventral nerve-cords. co.2. Second commissure between the ventral nerve-cords. co1. 2. Mass of cells developed on second commissure. cor. Cornea. c.s.d. Common duct for the two salivary glands. cu. Cuticle. d. Ventral protuberance of brain. d.l.m. Dorsal longitudinal muscle of pharynx. d.n. Median dorsal nerve to integument from supra-œsophageal ganglia. d.o. Muscular bands passing from the ventro-lateral wall of the pharynx at the region of its opening into the buccal cavity. E. Eye. E. Central lobe of white matter of brain. e.n. Nerves passing outwards from the ventral cords. ep. Epidermis. ep.c. Epidermis cells. F.1, F.2, &c. First and second pair of feet, &c. f. Small accessory glandular tubes of the male generative apparatus. F.g. Ganglionic enlargement on ventral nerve-cord, from which a pair of nerves to foot pass off. f.gl. Accessory foot-gland. F.n. Nerves to feet. g.co. Commissures between the ventral nerve-cords containing ganglion cells. g.o. Generative orifice. H. Heart. h. Cells in lateral division of body-cavity. hy. Hypoblast. i.j. Inner jaw. j. Jaw. j.n. Nerves to jaws. L. Lips. l. Lens. l.b.c. Lateral compartment of body-cavity. le. Jaw lever (cuticular prolongation of inner jaw lying in a backwardly projecting diverticulum of the buccal cavity). l.m. Bands of longitudinal muscles. M. Buccal cavity. M1. Median backward diverticulum of mouth or common salivary duct which receives the salivary ducts. me. Mesenteron. mes. Mesoblastic somite. m.l. Muscles of jaw lever. m.s. Sheets of muscle passing round the side walls of pharynx to dorsal body-wall. od. Oviduct. œ. Œsophagus. œs.co. Œsophageal commissures. o.f.g. Orifice of duct of foot-gland. o.j. Outer jaw. op. Optic ganglion. op.n. Optic nerve. or.g. Ganglionic enlargements for oral papillæ. or.n. Nerves to oral papillæ. or.p. Oral papillæ. o.s. Orifice of duct of segmental organ. ov. Ovary. P. Pads on ventral side of foot. p. Common duct into which the vasa deferentia open. p.c. Posterior lobe of brain. p.d.c. Posterior commissure passing dorsal to rectum. p.f. Internal opening of nephridium into body-cavity. ph. Pharynx. pi. Pigment in outer ends of epidermic cells. pi.r. Retinal pigment. p.n. Nerves to feet. p.p. Primary papilla. pr. Prostate. R. Rectum. re. Retinal rods. R. m. Muscle of claw. s. Vesicle of nephridium. s1. Part of 4th or 5th nephridium which corresponds to vesicle of other nephridia. [Pg 916] s.c.1. Region No. 1 of coiled tube of nephridium. s.c.2. Region No. 2 of ditto. s.c.3. Region No. 3 of ditto. s.c.4. Region No. 4 of ditto. s.d. Salivary duct. s.g. Salivary gland. sl.d. Reservoir of slime gland. sl.g. Tubules of slime gland. s.o.1, 2, 3, &c. Nephridia of 1st, 2nd, &c., feet. s.o.f. Terminal portion of nephridium. s.p. Secondary papilla. st. Stomach. st.e. Epithelium of stomach. sy. Sympathetic nerve running in muscles of tongue and pharynx. sy´. Origin of pharyngeal sympathetic nerves. T. Tongue. t. Teeth on tongue. te. Testis. tr. Tracheæ. tr.c. Cells found along the course of the tracheæ. tr.o. Tracheal stigma. tr.p. Tracheal pit. ut. Uterus. v.c. Ventral nerve cord. v.d. Vas deferens. v.g. Imperfect ganglia of ventral cord.
Plate 46.
Fig. 1. Peripatus capensis, x 4; viewed from the dorsal surface. (From a drawing by Miss Balfour.)
Plate 47.
Fig. 2. A left leg of Peripatus capensis, viewed from the ventral surface; x 30. (From a drawing by Miss Balfour.)
Fig. 3. A right leg of Peripatus capensis, viewed from the front side. (From a drawing by Miss Balfour.)
Fig. 4. The last left (17th) leg of a male Peripatus
capensis, viewed from the ventral side to shew the papilla at the apex
of which the accessory gland of the male, or enlarged crural gland, opens
to the exterior. (From a drawing by Miss Balfour.) Prof. Balfour left a
rough drawing (not reproduced) shewing the papilla, to which is appended
the following note. Figure shewing the accessory genital gland of male,
which opens on the last pair of legs by a papilla on the ventral side. The
papilla has got a slit-like aperture at its extremity.
Fig. 5. Ventral view of head and oral region of Peripatus capensis. (From a drawing by Miss Balfour.)
Plate 48.
Figs. 6 and 7 are from one drawing.
Fig. 6. Peripatus capensis dissected so as to shew the alimentary canal, slime glands, and salivary glands; x 3. (From a drawing by Miss Balfour.)
Fig. 7. The anterior end of Fig. 6 enlarged; x 6. (From a drawing by Miss Balfour.) The dissection is viewed from the ventral side, and the lips, L., have been cut through in the middle line behind and pulled outwards, so as to expose the jaws, j., which have been turned outwards, and the tongue, T., bearing a median row of chitinous teeth, which branches behind into two. The junction of the salivary ducts, s.d., and the opening of the median duct so formed into the buccal cavity is also shewn. The muscular pharynx, extending back into the space between the 1st and 2nd pairs of legs, is followed by a short tubular œsophagus. The latter opens into the large stomach with plicated walls, extending almost to the hind end of the animal. The stomach at its point of junction with the rectum presents an S-shaped ventro-dorsal curve.
[Pg 917] A. Anus. at. Antenna. F.1, F.2. First and second feet. j. Jaws. L. Lips. œ. Œsophagus. or.p. Oral papilla. ph. Pharynx. R. Rectum. s.d. Salivary duct. s.g. Salivary gland. sl.d. Slime reservoir. sl.g. Portion of tubules of slime gland. st. Stomach. T. Tongue in roof of mouth.
Fig. 8. Peripatus capensis, x 4; male. (From a drawing by Miss Balfour.) Dissected so as to shew the nervous system, slime glands, ducts of the latter passing into the oral papilla, accessory glands opening on the last pair of legs (enlarged crural glands), and segmental organs, viewed from dorsal surface. The first three pairs of segmental organs consist only of the vesicle and duct leading to the exterior. The fourth and fifth pairs are larger than the succeeding, and open externally to the crural glands. The ventral nerve-cords unite behind dorsal to the rectum.
A. Anus. a.g. Accessory generative gland, or enlarged crural gland of the 17th leg. at. Antenna. c.g. Supra-œsophageal ganglia with eyes. co. Commissures between the ventral nerve-cords. d.n. Large median nerve to dorsal integument from hinder part of brain. F.1, 2, &c. Feet. g.o. Generative orifice. œ. Œsophagus. œs.co. Œsophageal commissures. or.p. Oral papilla. p.d.c. Posterior dorsal commissure between the ventral nerve-cords. ph. Pharynx. p.n. Nerves to feet, one pair from each ganglionic enlargement. sl.d. Reservoir of slime gland. sl.g. Tubules of slime gland. s.o.1, 2, 3, &c. Segmental organs. v.c. Ventral nerve-cords. v.g. Imperfect ganglia of ventral cords.
Figs. 9 and 10. Left jaw of Peripatus capensis (male), shewing reserve jaws. (From a drawing by Miss Balfour.)
Fig. 9. Inner jaw.
Fig. 10. Outer jaw.
Plate 49.
Figs. 11-16. A series of six transverse sections through the head of Peripatus capensis.
Fig. 11. The section is taken immediately behind the junction of the supra-œsophageal ganglia, c.g., and passes through the buccal cavity, M., and jaws, o.j. and i.j.
Fig. 12. The section is taken through the hinder part of the buccal cavity at the level of the opening of the mouth into the pharynx and behind the jaws. The cuticular rod-like continuation (le.) of the inner jaw lying in a backwardly directed pit of the buccal cavity is shewn; on the right hand side the section passes through the opening of this pit.
Fig. 13. The section passes through the front part of the pharynx, and shews the opening into the latter of the median backward diverticulum of the mouth (M1), which receives the salivary ducts. It also shews the commencement of the ventral nerve-cords, and the backwardly projecting lobes of the brain.
Fig. 14. The section passes through the anterior part of the pharynx at the level of the second commissure (co.2), between the ventral nerve-trunks, and shews the mass of cells developed on this commissure, which is in contact with the epithelium of the backward continuation of the buccal cavity (M1).
[Pg 918] Fig. 15. Section through the point of junction of the salivary ducts with the median oral diverticulum.
Fig. 16. Section behind the pharynx through the œsophagus.
b.c. Body-cavity. C. Cutis. c.b.c. Central compartment of body-cavity. c.g. Supra-œsophageal ganglia. c.m. Layer of circular muscles. co. Commissure between ventral nerve-cords. co. 2. Second commissure between the ventral nerve-cords. co1. 2. Mass of cells developed on second commissure (probably sensory). c.s.d. Common duct for the two salivary glands. d.l.m. Dorsal longitudinal muscles of pharynx. d.o. Muscles serving to dilate the opening of the pharynx. Ep. Epidermis. e.n. Nerve passing outwards from ventral nerve-cord. H. Heart. i.j. Inner jaw. j.p. Jaw papillæ. L. Lips of buccal cavity. l.b.c. Lateral compartment of body-cavity. le. Rod-like cuticular continuation of inner jaw, lying in a pit of the buccal cavity. l.m. Bands of longitudinal muscles. M. Buccal cavity. M1. Median backward continuation of buccal cavity. m.l. Muscles of jaw lever. m.s. Muscular sheets passing from side walls of pharynx to dorsal body-wall. œ. Œsophagus. œs.co. Œsophageal commissures. o.j. Outer jaw. ph. Pharynx. s.d. Salivary duct. s.g. Salivary gland. sl.d. Reservoir of slime gland. sy. Sympathetic nerves running in muscles of tongue or pharynx. sy1. Origin of sympathetic nerves to pharynx. T. Tongue. v.c. Ventral nerve-cords.
Figs. 17, 18. Two longitudinal horizontal sections through the head of Peripatus capensis. Fig. 17 is the most ventral. They are both taken ventral to the cerebral ganglia. In Fig. 17 dorsal tracheal pits are shewn with tracheæ passing off from them. (Zeiss a a, Hartnack's camera.) C. Cutis. c.s.d. Common salivary duct. ep. Epidermis. i.j. Inner jaw. M. Buccal cavity. M1. Median backward diverticulum of mouth. o.j. Outer jaw. s.d. Salivary ducts. T. Tongue. t. Teeth on tongue. tr. Tracheæ. tr.p. Tracheal pits.
Plate 50.
Fig. 19. "A, B, C, D, E, F, G." Seven transverse sections illustrating the structure of the supra-œsophageal ganglia. (Zeiss A, Hartnack's camera.) a. Dorso-lateral horn of white matter. b. Ventro-lateral horn of white matter. c. Postero-dorsal lobe of white matter. d. Ventral protuberance of brain. e. Central lobe of white matter. o.p. Optic ganglion.
A. Section through
anterior portions of ganglia close to the origin of the antennary nerve.
B. Section a little in front of the point
where the two ganglia unite. C. Section
close to anterior junction of two ganglia. D. Section through origin of optic nerve on the right
side. E. Section shewing origin of the
optic nerve on the left side. F. Section
through the dorso-median lobe of white matter. G. Section near the termination of the dorsal tongue
of ganglion cells.
Plate 51.
Fig. 20. Portion of a transverse section through the hinder part of Peripatus capensis (male). The section passes through a leg, and shews the opening of the segmental organ (o.s.), and of a crural gland, o.f.g., and the forward continuation of [Pg 919] the enlarged crural gland of the 17th leg (f.gl.). (Zeiss a a, Hartnack's camera.) a.g. accessory gland of male (modified crural gland of last leg). C. Cutis. cl. Claw. cu. Cuticle. ep. Epidermis. f.gl. Crural gland. h. Cells in lateral compartment of body-cavity. o.f.g. Orifice of accessory foot gland. o.s. Opening of segmental organ. P. Three spinous pads on ventral surface of foot. pr. Prostate. R.M. Retractor muscle of claw. s. Vesicle of nephridium. s.c.i. Region No. 1 of coiled part of nephridium. sl.g. Tubule of slime gland. s.o.t. Terminal portion of nephridium. st. Stomach. st.e. Epithelium of stomach. v.c. Ventral nerve-cord. v.d. Vas deferens.
Fig. 21. Longitudinal vertical section through the
supra-œsophageal ganglion and œsophageal commissures of
Peripatus capensis. (Zeiss a a, Hartnack.)
at. Antenna. e. Central lobe of white matter.
j. Part of jaw. s.g. Salivary
gland.
Fig. 22: drawn by Miss Balfour. Brain and anterior part of the ventral nerve-cords of Peripatus capensis enlarged and viewed from the ventral surface. The paired appendages (d) of the ventral surface of the brain are seen, and the pair of sympathetic nerves (sy1) arising from the ventral surface of the hinder part.
From the commencement of the œsophageal commissures (œs.co.) pass off on each side a pair of nerves to the jaws (j.n.).
The three anterior commissures between the ventral nerve-cords are placed close together; immediately behind them the nerve-cords are swollen, to form the ganglionic enlargements from which pass off to the oral papillæ a pair of large nerves on each side (or.n.).
Behind this the cords present a series of enlargements, one pair for each pair of feet, from which a pair of large nerves pass off on each side to the feet (p.n). at.n. Antennary nerves. co. Commissures between ventral cords. d. Ventral appendages of brain. E. Eye. e.n. Nerves passing outwards from ventral cord. F.g. Ganglionic enlargements from which nerves to feet pass off. j.n. Nerves to jaws. or.g. Ganglionic enlargement from which nerves to oral papillæ pass off. or.n. Nerves to oral papillæ. p.c. Posterior lobe of brain. p.n. Nerves to feet. s.y. Sympathetic nerves.
Fig. 23. Longitudinal horizontal section through the
head of Peripatus capensis, shewing the structure of the brain, the
antennary and optic nerves, &c. (Zeiss
a a, Hartnack's camera.)
at.
Antenna. at.n. Antennary nerve. cor. Cornea. e. Central mass of white
matter. l. Lens. op.n. Optic
nerve. ph. Pharynx. p.p. Primary papilla covered with secondary papillæ
and terminating in a long spine. sy.
Pharyngeal sympathetic nerves.
Fig. 24. Eye of Peripatus capensis, as shewn
in a longitudinal horizontal section through the head. The figure is so far
diagrammatic that the lens is represented as filling up the whole space
between the rods and the cornea. In the actual section there is a
considerable space between the parts, but this space is probably
artificial, being in part caused by the shrinkage of the lens and in part
by the action of the razor. (Zeiss C,
Hartnack's camera.)
(It appears that the ganglionic region of the eye
is covered by a thin capsule, which is omitted in the figure.)
cor. Cornea. l. Lens. op. Optic ganglion. op.n. Optic nerve. pi.r. Pigment. Re. rods. s.p. Secondary papillæ.
[Pg 920] Fig. 25. Longitudinal horizontal section through the dorsal skin, shewing the peculiar arrangement of the circular muscular fibres. (Zeiss A, Hartnack's camera.)
Plate 52.
Fig. 26. Portion of ventral cord of Peripatus capensis enlarged, shewing two ganglionic enlargements and the origin of the nerves and commissures. (From a drawing by Miss Balfour.)
co. Commissures. E.n. Nerves passing out from ventral cords. F.n. Nerves to feet. g.co. Commissures between the ventral cords containing ganglion cells. v.g. Ganglionic enlargements.
Fig. 27. Segmental organ from the 5th pair of legs of Peripatus capensis. This nephridium resembles those of the 4th legs, and differs from all the others in its large size and in the absence of any dilatation giving rise to a collecting vesicle on its external portion (enlarged). The terminal portion has the same histological characters as in the case of the hinder segmental organs. (From a drawing by Miss Balfour.)
Fig. 28. Segmental organ or nephridium from the 9th pair of legs of Peripatus capensis, shewing the external opening, the vesicle, the coiled portion and the terminal portion with internal opening (enlarged). (From a drawing by Miss Balfour.)
o.s. External opening of segmental organ. p.f. Internal opening of nephridium into the body-cavity (lateral compartment). s. Vesicle of segmental organ. s1. Portion of segmental organ of 4th and 5th legs, corresponding to vesicle of the other nephridia. s.c.1. First or external portion of coiled tube of nephridium, lined by columnar epithelium with small nuclei; the cells project for very different distances, giving the inner boundary of this region a ragged appearance. s.c.2. Region No. 2 of coiled tube of nephridium, lined by small closely-packed columnar cells. s.c.3. Region No. 3 of coiled tube of segmental organ, lined by large flat cells with large disc-shaped nuclei. s.c.4. Region No. 4 of coiled tube of nephridium; this region is very short and lined by small columnar cells. s.o.t. Terminal portion of nephridium.
Fig. 29. Portion of nephridium of the hindermost leg
of Peripatus capensis, seen in longitudinal and vertical section.
The figure is given to shew the peritoneal funnel of the nephridium.
Portions of the collecting sack (s.) and other parts are also
represented. (Zeiss B, Hartnack's
camera.)
p.f. Peritoneal funnel. s. Vesicle. s.c.1, s.c.2, s.c.3. Portions of coiled tube.
Fig. 30. Section through a tracheal pit and diverging
bundles of tracheal tubes
taken transversely to the long axis of the
body. (Zeiss E, oc. 2.) (From a rough drawing by Prof. Balfour.)
tr. Tracheæ, shewing rudimentary spiral fibre. tr.c. Cells resembling those lining the tracheal pits, which occur at intervals along the course of the tracheæ. tr.s. Tracheal stigma. tr.p. Tracheal pit.
Fig. 31. Sense organs and nerves attached from
antenna of Peripatus capensis (Zeiss, immersion 2, oc. 2.)
(From a rough drawing by Prof. Balfour.) The
figure shews the arrangement of the epidermis cells round the base of the
spine. The spine is seen to be continuous with the inner layer of the
cuticle.
[Pg 921] Fig. 32. Section through the skin of Peripatus capensis; it shews the secondary papillæ covered with minute spinous tubercles and the relation of the epidermis to them. (The cuticle in the process of cutting has been torn away from the subjacent cells.) The cells of the epidermis are provided with large oval nuclei, and there is a deposit of pigment in the outer ends of the cells. The granules in the protoplasm of the inner ends of the cells are arranged in lines, so as to give a streaked appearance. (Zeiss E, oc. 2.) (From a rough drawing by Prof. Balfour.)
c. Dermis. cu. Cuticle. ep.c. Epidermis cells. pi. Deposit of pigment in outer ends of epidermis cells. s.p. Secondary papillæ.
Fig. 33. Female generative organs of Peripatus
capensis, × 5. (From a rough drawing by Prof. Balfour.) The following
note was appended to this drawing: Ovary rather to dorsal side, lying in
a central compartment of body-cavity and attached to one of the
longitudinal septa, dividing this from the lateral compartment between the
penultimate pair of legs and that next in front. The oviducts cross before
opening to the exterior, the right oviduct passing under the rectum and the
left over it. They meet by opening into a common vestibule, which in its
turn opens below the anus. On each side of it are a pair of short papillæ
(aborted feet?).
F. 16, 17. Last two pairs of legs. od. Oviduct. ov. Ovary. ut. Uterus. v.c. Nerve-cord.
Plate 53.
Figs. 34-39. Five young embryos of Peripatus capensis; ventral view. All, excepting Fig. 37, from drawings by Miss Balfour. In figures 34 to 38a denotes what is probably the anterior extremity.
Fig. 34, Stage A. Youngest embryo found, with slightly elongated blastopore.
Fig. 35, Stage B. Embryo with three mesoblastic somites and elongated blastopore. The external boundaries of the somites are not distinct.
Fig. 36, Stage C. Embryo with five somites. The blastopore is closing in its middle portion.
Fig. 37, Stage D. The blastopore has completely closed in its middle portion, and given rise to two openings, the future mouth and anus. (From a rough drawing left by Professor Balfour.) (Zeiss A, Camera Oberhaus. on level of stage.)
The following note was appended to this drawing in his
handwriting: Young larva of Peripatus capensis. I could not tell
for certain which was the anterior end. Length, 1.34 mm.
Fig. 38, Stage E. Embryo with about thirteen mesoblastic somites in which the flexure of the hind part of the body has commenced. The remains of the original blastopore are present as the mouth, placed between the second pair of mesoblastic somites, and the anus placed on the concavity of the commencing flexure of the hind part of the body.
[Pg 922] Fig. 39. Side view of same embryo.
Figs. 40-42. Drawings by Professor Balfour of three transverse sections through the embryo from which fig. 36 was taken. (Zeiss c, Camera.) Figs. 40 and 42 pass through the region of the blastopore.
bl. Blastopore. ep. Epiblast. hy. Hypoblast. me. Mesenteron. mes. Mesoblastic somite.
Fig. 43. Male generative organs of Peripatus capensis, viewed from the dorsal surface. (From a drawing by Miss Balfour.)
a.g. Enlarged crural glands of last pair of legs. F.16, 17. Last pairs of legs. f. Small accessory glandular tubes. p. Common duct into which vasa deferentia open. p.r. Prostate. te. Testes. v.c. Nerve-cord. v.d. Vas deferens.
[572] Comparative Embryology; original edition, Vol. I. p. 318. [This edition, Vol. II. p. 385.]
[573] We have seen nothing in any of our sections which we can identify as of so-called mesenchymatous origin.
[574] This edition, Vol. II. p. 457.
[575] This edition, Vol. III. p. 352.
[576] This edition, Vol. III. p. 356.
[577] This edition, Vol. III. pp. 378, 379.
[578] The explanations of the figures printed within inverted commas are by Professor Balfour, the rest are by the Editors.
CAMBRIDGE: PRINTED BY C. J. CLAY, M.A., AND SON, AT THE UNIVERSITY PRESS.
TRANSCRIBER'S NOTES:
Hyphens were spaced in ranges of small numbers to ease readability,
e.g., 1/2000-1/3000 of an inch
was changed to 1/2000 - 1/3000 of
an inch
.
Raised dots in numbers were converted to decimals.
Use of periods and commas in the abbreviations within and referring to figures and plates is inconsistent. Often, punctuation marks do not match the illustrations to which they refer. Periods were retained; commas were added to separate figure numbers from abbreviations within the figure. Spacing within the abbreviations was standardized.
Footnotes were renumbered sequentially, and were moved to the end of the chapter. There is no anchor for footnote 496; anchor was placed at the spot the transcriber deemed it likely belonged. Footnote 351 incorrectly identifies the page number of the article on urinogential organs of vertebrates. The number provided in the original text of the footnote was retained, but the link was corrected.
Changes for consistency within the text of the book:
body cavity to body-cavity
body wall to body-wall
choroid-slit to choroid slit
develope(s) to develop(s)
dog fish to dog-fish
Elasmobranchs to Elasmobranchii
Entwickelung to Entwicklung
head-fold to head fold
inter-renal to interrenal
juxta-position to juxtaposition
lenslike to lens-like
re-agent(s) to reagent(s)
omphalo-meseraic to omphalomeseraic
pleuroperitoneal to pleuro-peritoneal
proto-vertebra(æ) to protovertebra(æ)
re-appear to reappear
semi-lunar to semilunar
side-fold to side fold
spongework to sponge-work
subgerminal to sub-germinal
sub-intestinal to subintestinal
sub-kingdom to subkingdom
sub-notochordal to subnotochordal
suboesophageal to sub-oesophageal
supraoesophageal to supra-oesophageal
urino-genital to urinogenital
Urogenital-system to Urogenitalsystem, except where cited as a title of a work.
Verwandschaft to Verwandtschaft
widespread to wide-spread
wood-cut(s) to woodcut(s)
zool. zoot. to zool.-zoot.
italics removed from eight instances of vide
italics, where missing, were added to loc. cit., i.e. and e.g.
Other changes:
[TN1] changed from 'reremainder'
[TN2] changed
from 'on'
[TN3] changed
from 'splachnopleure'
[TN4] changed
from 'Sitzen.'
[TN5] changed
from 'diffiulty'
[TN6] changed
from 'it'
[TN7] changed
from 'primive'
[TN8] and [TN10] changed
from 'opthalmicus'
[TN9] Figure
number is missing in the original.
[TN11] changed
from 'Ureierernester'
[TN12] changed
from 'vascula'
[TN13] changed
from 'Metozoa'
[TN14]
duplicate word 'of' removed
[TN15] changed
from 'protodæum'
[TN16] changed
from 'is it'
[TN17] changed
from 'is is'
[TN18] changed
from 'continous'
[TN19] changed
from 'Zussammenhang'
[TN20] changed
from 'Tranverse'
[TN21] changed
from 'odontophor'
[TN22] changed
from 'lens'
[TN23] changed
from 'Platyelminthes'